Abstract
Purpose
Obstructive sleep apnea (OSA) is underdiagnosed in females due to different clinical presentation. We aimed to determine the effect of gender on clinical and polysomnographic features and identify predictors of OSA in women.
Methods
Differences in demographic, clinical, and polysomnographic parameters between 2052 male and 775 female OSA patients were compared.
Results
In female OSA patients, age (56.1 ± 9.7 vs. 50.4 ± 11.6 years, p < 0.0001) and body mass index (36.3 ± 8.6 vs. 31.8 ± 5.9 kg/m2, p < 0.0001) were increased, whereas men had higher waist-to-hip ratio and neck circumference (p < 0.0001). Hypertension, diabetes mellitus, thyroid disease, and asthma were more common in females (p < 0.0001). Men reported more witnessed apnea (p < 0.0001), but nocturnal choking, morning headache, fatigue, insomnia symptoms, impaired memory, mood disturbance, reflux, nocturia, and enuresis were more frequent in women (p < 0.0001).
The indicators of OSA severity including apnea-hypopnea index (AHI) (p < 0.0001) and oxygen desaturation index (p = 0.007) were lower in women. REM AHI (p < 0.0001) was higher, and supine AHI (p < 0.0001) was lower in females. Besides, women had decreased total sleep time (p = 0.028) and sleep efficiency (p = 0.003) and increased sleep latency (p < 0.0001). In multivariate logistic regression analysis, increased REM AHI, N3 sleep, obesity, age, morning headache, and lower supine AHI were independently associated with female gender.
Conclusions
These data suggest that frequency and severity of sleep apnea is lower in female OSA patients, and they are presenting with female-specific symptoms and increased medical comorbidities. Therefore, female-specific questionnaires should be developed and used for preventing underdiagnosis of OSA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common disorder which is characterized by instability of the upper airway during sleep resulting in reduction or elimination of airflow, oxygen desaturation, and sleep disruption. The prevalence of OSA defined at an apnea-hypopnea index (AHI) ≥5 was a mean of 22% in men and 17% in women in epidemiological studies, and OSA with excessive daytime sleepiness occurred in 6% of men and in 4% of women [1]. There is convincing data to support OSA as an independent risk factor for cardiovascular disorders including hypertension, congestive heart failure, coronary heart disease, arrhythmias, stroke, and fatal and nonfatal cardiovascular events [2–4]. In addition, several studies have assessed potential independent contribution of OSA to the pathogenesis of metabolic abnormalities, including type 2 diabetes, glucose intolerance, insulin resistance, metabolic syndrome, and nonalcoholic fatty liver disease [5, 6]. Sleep apnea consequences are associated with increased morbidity and mortality, and early diagnosis and management of OSA are very important.
Obstructive sleep apnea was previously described as a disease primarily of men. It is now widely recognized that OSA in women is not as rare as was originally believed. However, it is clinically underdiagnosed as women manifest OSA differently [7, 8]. Men are predominantly referred to sleep clinics with classical symptoms, namely, sleepiness, snoring, and witnessed apneas. However, female OSA patients more frequently report different symptoms, such as insomnia, depression, morning headache, nightmare, fatigue, and mood disturbance and thus are less likely to be referred for the evaluation of sleep disordered breathing. So, the diagnosis of OSA may be missed in women, and they may have worse clinical outcomes. Knowledge about these gender-related differences in clinical features of OSA may contribute to an increased awareness, improved diagnosis, and its therapeutic consequences [9–11]. Polysomnographic features of female OSA patients are also different than males. Women have less severe syndrome with lower AHI, and their AHI is lower in supine position compared to men. Besides, AHI during rapid eye movement (REM) sleep is higher, and sleep efficiency and latency are worse than males [12, 13].
Numerous sleep studies have been conducted in men, and the most generally accepted characteristics and the clinical guidelines established for evaluation and diagnosis of OSA are based primarily on studies in male patients. Understanding gender differences in OSA is currently a critical area of exploration [7]. Therefore, the present study was undertaken to clarify the clinical under-recognition in females by examining the differences in clinical presentation and polysomnographic findings between male and female OSA patients. The objectives of this study were to evaluate the effect of gender on demographic, clinical, and polysomnographic features and to identify predictors of OSA in females in a large number of patients with OSA.
