Abstract
Background
It has been reported that the clinical expression of obstructive sleep apnea (OSA) may differ in women and men.
Objective
The objective of this study was to evaluate the influence of gender on reported OSA-related symptoms in a large clinical population of patients.
Methods
The database from the sleep laboratory of a tertiary care center was examined. Adult patients who had undergone a diagnostic polysomnography and completed the Berlin questionnaire, a sleep questionnaire, and the Epworth sleepiness scale were selected. Multiple logistic regression analysis was performed to assess the relationship between OSA-associated symptoms and different potential explanatory variables.
Results
The study sample included 1084 patients, median age was 53 years, 46.5% (504) were women, 72.7% (788) had OSA (apnea/hypopnea index ≥ 5), and 31.2% were obese. After adjusting for age, body mass index, and apnea/hypopnea index, men were more likely to report snoring (OR 4.06, p < 0.001), habitual or loud snoring (OR 2.34, p < 0.001; 2.14, p < 0.001, respectively) and apneas (OR 2.44, p < 0.001), than women. After controlling for multiple variables, female gender was an independent predictive factor for reported tiredness (OR 0.57, p 0.001), sleep onset insomnia (OR 0.59, p 0.0035), and morning headaches (OR 0.32, p < 0.001). Reports of excessive daytime sleepiness, nocturia, midnight insomnia, and subjective cognitive complaints were not significantly associated with gender.
Conclusion
Women with OSA were more likely to report tiredness, initial insomnia, and morning headaches, and less likely to complain of typical OSA symptoms (snoring, apneas) than men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on available general population-based studies, the prevalence of obstructive sleep apnea (OSA) associated with accompanying daytime sleepiness is approximately 11% for adult men and 4% for adult women [1]. It has been suggested that the higher prevalence of OSA in males may result from the fact that women do not show the classic symptomatology or consult for nonspecific symptoms, and thus they may be underdiagnosed. Also, men have a longer, collapsible oropharynx, which is the pharyngeal segment involved in OSA, and a larger, fatter, posterior tongue than women and, hence, more chance of developing OSA [2].
Patients with obstructive sleep apnea usually consult for symptoms such as habitual or loud snoring, apneas, tiredness or fatigue, and excessive daytime sleepiness. Sometimes, the initial complaints are nonspecific symptoms such as insomnia, frequent awakenings at night, nocturia, morning headaches, or neurocognitive impairment [3]. Whereas some studies have pointed out that women complain more frequently of insomnia [4,5,6,7,8,9] and daytime tiredness [7,8,9] compared to men, others have shown discordant results regarding the reporting of snoring [6, 10, 11], apnea [4,5,6, 10], and excessive daytime sleepiness [4, 6, 7].
Several studies have conducted comparative analyses of the prevalence of symptoms between women and men with OSA, with [4, 6] or without [9, 12,13,14] covariate adjustment; other researchers have performed multiple regression analysis to determine whether gender independently predicted OSA symptoms [7, 8, 10, 11, 15]. In order to extend previous observations and clarify some controversies, we aimed to evaluate the influence of gender on reported OSA-related symptoms in a large population of patients referred to the sleep laboratory of our institution.
Methods
Patient selection
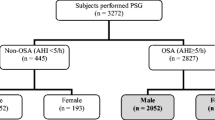
We examined the database from Hospital Alemán’s sleep laboratory from January 2013 to January 2016 and preselected 2154 patients older than 17 years who had a polysomnography (PSG) and answered a sleep questionnaire. Since there was male predominance in the initial sample (1454), 700 males were chosen at random to equal the number of females (700). The subjects who had undergone diagnostic PSG, the Berlin questionnaire, a sleep questionnaire, and the Epworth sleepiness scale (ESS) were selected. Exclusion criteria comprised patients who did not answer questions 1 to 7 of the Berlin questionnaire or the ESS, marked more than one answer on any of the questions, had suspected narcolepsy, performed a split-night PSG, or had a PSG with less than 180 min of total sleep time. Of the 1400 patients selected, 316 were excluded for different reasons. Figure 1 shows the study flow chart.