Methods
Study population
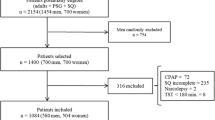
In this observational study, we retrospectively evaluated 3272 consecutive subjects admitted to the sleep laboratory of a university hospital for evaluation of presumed sleep-breathing disorder and underwent in-laboratory polysomnography between September 2006 and September 2015. The subjects with an AHI of <5 events/h (n = 445) were excluded. Out of 2827 OSA patients, 2052 (72.6%) were men and 775 (27.4%) were women. Flow chart of the study is presented in Fig. 1.
Demographic data (age, gender, smoking history, alcohol and psychotropic drug use), anthropometric measurements [height, weight, body mass index (BMI), circumferences of neck, waist, and hip], and medical history were evaluated. Pulmonary function tests, arterial blood gas analysis, and full-night in-laboratory polysomnography were performed in all patients as routine investigations of our sleep laboratory. The symptoms of insomnia including difficulty initiating sleep, maintaining sleep, and early morning awakenings were evaluated during medical history. Besides, the patients with OSA and insomnia symptoms and sleep onset latency ≥30 min and/or sleep efficiency ≤85% were considered as “comorbid insomnia and OSA.” Subjective daytime sleepiness was assessed by using the Turkish version of Epworth Sleepiness Scale (ESS), and scores higher than 10 were considered as sleepiness [14]. Demographic and anthropometric features, pulmonary function tests, blood gas analysis, and polysomnographic findings of female OSA patients were compared to male patients. The relationship between gender and other variables was examined with univariate and multivariate logistic regression analysis.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee (Ethics Committee of Ege University School of Medicine) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the present study. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Polysomnography
All patients underwent full-overnight in-laboratory polysomnography (Compumedics E Series, Australia or Alice 5 Diagnostic Sleep System, Philips, Respironics, USA). The electrodes of electroencephalography were positioned according to the international 10–20 system. Polysomnography consisted of monitoring of sleep by electroencephalography, electrooculography, electromyography, airflow, and respiratory muscle effort and included measures of electrocardiographic rhythm and blood oxygen saturation. Thoracoabdominal plethysmograph, oro-nasal temperature thermistor, and nasal-cannula-pressure transducer system were used to identify apneas and hypopneas. Transcutaneous finger pulse oximeter was used to measure oxygen saturation. Sleep was recorded and scored according to the standard method, and alternative criteria were used for hypopnea definition [15]. AHI was the sum of the number of apneas and hypopneas per hour of sleep.
Statistical analysis
Statistical analysis was performed with IBM SPSS 20.0 for Windows packaged software. All analyses comparing the study groups were performed to compare female and male OSA patients. Numerical variables were summarized with mean ± standard deviation and categorical variables with frequency and percentage. The significance of differences among groups was assessed by Student’s t test, and analysis of categorical variables was examined by chi-square test. A value of p < 0.05 was considered significant for all statistical analysis. Multiple logistic regression analysis was used to determine the relationship between the demographic, anthropometric, and polysomnographic parameters with AHI in both groups. Besides, odds ratio (OR) and 95% confidence intervals (95% CI) were calculated to show the association.
Results
Characteristics of female and male OSA patients
OSA was predominant in males with a male-to-female ratio of 2.6:1. The baseline characteristics of the patients were compared according to gender, and it was observed that women were older (56.1 ± 9.7 vs. 50.4 ± 11.6 years, respectively, p < 0.0001) and more obese (36.3 ± 8.6 vs. 31.8 ± 5.9 kg/m2, respectively, p < 0.0001) than men. The percentage of obese women was also higher (75.9 vs. 57.9%, p < 0001).
Although the circumferences of waist (p = 0.007) and hip (p < 0.0001) were higher in female OSA patients, waist-to-hip ratio (p < 0.0001), neck circumference (p < 0.0001), and neck/height ratio (p = 0.001) were significantly increased in males. We also compared all the anthropometric parameters in women before and after menopause and found no significant difference. The rates of cigarette (p < 0.0001) and alcohol (p < 0.0001) consumption were higher in men, whereas women used more psychotropic drugs (p < 0.0001).
In spirometry, lung volumes were lower (p = 0.002), and in blood gas analysis, mean PaO2 was decreased in women (p = 0.001). Mean Epworth sleepiness scores (10.2 ± 5.8 vs. 10.4 ± 5.9, p > 0.05) and patients with scores greater than 10 were similar in female and male patients (47 vs. 48%, p > 0.05). The percentage of women with hypertension, diabetes mellitus, thyroid disease, and asthma was higher (p < 0.0001), whereas COPD observed more frequently in men (p < 0.0001). The rates of coronary artery disease and congestive heart failure were similar in both groups. The characteristics of the patients are shown in Table 1.
Sleep apnea-related symptoms of female and male OSA patients
As shown in Table 2, there was no difference in terms of daytime sleepiness and snoring symptoms between genders, but bedpartners of male patients reported more witnessed apneas compared to females (p < 0.0001). However, nocturnal choking (p < 0.0001), morning headache (p < 0.0001), nonrestorative sleep (p < 0.0001), impaired concentration (p = 0.019), impaired memory (p < 0.0001), and mood changes and irritability (p < 0.0001) observed more common in women. Additionally, women reported morning dry mouth (p = 0.030), nocturnal reflux (p < 0.0001), nocturia (p < 0.0001), and enuresis (p < 0.0001) more frequently.
Female OSA patients reported higher percentage of insomnia symptoms including difficulty initiating sleep, maintaining sleep, and early morning awakenings than males (34 vs. 23%, p < 0.0001). The patients with insomnia symptoms and sleep onset latency ≥30 min and/or sleep efficiency ≤85% were compared in terms of comorbid insomnia and OSA diagnosis, and it was observed that women also had higher rates of comorbid insomnia than men (21 vs. 12%, p < 0.0001).
Sleep parameters of female and male OSA patients
The indicators of OSA severity including AHI and oxygen desaturation index (ODI) were lower in women (p < 0.0001 and p = 0.007, respectively) compared to men. OSA severity increased in both genders with age, but the difference in AHI between women and men decreased after the age of 60 years (Fig. 2). In fact, comparison of AHI and ODI of the postmenopausal women with men of the same ages showed no significant difference. We also evaluated OSA severity in women before and after menopause and found that females after menopause had significantly higher AHI (38.4 ± 28.7 vs. 31.6 ± 32.4, p = 0.034) and ODI (37.6 ± 29.9 vs. 30.7 ± 33.0, p = 0.049) than those before menopause.
In the other polysomnographic parameters, the lowest nocturnal oxygen saturation (SpO2) (%) was lower (p = 0.001), and sleep time with SpO2 <90% (%) were higher in women (p = 0.058). Supine AHI was decreased (p < 0.0001), and REM AHI was increased in female patients (p < 0.0001). Total sleep time (p = 0.028) and the percentage of sleep efficiency (p = 0.003) were decreased, and sleep latency (p < 0.0001) was increased in female OSA patients. In addition, the percentages of stages N1 and N2 sleep were lower (p < 0.0001), but N3 sleep was higher in women (p < 0.0001). The sleep parameters are shown in Table 3.
Predictive factors for OSA in females
The relationship between female gender and other variables was examined with univariate logistic regression analysis. Twenty one variables [witnessed apnea, nocturnal choking, morning headache, nonrestorative sleep, insomnia symptoms, mood changes, AHI, ODI, REM AHI, supine AHI, sleep efficiency (%), sleep latency, N1 sleep (%), N2 sleep (%), N3 sleep (%), lowest SpO2 (%), obesity, different age groups (≤39, 40–49, 50–59, 60–69 years)] were selected for multivariate logistic regression analysis. Consequently, increased REM AHI, N3 sleep, obesity, age, morning headache, and lower supine AHI were found to be independently associated with female gender in multivariate analysis (Table 4).
Discussion
Clinical presentation and polysomnographic features of sleep apnea in females are quite different than males. Knowledge about gender-related differences may contribute to an increased awareness and improved diagnosis. In the present study, we assessed the differences in clinical and polysomnographic findings between 2052 male and 775 female OSA patients and observed that more diagnoses of OSA were rendered in males. Female OSA patients were older and more obese than men. Women presented with increased medical comorbidities and female-specific sleep apnea symptoms. Besides, female patients had less severe sleep apnea syndrome in all ages, and sleep quality was worse in women. The present study suggests that women are presenting with quite different OSA phenotype compared to males.