Measurements
All the patients enrolled in this study underwent a diagnostic PSG which included EEG (F4/A1, C4/A1, O2/A1), EOG (two channels), chin EMG, leg EMG (two channels), ECG, airflow by nasal pressure, and an oral thermistor, thoracic and abdominal movements (two channels with piezoelectric sensors), snoring, oxygen saturation (SO2), and body position. The PSGs were registered from 10:30–11:30 p.m. to 5:00–6:00 a.m. Prior to PSG, the patients were given the following instructions: (1) to avoid napping and alcoholic or caffeinated beverages, (2) to continue taking the usual medication, (3) to eat supper between 8 and 9 p.m., and (4) to report to the sleep laboratory between 9 and 10:30 p.m. The PSG analysis was performed manually by four trained physicians following international criteria [16]. OSA was defined as an apnea/hypopnea index (AHI) ≥ 5. On arrival at the sleep lab, the patients completed a Spanish version of the ESS [17] and the Berlin questionnaire [18], and a sleep medical record (see appendix). Body weight and height were recorded in all subjects wearing only light clothes and no shoes. The Spanish version of the Berlin questionnaire was validated against PSG, and it showed to have similar sensitivity and specificity to reports in the original literature [19, 20]. Comorbidities were detected either by self-reporting and/or the medication that the patient was taking. Restless leg syndrome was assumed when patients complained of uncomfortable sensations in their legs before sleeping and reported behaviors to relieve this discomfort. Menopause was considered when it was reported on the sleep questionnaire or when women were older than 50 years [21]. Definitions used for nocturnal and diurnal symptoms associated with OSA are shown in Table 1.
Statistical analysis
A frequency histogram and the Kolmogorov–Smirnov test were used to assess if the study variables had a normal distribution. The Mann–Whitney or the chi-square tests were used to compare differences between women and men.
Multiple logistic regression analysis was performed to assess the relationship between symptoms associated with OSA (present = 1, absent = 0) and various potential explanatory variables. Typical symptoms of OSA (snoring, apnea) were adjusted for age, gender (male = 1, female = 0), BMI, and AHI. In order to predict other less-specific symptoms of OSA (tiredness, excessive diurnal somnolence, insomnia, nocturia, morning headaches, subjective cognitive complaints), logistic regression models included other factors that could potentially explain their presence or absence (mean SO2; minimal SO2; T90; use of sedatives, antidepressants, or analgesics; cardiovascular comorbidities; diabetes; hypothyroidism; rhinitis; pulmonary pathology’ restless leg syndrome; and gastroesophageal reflux symptoms). For the purpose of this investigation, the term comorbidities, used as predictor in logistic regression models, referred to any of the following conditions: hypertension, coronary disease, stroke, heart failure, diabetes, hypothyroidism, rhinitis, asthma, COPD, gastro-esophageal reflux, nocturia, and chronic use of analgesics.
The statistical analysis was carried out with a commercial computer program, MedCalc Statistical Software version 16.8 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2016).
Results
The study sample included 1084 patients, median age was 53 years, 46.5% (504) were women, 72.7% (788) had OSA (AHI ≥ 5), and 31.2% were obese. The characteristics of study population are shown in Table 2.
Gender differences in OSA population
Tables 2, 3, and 4 show gender differences in terms of clinical manifestations and PSG features. Women presented older age, lower AHI, and higher prevalence of morbid obesity than men. Seventy-one percent of the women with obstructive sleep apnea were menopausal. Women reported more hypothyroidism and restless legs syndrome than men and complained more often of tiredness, insomnia, morning headache, and subjective cognitive complaints. They also reported taking more sedatives/antidepressants, analgesics, and antacids.
Factors associated with OSA symptoms
Table 5 shows the results of the different logistic regression models used to identify the explanatory variables of OSA-related symptoms.
Specific OSA symptoms
Snoring (S) and habitual snoring (HS) were significantly associated with male gender (S: coefficient 1.40, OR 4.06, p < 0.001; HS: coefficient 0.85, OR 2.34, p < 0.001), BMI (S: coefficient 0.038, OR 1.04, p 0.039; HS: coefficient 0.076, OR 1.08, p < 0.001), and AHI (S: coefficient 0.067, OR 1.07, p < 0.001; HS: coefficient 0.026, OR 1.03, p < 0.001). Loud snoring had a significant relationship with age (coefficient − 0.0095, OR 0.99, p 0.041), male gender (coefficient 0.76, OR 2.14, p < 0.001), BMI (coefficient 0.031, OR 1.03, p 0.015), and AHI (coefficient 0.025, OR 1.03, p < 0.001). Apnea also showed a significant association with age (coefficient − 0.015, OR 0.98, p 0.004), male gender (coefficient 0.89, OR 2.44, p < 0.001), and AHI (coefficient 0.035, OR 1.03, p < 0.001).