In many studies, it was shown that men had a higher rate of OSA compared to women, especially at ages corresponding to premenopausal women, and the male-to-female ratio is approximately 2:1 or 3:1 [16–18]. We found that more men were rendered more OSA diagnoses than women in a large number of patients with OSA seen in a university hospital. The diagnostic rates of OSA were 80% in females and 89% in males referred for polysomnography. It has long been recognized that men have greater vulnerability than women toward developing OSA, but it was also estimated that 93% of women with moderate-to-severe sleep apnea have not been clinically diagnosed [19]. Several explanations exist for lower diagnostic rate in women. First, premenopausal and postmenopausal women are included together in the studies, and the rate is lower in premenopausal period. Second, bedpartners of female patients do not complain of snoring or observe breathing pauses in sleep, and this may contribute to the clinical under-recognition of the disorder in women. Finally, it is known that men and women with OSA have distinct symptom profiles, with women possibly not reporting the classical symptoms, namely, loud snoring, sleepiness, and witnessed apneas. Shepertycky et al. [20] assessed 130 female and 130 male OSA patients. They found that snoring and sleepiness were similarly common in women and men, but women more often described their main presenting symptom as insomnia and admitted less often with a primary complaint of witnessed apnea. Quintana-Gallego et al. [21] also showed in a large cohort that frequency of snoring and daytime sleepiness was similar in both genders, although witnessed apneas were more frequent in males. Besides, they found that fatigue, morning headache, insomnia symptoms, and depression were more frequent in women. The results of the present study were similar, and there was no difference in terms of daytime sleepiness and snoring symptoms between genders, but male patients presented with more witnessed apneas. Additionally, other symptoms of OSA, such as morning headache, fatigue, impaired concentration, impaired memory, mood changes, irritability, dry mouth, nocturnal reflux, nocturia, and enuresis observed more frequently in females. OSA patients with insomnia symptoms and/or altered sleep architecture in polysomnography indicating insomnia (such as increased sleep latency, decreased sleep efficiency, increased wake after sleep onset) have been diagnosed as comorbid insomnia and OSA. There has been a growing recognition of co-occurring insomnia disorder and sleep apnea, and the rate of insomnia has been reported as 39–58% in OSA patients [22, 23]. In the present study, female OSA patients with insomnia symptoms and also women with insomnia symptoms as well as polysomnographic parameters indicating insomnia disorder were significantly higher than men. Thus, increased complaints of insomnia, fatigue, headache, and dissatisfaction with life could lead primary care physicians to under or misdiagnose OSA in women [8].
Obesity is an important risk factor for OSA. It was shown that women with OSA are more likely to be obese than men with OSA of similar severity. However, there are different findings in terms of correlation between BMI and AHI in women [18, 24–26]. Mazzuca et al. [27] showed that BMI and waist circumference were the most useful anthropometric variables associated with OSA in men, whereas hip circumference was the best marker of OSA in women. In another study, women had less severe OSA compared to men despite having a higher BMI and age, and waist-to-hip ratio was more predictive of severity of OSA in men than in women [28]. In our study, it was also observed that female OSA patients were older and more obese than males. Thus, in order to prevent potential diagnostic delays, women with a BMI >30 kg/m2 and age >50 should be considered at risk for OSA, even in the absence of typical OSA symptomatology. In addition, waist-to-hip ratio, neck circumference, and neck/height ratio were significantly lower in females. These findings suggest that there is a gender difference in fat distribution, and men have mainly central obesity. So, it can be postulated that weight loss in women may not be associated with as much marked improvement in OSA severity as men like it was found by Newman et al. [29] in a large population.
In many studies, comorbidities like depression, cardiac disease, hypertension, diabetes mellitus, hypothyroidism, and asthma were found to be more common in female OSA patients, although women had milder OSA compared to men [20, 21, 25, 30]. The present results also demonstrated that female patients experienced more hypertension, diabetes mellitus, thyroid disease, and asthma than males. Men had increased tobacco abuse and COPD, whereas the rates of coronary artery disease and congestive heart failure were similar in both genders. In a prospective study of 1116 women for 88 months, severe OSA independently associated with cardiovascular mortality, and that adequate continuous positive airway pressure (CPAP) therapy reduced cardiovascular death in female OSA patients [31]. Therefore, early diagnosis and management of OSA in women are very important.
O’Connor et al. [16] assessed the influence of gender on polysomnographic features of OSA in a large population. They found that OSA was less severe in women because of milder OSA during NREM sleep, and women had a greater clustering of respiratory events during REM sleep than men. Subramanian et al. [32] studied the effect of slow wave sleep (SWS) on OSA and showed that the difference between REM AHI and SWS AHI was greater in women than in men. Evaluation of the polysomnographic parameters in our study revealed that supine AHI was lower, REM AHI was higher, and sleep efficiency and latency were worse in females than males. Besides, indicators of OSA severity, namely, AHI and ODI were lower in female patients. OSA severity increased in both genders with age, but the difference in AHI between women and men decreased after the age of 60 years. We also compared OSA severity in women before and after menopause and found that postmenopausal females had significantly higher AHI and ODI than those before menopause, although their obesity parameters were similar. These observations were in agreement with previous studies demonstrated that women have less severe sleep apnea and worse sleep quality compared to men [13, 30].