Nonspecific OSA symptoms
Tiredness showed a marked relationship with age (coefficient − 0.014, OR 0.98, p 0.02), female gender (coefficient − 0.55, OR 0.57, p 0.001), insomnia (coefficient 0.83, OR 2.29, p < 0.001), restless leg syndrome (coefficient 0.59, OR 1.80, p 0.001), and ESS (coefficient 0.77 OR 2.16, p < 0.001).
Excessive diurnal somnolence was associated with age (coefficient − 0.0012, OR 0.99, p 0.019), BMI (coefficient 0.033, OR 1.03, p 0.022), AHI (coefficient 0.017, OR 1.02, p < 0.001), mean SO2 (coefficient 0.13, OR 1.14, p 0.007), insomnia (coefficient − 0.42, OR 0.66, p 0.012), and tiredness (coefficient 0.77, OR 2.16, p 0.001).
Sleep onset insomnia was significantly related with age (coefficient − 0.015, OR 0.98, p 0.017), female gender (coefficient − 0.53, OR 0.59, p 0.0035), use of sedatives or antidepressants (coefficient 0.47, OR 1.60, p 0.006), frequent awakenings (coefficient 0.99, OR 2.70, p < 0.001), sleeping movements (coefficient 0.99, 2.69, p < 0.001), restless leg syndrome (coefficient 0.47, OR 1.60, p 0.01), tiredness (coefficient 0.58, OR 1.77, p 0.001), and ESS (coefficient − 0.66, OR 0.52, p 0.001).
Maintenance insomnia showed a meaningful relationship with AHI (coefficient − 0.021, OR 0.95, p 0.003), use of sedatives or antidepressants (coefficient 0.72, OR 2.06, p 0.001), frequent awakenings (coefficient 2.25, OR 9.5, p < 0.001), and tiredness (coefficient 0.70, OR 2.02, p 0.001).
Subjective cognitive complaints correlated with AHI (coefficient − 0.012, OR 0.99, p 0.026), T90 (coefficient 0.018, OR 1.02, p 0.004), insomnia (coefficient 0.47, OR 1.60, p 0.004), sleeping movements (coefficient 0.35, OR 1.42, p 0.02), frequent awakenings (coefficient 0.36, OR 1.44, p 0.02), tiredness (coefficient 0.48, OR 1.62, p < 0.001), ESS (coefficient 0.78, OR 2.17, p < 0.001), consumption of sedatives or antidepressants (coefficient 0.49, OR 1.64, p 0.002), and comorbidities (coefficient 0.44, OR 1.55, p 0.045).
Nocturia presented a marked association with age (coefficient 0.040, OR 1.04, p < 0.001), BMI (coefficient 0.039, OR 1.04, p 0.001), mean SO2 (coefficient − 0.11, OR 0.89, p 0.001), insomnia (coefficient − 0.43, OR 0.65, p 0.02), frequent awakenings (coefficient 1.92, OR 6.8, p < 0.001), and sleeping movements (coefficient 1.19, OR 3.28, p < 0.001).
Morning headaches were associated with age (coefficient − 0.021, OR 0.98, p 0.005), gender (coefficient − 1.15, OR 0.32, p < 0.001), ESS (coefficient 0.75, OR 2.11, p 0.001), insomnia (coefficient 0.83, OR 2.28, p 0.001), and restless leg syndrome (coefficient 0.93, OR 2.52, p < 0.001). Figures 2 and 3 show the influence of gender on the symptoms associated with OSA.
Discussion
In our population that included patients without OSA to avoid leaving out of the analysis patients who could potentially have had airway resistance syndrome (AHI < 5 plus sleepiness), we observed that gender was an independent, explanatory variable of several specific and nonspecific OSA-related symptoms. After adjusting for age, BMI, and AHI, women were less likely to report snoring, habitual or loud snoring, and apneas than males. After controlling for multiple variables, female gender was an independent predictive factor of tiredness, sleep onset insomnia, and morning headaches. On the other hand, the report of excessive daytime sleepiness, nocturia, maintenance insomnia, and subjective cognitive complaints did not show a significant association with gender.
Our observations are supported by early publications. It has been reported that male gender constitutes an independent risk factor for typical symptoms of OSA, such as habitual or loud snoring [10, 22] and witnessed apnea [4, 6, 9, 10], after adjusting for covariables such as age, BMI, alcohol consumption or sedatives, cigarette smoking, and/or AHI. Several potential explanations may account for these findings. We speculated that during the development of the obstructive respiratory event, males could develop a higher intrathoracic negative pressure than females. Thus, the higher forces applied to the vibratory structures of the upper airway could cause more intense and perceptible respiratory sounds. In line with this theory, Itasaka [23] and Stoohs [24] observed that, in subjects with OSA, the intensity of snoring showed a strong positive correlation with inspiratory effort evaluated by esophageal pressure. It has also been described that men had significantly more and longer apneas than their female counterparts [25]. Therefore, women could perceive more easily the breathing pauses than males and so report it to their bedpartner. Third, women may consider their own snoring “unladylike” and therefore be less likely to mention it [2]. In addition, women are more likely to attend clinical appointments on their own, whereas men often do so with their partner [14]. Consequently, information from a partner on snoring and witnessed apneas may be less readily available for women. Finally, the definitions used for habitual or loud snoring are not uniform in published studies and this could add another source of variation in symptom reporting.
Most publications from clinical or community populations have found that women with OSA complain more of tiredness or fatigue than their male counterparts, independently of other confounders [7, 8, 13]. Several hypotheses may be raised to try to explain this observation. Prior studies have indicated that women are frequently more likely to report physical symptoms [26,27,28]. Also, cultural differences may make men less willing than women to admit that they have any of the problems we asked about, or there may be a real gender-based neurophysiologic bias in the way daytime effects of disturbed sleep are perceived. An unexpected finding was the lack of association between tiredness and OSA severity indicators such as AHI, and various measurements of oxygen desaturation. Nevertheless, other investigators have published similar findings [8, 39,40,41,42]. The absence of association between OSA and fatigue suggests that this subjective complaint may be produced by other factors. However, the fact that the sensation of tiredness or fatigue is relieved in some patients who use CPAP adequately is a strong argument for a causal relationship between sleep apnea and this symptomatology [43, 44].
There is contradictory information in the literature regarding the influence of gender on the report of excessive daytime sleepiness (EDS) in subjects with OSA. Although men more frequently had sleepiness than women (see Table 4), in the multiple regression model, the gender was not a predictor of EDS. This finding was maintained even when the cut-off point to define EDS was changed to greater than 9 or 11 (data not shown). In line with our finding, some authors [4, 6] have observed, in models fitted by confounders, that the complaint of excessive daytime sleepiness was not related to gender. A similar finding was reported in a community population study [29]. However, other studies have reported that both women [8] and men [7] with OSA are more likely to report sleepiness. These discrepancies could be related to the way EDS was defined, differences in the variables included in the predictive models of EDS or study population characteristics. Baldwin et al. [7] considered sleepiness as either “feeling frequently or almost always sleepiness during the day” or an ESS greater than 10. While an ESS greater than 10 was related with male gender (adjusted OR = 0.77; CI, 0.66–0.90 for women), the statement “feeling frequently or almost always sleepiness during the day” was not significantly associated with gender (adjusted OR = 1.06; CI, 0.86–1.32). On the other hand, the study by Chervin et al. [8] did not include BMI as a predictor of EDS in the multiple regression analysis and it was described as an independent predictor of EDS [29].