The most important strength of our study is that it was one of the largest studies performed in a university hospital where demographic and anthropometric features, blood gas analysis, spirometry, and polysomnographic data were available in all patients. A potential weakness of the present study is referral bias resulting from our clinic population not being representative of the community. However, our population sample size was very large, and the population was accumulated over a long period, with more moderate-to-severe OSA patients. Second, we evaluated all classical and other symptoms of sleep apnea during recoding medical history of the patients, but we usually asked classical symptoms at the admission to the sleep laboratory. Therefore, PSG may not have been performed in some women with nonclassical OSA symptoms. Third, this was an observational study with a cross-sectional design, and data were evaluated retrospectively. We could have included only male OSA patients matched for age, BMI, and AHI in the study, but in this case, the number of the study population would have been smaller.
In conclusion, it was demonstrated in the present study that at the time of diagnosis, female OSA patients are older, more obese, have a higher rate of medical comorbidities, and they are referred to sleep clinics with different clinical presentation. Although there may be delays in referrals of women for OSA evaluation, they have less severe sleep apnea syndrome in all ages and worse sleep quality than men. Our findings suggest that females are a different OSA phenotype; they are symptomatic at relatively low AHI and have a different symptom profile. Therefore, primary care physicians should be aware of different clinical presentation of OSA in women, and female-specific questionnaires should be developed and used for preventing underdiagnosis of OSA.
Abbreviations
- AHI:
-
apnea-hypopnea index
- BMI:
-
body mass index
- CI:
-
confidence interval
- CPAP:
-
continuous positive airway pressure
- ESS:
-
Epworth sleepiness score
- FEV1 :
-
forced expiratory volume in one second
- FVC:
-
forced vital capacity
- N:
-
non-rapid eye movement
- ODI:
-
oxygen desaturation index
- OR:
-
odds ratio
- OSA:
-
obstructive sleep apnea
- PaCO2 :
-
arterial partial pressure of carbon dioxide
- PaO2 :
-
arterial partial pressure of oxygen
- REM:
-
rapid eye movement
- SWS:
-
slow wave sleep
- SpO2 :
-
oxygen saturation
References
Franklin KA, Lindberg E (2015) Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. J Thorac Dis 7:1311–1322
Lévy P, Ryan S, Oldenburg O, Parati G (2013) Sleep apnoea and the heart. Eur Respir Rev 22:333–352
Selim B, Won C, Yaggi HK (2010) Cardiovascular consequences of sleep apnea. Clin Chest Med 31:203–220
Lyons OD, Ryan CM (2015) Sleep apnea and stroke. Can J Cardiol 31:918–927
Bonsignore MR, Borel AL, Machan E, Grunstein R (2013) Sleep apnoea and metabolic dysfunction. Eur Respir Rev 22:353–364
Kent BD, McNicholas WT, Ryan S (2015) Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J Thorac Dis 7:1343–1357
Ye L, Pien GW, Weaver TE (2009) Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med 10:1075–1084
Jordan AS, McEvoy RD (2003) Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev 7:377–389
Valipour A (2012) Gender-related differences in the obstructive sleep apnea syndrome. Pneumologie 66:584–588
Ralls FM, Grigg-Damberger M (2012) Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Curr Opin Pulm Med 18:568–573
Saaresranta T, Anttalainen U, Polo O (2015) Sleep disordered breathing: is it different for females? ERJ Open Res 1:00063–02015
Yamakoshi S, Kasai T, Tomita Y, Takaya H, Kasagi S, Kawabata M et al (2016) Comparison of clinical features and polysomnographic findings between men and women with sleep apnea. J Thorac Dis 8:145–151
Vagiakis E, Kapsimalis F, Lagogianni I, Perraki H, Minaritzoglou A, Alexandropoulou K et al (2006) Gender differences on polysomnographic findings in Greek subjects with obstructive sleep apnea syndrome. Sleep Med 7:424–430
Izci B, Ardic S, Firat H, Sahin A, Altinors M, Karacan I (2008) Reliability and validity studies of the Turkish version of the Epworth Sleepiness Scale. Sleep Breath 12:161–168
Iber C, Ancoli-Israel S, Chesson AL (2007) In: Quan SF for the American Academy of Sleep Medicine (ed) The AASM manual 2007 for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine, Westchester, Illinois
O’Connor C, Thornley KS, Hanly PJ (2000) Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 161:1465–1472