It has been reported that female gender is an independent predictor of insomnia in general and clinical population after adjusting for numerous confounding variables [4, 6, 7, 15]. However, little attention has been paid to the relationship between subtype of insomnia and gender. We found that sleep onset insomnia, but not maintenance insomnia, was independently associated with female gender. This finding could indicate that OSA would not be a cause of initial insomnia. Given that OSA is a disorder that occurs after sleep onset, it would seem more likely that sleep maintenance insomnia would be causally connected with OSA. Indeed, Chung et al. [30] showed that the most common insomnia complaint was frequent awakenings during the night and the least common complaint was difficulty falling asleep. Also, after 2 years of CPAP treatment, OSA patients often improve midnight insomnia but not early or late insomnia [31]. Thus, it has been hypothesized that sleep maintenance insomnia has causal links with OSA, but sleep onset insomnia might be an independent co-occurring sleep disorder [30]. However, in a community-based study [7], different subtypes of insomnia were associated with female gender after controlling for multiple variables including the AHI.
We did not find a relationship between subjective cognitive complaints and gender. Our results are consistent with a paper that assessed the relation between habitual snoring, sleep apnea, and cognitive complaints (concentration and memory problems) in an adult population-based sample [32]. After adjusting for potential confounders such as gender, both concentration and/or memory problems were related to depression, insomnia, unintended sleepiness, habitual snoring, and sleep apnea. Similarly, in a large cohort of adults, the relationship between objective neurocognitive tests and severity of OSA did not show any gender influence on cognitive performance [33].
We did not observe any association between gender and nocturia reports. This is in line with a recent systematic review of factors associated with nocturia in community-based cohorts [34]. Also, the Sleep Health Heart Study evaluated the association between nocturia, cardiovascular morbidity, and OSA and no relationship between nocturia and gender was found after adjusting for several covariates [35].
Female gender constituted a risk factor for morning headaches. In addition, we observed no association between the report of morning headaches and the severity of OSA measured through AHI and other indicators of oxygen desaturation. Such findings are in agreement with previous studies [36, 37]. We speculate that these observations may reflect the fact that migraine and tension headaches are more prevalent conditions in women than in men [38].
Clinical importance and limitation
The fact that obstructive sleep apnea has different clinical presentations in women and men may have important clinical implications. First, reliance on snoring or self-reported apnea may result in more men being referred for sleep study than women. Second, the fact that women present more nonspecific symptoms, such as tiredness or insomnia, could make physicians turn to other diagnostic possibilities like anxiety or depression, rather than OSA. Finally, understanding gender differences will contribute to the better clinical recognition of OSA in women as well as the provision of proper health care and therapeutic practice.
The present study presents limitations due to its retrospective design, selection bias, and the fact that the study site was a tertiary care center with its inherent referral bias and limited generalizability.
In summary, after adjusting for numerous covariables, we found that women with OSA were more likely to report tiredness, sleep onset insomnia, and morning headaches, and less likely to complain of typical OSA symptoms (snoring, apneas) compared to men. We did not observe any gender influence in the self-report of daytime sleepiness, subjective neurocognitive complaints, maintenance insomnia, or nocturia.
Further studies describing the clinical manifestations of different severity levels of OSA in men and women will substantially contribute to its identification and treatment in women across a wide disease spectrum.
References
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177(9):1006–1014. https://doi.org/10.1093/aje/kws342
Lin CM, Davidson TM, Ancoli-Israel S (2008) Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 12(6):481–496. https://doi.org/10.1016/j.smrv.2007.11.003
Consenso Nacional sobre el Síndrome Apneas-Hipopneas del Sueño del Grupo Español de Sueño. Definición y concepto, fisiopatología, clínica y exploración del SAHS (2005) Arch bronconeumol 41, Extraordinario 4: 12–29
Shepertycky MR, Banno K, Kryger MH (2005) Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep 28(3):309–314
Alotair H, Bahammam A (2008) Gender differences in Saudi patients with obstructive sleep apnea. Sleep Breath 12(4):323–339. https://doi.org/10.1007/s11325-008-0184-8
Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P (2007) Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep 30(3):312–319. https://doi.org/10.1093/sleep/30.3.312
Baldwin CM, Kapur VK, Holberg CJ, Rosen C, Nieto FJ (2004) Sleep Heart Health Study Group. Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep 15;27:305-311
Chervin RD (2000) Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest 118(2):372–379. https://doi.org/10.1378/chest.118.2.372
Ambrogetti A, Olson LG, Saunders NA (1991) Differences in the symptoms of men and women with obstructive sleep apnoea. Aust NZ J Med 21(6):863–866. https://doi.org/10.1111/j.1445-5994.1991.tb01408.x
Redline S, Kump K, Tishler PV, Browner I, Ferrette V (1994) Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 149(3):722–726. https://doi.org/10.1164/ajrccm.149.3.8118642
Young T, Hutton R, Finn L, Badr S, Palta M (1996) The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med 156(21):2445–2451. https://doi.org/10.1001/archinte.1996.00440200055007
Walker RP, Durazo-Arvizu R, Wachter B, Gopalsami C (2001) Preoperative differences between male and female patients with sleep apnea. Laryngoscope 111(9):1501–1505. https://doi.org/10.1097/00005537-200109000-00001
Lichstein KL, Means MK, Noe SL, Aguillard RN (1997) Fatigue and sleep disorders. Behav Res Ther 35(8):733–740. https://doi.org/10.1016/S0005-7967(97)00029-6
Quintana-Gallego E, Carmona-Bernal C, Capote F, Sánchez-Armengol A, Botebol-Benhamou G, Polo-Padillo J, Castillo-Gómez J (2004) Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med 98(10):984–989. https://doi.org/10.1016/j.rmed.2004.03.002
Krell SB, Kapur VK (2005) Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath 9(3):104–110. https://doi.org/10.1007/s11325-005-0026-x
Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st edn. American Academy of Sleep Medicine, Westchester
Chica-Urzola HL, Escobar-Córdoba F, Eslava-Schmalbach J (2007) Validating the Epworth sleepiness scale. Rev Salud Publica (Bogota) 4:558–567
Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP (1999) Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 131(7):485–491. https://doi.org/10.7326/0003-4819-131-7-199910050-00002
Varela B, Nigro CA, Dibur E, Malnis S, Rhodius EE (2010) Utilidad del cuestionario de Berlín y el Mallanpati para el diagnóstico del síndrome apnea/hipopnea obstructiva del sueño. Revista Americana de Medicina Respiratoria, Suplemento, Page 52:TL099
Abrishami A, Khajehdehi A, Chung F (2010) A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth 57(5):423–438. https://doi.org/10.1007/s12630-010-9280-x
Schildkraut JM, Cooper GS, Halabi S, Calingaert B, Hartge P, Whittemore AS (2001) Age at natural menopause and the risk of epithelial ovarian cancer. Obstet Gynecol 98(1):85–90
Wilson K, Stoohs RA, Mulrooney TF, Johnson LJ, Guilleminault C, Huang Z (1999) The snoring spectrum: acoustic assessment of snoring sound intensity in 1139 individuals undergoing polysomnography. Chest 115(3):762–770. https://doi.org/10.1378/chest.115.3.762
Itasaka Y, Miyazaki S, Ishikawa K, Togawa K (1999) Intensity of snoring in patients with sleep-related breathing disorders. Psychiatry Clin Neurosci 53(2):299–300. https://doi.org/10.1046/j.1440-1819.1999.00510.x
Stoohs R, Skrobal A, Guilleminault C (1993) Does snoring intensity predict flow limitation or respiratory effort during sleep? Respir Physiol 92(1):27–38. https://doi.org/10.1016/0034-5687(93)90117-S
Ware JC, McBrayer RH, Scott JA (2000) Influence of sex and age on duration and frequency of sleep apnea events. Sleep 23(2):165–170
Kroenke K, Spitzer RL (1998) Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med 60(2):150–155. https://doi.org/10.1097/00006842-199803000-00006
Wool CA, Barsky AZ (1994) Do women somatize more than men? Gender differences in somatization. Psychosomatics 35(5):445–452. https://doi.org/10.1016/S0033-3182(94)71738-2