Wahner-Roedler DL, Olson EJ, Narayanan S, Sood R, Hanson AC, Loehrer LL et al (2007) Gender-specific differences in a patient population with obstructive sleep apnea-hypopnea syndrome. Gend Med 4:329–338
Gabbay IE, Lavie P (2012) Age- and gender-related characteristics of obstructive sleep apnea. Sleep Breath 16:453–460
Young T, Evans L, Finn L, Palta M (1997) Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 20:705–706
Shepertycky MR, Banno K, Kryger MH (2005) Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep 28:309–314
Quintana-Gallego E, Carmona-Bernal C, Capote F, Sánchez-Armengol A, Botebol-Benhamou G, Polo-Padillo J et al (2004) Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med 98:984–989
Wickwire EM, Collop NA (2010) Insomnia and sleep-related breathing disorders. Chest 137:1449–1463
Luyster FS, Buysse DJ, Strollo PJ Jr (2010) Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med 6:196–204
Walker RP, Durazo-Arvizu R, Wachter B, Gopalsami C (2001) Preoperative differences between male and female patients with sleep apnea. Laryngoscope 111:1501–1505
Franklin KA, Sahlin C, Stenlund H, Lindberg E (2013) Sleep apnoea is a common occurrence in females. Eur Respir J 41:610–615
Dursunoglu N, Ozkurt S, Sarikaya S (2009) Is the clinical presentation different between men and women admitting to the sleep laboratory? Sleep Breath 13:295–298
Mazzuca E, Battaglia S, Marrone O, Marotta AM, Castrogiovanni A, Esquinas C et al (2014) Gender-specific anthropometric markers of adiposity, metabolic syndrome and visceral adiposity index (VAI) in patients with obstructive sleep apnea. J Sleep Res 23:13–21
Subramanian S, Jayaraman G, Majid H, Aguilar R, Surani S (2012) Influence of gender and anthropometric measures on severity of obstructive sleep apnea. Sleep Breath 16:1091–1095
Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T (2005) Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 165:2408–2413
Alotair H, Bahammam A (2008) Gender differences in Saudi patients with obstructive sleep apnea. Sleep Breath 12:323–329
Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM (2012) Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med 156:115–122
Subramanian S, Hesselbacher S, Mattewal A, Surani S (2013) Gender and age influence the effects of slow-wave sleep on respiration in patients with obstructive sleep apnea. Sleep Breath 17:51–56
Acknowledgements
The authors thank sleep technicians Bahar Yoruk, Yakup Coskun, and Merve Ozdemir in the Sleep Disorders Laboratory, Department of Chest Diseases, Ege University School of Medicine, for their valuable help in gathering the data related to the health of the patients. The authors also thank Timur Kose from the Department of Biostatistics, Ege University School of Medicine, for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study, and no identifying information about participants was available in the article.
Additional information
Comments
This study reports gender associated differences in a cohort of patients with severe OSA. There are many significant differences in clinical presentation and polysomnographic variables between the male and female patients. Women were older, had a higher BMI, increased rate of medical comorbidities and lower AHI; yet, the question is, do these findings accurately characterize OSA in females or are only the most severely affected women referred for polysomnography. It is increasingly recognized that women do not necessarily manifest the characteristic symptoms of OSA. Symptoms not necessarily indicative of OSA but more so insomnia were significantly higher in women than men in this study. Additionally, the increased use of psychotropic medications in women suggests they were treated for a behavioral medicine disorder, insomnia or both. This further suggests women with sleep disturbances refractory to treatment made up a substantial proportion of the female cohort. Current screening questionnaires are likely inadequate in women, which could contribute to delays in diagnosis. Sleep medicine professionals must educate providers to have a heightened clinical suspicion for OSA in women, especially those with insomnia or behavioral medicine disorders that are not responding to treatment.
Vincent Mysliwiec
Tacoma, WA, USA
Rights and permissions
About this article
Cite this article
Basoglu, O.K., Tasbakan, M.S. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath 22, 241–249 (2018). https://doi.org/10.1007/s11325-017-1482-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1482-9