Nakao M, Fricchione G, Zuttermeister PC, Myers P, Barsky AJ, Benson H (2001) Effects of gender and marital status on somatic symptoms of patients attending a mind/body medicine clinic. Behav Med 26(4):159–168. https://doi.org/10.1080/08964280109595763
Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A (2005) Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab 90(8):4510–4515. https://doi.org/10.1210/jc.2005-0035
Chung KF (2005) Insomnia subtypes and their relationships to daytime sleepiness in patients with obstructive sleep apnea. Respiration 72(5):460–465. https://doi.org/10.1159/000087668
Björnsdóttir E, Janson C, Sigurdsson JF, Gehrman P, Perlis M, Juliusson S, Arnardottir ES, Kuna ST, Pack AI, Gislason T, Benediktsdóttir B (2013) Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep 36(12):1901–1909. https://doi.org/10.5665/sleep.3226
Jennum P, Sjøl A (1994) Self-assessed cognitive function in snorers and sleep apneics. An epidemiological study of 1,504 females and males aged 30-60 years: the Dan-MONICA II Study. Eur Neurol 34(4):204–208. https://doi.org/10.1159/000117039
Quan SF, Chan CS, Dement WC, Gevins A, Goodwin JL, Gottlieb DJ, Green S, Guilleminault C, Hirshkowitz M, Hype PR, Kay GG, Leary EB, Nichols DA, Schweitzer PK, Simon RD, Walsh JK, Kushida CA (2011) The association between obstructive sleep apnea and neurocognitive performance—the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 34(3):303–314. https://doi.org/10.1093/sleep/34.3.303
Yoshimura K (2012) Correlates for nocturia: a review of epidemiological studies. Int J Urol 19(4):317–329. https://doi.org/10.1111/j.1442-2042.2011.02956.x
Parthasarathy S, Fitzgerald M, Goodwin JL, Unruh M, Guerra S, Quan SF (2012) Nocturia, sleep-disordered breathing, and cardiovascular morbidity in a community-based cohort. PLoS One 7(2):e30969. https://doi.org/10.1371/journal.pone.0030969
Beiske KK, Russell MB, Stavem K (2013) Prevalence and predictors of headache in patients referred to polysomnography. J Headache Pain 14(1):90. https://doi.org/10.1186/1129-2377-14-90
Sand T, Hagen K, Schrader H (2003) Sleep apnoea and chronic headache. Cephalalgia 23(2):90–95. https://doi.org/10.1046/j.1468-2982.2003.00460.x
Jarsen R, Stovner LJ (2008) Epidemiology and comorbidity of headache. Lancet Neurol 7:354–361
Hossain JL, Ahmad P, Reinish LW, Kayumov L, Hossain NK, Shapiro CM (2005) Subjective fatigue and subjective sleepiness: two independent consequences of sleep disorders? J Sleep Res 14(3):245–253. https://doi.org/10.1111/j.1365-2869.2005.00466.x
Lichstein KL, Means MK, Noe SL, Aguillard RN (1997) Fatigue and sleep disorders. Behav Res Ther 35(8):733–740. https://doi.org/10.1016/S0005-7967(97)00029-6
Bardwell WA, Moore P, Ancoli-Israel S, Dimsdale JE (2003) Fatigue in obstructive sleep apnea: driven by depressive symptoms instead of apnea severity? Am J Psychiatry 160(2):350–355. https://doi.org/10.1176/appi.ajp.160.2.350
Bardwell WA, Ancoli-Israel S, Dimsdale JE (2007) Comparison of the effects of depressive symptoms and apnea severity on fatigue in patients with obstructive sleep apnea: a replication study. J Affect Disord 97(1-3):181–186. https://doi.org/10.1016/j.jad.2006.06.013
Chotinaiwattarakul W, O'Brien LM, Fan L, Chervin RD (2009) Fatigue, tiredness, and lack of energy improve with treatment for OSA. J Clin Sleep Med 5(3):222–227
Tomfohr LM, Ancoli-Israel S, Loredo JS, Dimsdale JE (2011) Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: data from a randomized controlled trial. Sleep 34(1):121–126. https://doi.org/10.1093/sleep/34.1.121
Acknowledgements
The authors wish to thank Mrs. María Dolores Marinaro and polysomnography technicians Sergio González, Marcelo González, and Anabella Arce for their assistance in collecting data.
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of our institutional committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Hospital Alemán, Hospital Británico and Hospital de Clínicas are part of Grupo Argentino para la Investigación de la Apnea del Sueño (GAIAS).
Electronic supplementary material
ESM 1
(DOC 91 kb)
Rights and permissions
About this article
Cite this article
Nigro, C.A., Dibur, E., Borsini, E. et al. The influence of gender on symptoms associated with obstructive sleep apnea. Sleep Breath 22, 683–693 (2018). https://doi.org/10.1007/s11325-017-1612-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1612-4