Abstract
Purpose
This study was aimed to assess potential correlations between periodic leg movement (PLM) index, hepcidin levels, and iron status in patients with obstructive sleep apnea syndrome (OSAS).
Methods
Forty-four newly diagnosed OSAS patients and 49 non-apneic controls were enrolled in this study. All patients underwent polysomnographic evaluation. The hepcidin, iron, ferritin, total iron binding capacity, and C-reactive protein levels were measured.
Results
The mean age was 47.4 ± 7.2 years (18–68) in the OSAS group and 44.9 ± 11.1 years (23–65) in the control group. There were no differences in age, gender, and smoking between OSAS patients and controls. Mean apnea–hypopnea index (AHI) was 25.1 events/h. Mean serum hepcidin levels were significantly higher in OSAS subjects (725.9 ng/ml) than in control subjects (646.0 ng/ml) (p < 0.001). Serum iron levels were significantly lower in the OSAS and PLM disorder groups than in control subjects (p < 0.001). Serum hepcidin levels were significantly correlated with AHI (r = 0.453) and PLM index (r = 0.114). Serum iron levels were significantly negatively correlated with AHI (r = −0.169) and PLM index (r = −0.180).
Conclusions
In our study, the level of hepcidin was increased in patients with OSAS. Our study indicates that levels of hepcidin correlate with the AHI and PLM index severity of OSAS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is diagnosed in the majority of patients seeking treatment at sleep disorder centers. OSAS causes excessive daytime sleepiness in at least 3 % of affected adults, while others with this condition do not experience overt sleepiness. Between one quarter and one-half of the patients evaluated by polysomnography for suspected OSAS also have periodic leg movements (PLM) during sleep [1, 2].
PLM disorder is characterized by periodic episodes of repetitive limb movements during sleep. These movements most often occur in the lower extremities (e.g., toes, ankles, knees, and hips), but can occasionally occur in the upper extremities. These movements may be associated with an arousal, which can disrupt sleep and lead to excessive daytime sleepiness [3].
Restless leg syndrome (RLS) is characterized by the occurrence of periodic leg movements during sleep (PLMS). PLMS occurs in approximately 80 to 90 % of patients with RLS and supports the diagnosis of RLS [3].
Both RLS and PLM during sleep are more prevalent in certain populations, including pregnant women, those with iron deficiency anemia, and those with end-stage renal disease, in which inadequate iron status is likely to develop [4–6]. Ekbom reported an association between iron deficiency and RLS as early as 1969 [7]. Some studies suggest that iron therapy can reduce RLS symptoms in patients with ferritin levels less than 45 μg/L [8, 9].
However, the relationship between hepcidin, iron status, and PLM disorder in patients with OSAS has not been well studied. Initial studies identified hepcidin (hepatic bactericidal protein) as a urinary antimicrobial peptide that is rich in cysteine. Further studies indicated that hepcidin is overexpressed in mice with iron overload and plays a significant role in iron homeostasis in knockout animals with iron storage disease. Complete abrogation of hepcidin leads to excessive intestinal absorption of iron and an increase in the amount of iron released by macrophages, which causes iron overload [10–12]. Hepcidin functions via interactions between hepcidin and ferroportin [13].
Iron regulates hepcidin homeostasis. Hepcidin production is stimulated by increases in plasma iron levels and by increases in iron storage [14]. Hepcidin production is suppressed during iron deficiency. In addition, the proper function of the feedback loop between iron and hepcidin ensures appropriate physiological concentrations of iron in the plasma [15]. Furthermore, hepcidin levels are elevated during inflammation and/or infection, which can cause iron dysregulation with hypoferremia and anemia-related inflammatory disease [16].
Jairam et al. compared the body iron stores, degree of inflammatory activation, and pro hepcidin levels in patients newly diagnosed with end-stage renal disease with those of a normal population [17]. They showed that the diseased patients had marked inflammatory activation, including high hepcidin levels, which could explain their functional iron deficiency.
While hepcidin levels are markedly high during inflammation, its expression is downregulated by hypoxia by mechanisms that are still being determined. Since both inflammation and hypoxia are active forces in the development of OSAS, we hypothesize that hepcidin plays a regulatory role in this condition. The present study aimed to assess potential correlations between the PLM index, hepcidin levels, and iron status in patients with OSAS.
Methods
Patient selection
All subjects were evaluated for OSAS at the Sleep Disorders Centre of Dicle University either because they were referred by their physician or because they came themselves due to problems with snoring or daytime sleepiness. Subjects were eligible for this study if their total sleep time was at least 240 min on the polysomnographic (PSG) recording. The Ethics Committee of Dicle University Medical Faculty approved the study protocol, and informed consent was obtained from each participant.
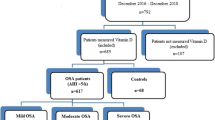
Forty-four newly diagnosed OSAS patients and 49 age-matched non-OSAS control subjects were enrolled in this study.
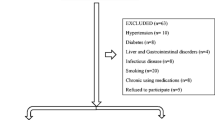
Subjects were excluded from this study if they had any known central sleep apnea, upper airway resistance, hematologic disorders, neurologic disorders, cardiovascular disease, lung disease, and renal or metabolic disease. Additionally, patients were excluded from this study if they were on any type of medications that may affect PLM disorder (e.g., dopaminergic medications, opiates, benzodiazepines, and tricyclic antidepressants). The body mass index (BMI) of each participant was calculated by dividing the weight (kg) by the height (m2).
Polysomnographic evaluation
All participants underwent an all-night sleep recording, which was performed using diagnostic equipment with integrated digitalized video (E-Series, 44-channel polysomnograph; Compumedics™, Melbourne, Victoria, Australia).
The effects of sleep stage on PSG and apnea–hypopnea events for adults were determined according to the updated criteria of the American Academy of Sleep Medicine [18].
The universally accepted criteria for diagnosis of PLM disorder are as follows:
-
1.
There should be at least four leg movements in a 90-s period;
-
2.
Contractions should be more than 0.5 s and less than 5 s;
-
3.
When contractions are recorded from both of the anterior tibialis muscles, they should be separated by an interval of at least 5 s for them to be counted as two separate movements;
-
4.
Contractions can either be associated with EEG arousals, or in severe cases, even overt arousals;
-
5.
The PLM index (PLMI) is calculated by dividing the total number of PLMs by the sleep time in hours; and
-
6.
The diagnosis of PLM disorder can be made when patients present with insomnia, tiredness, and daytime sleepiness as well as high PLMI [3].
The cutoff value of PLMI for PLMD is set at 15 episodes per hour [19].
Laboratory Analysis Blood samples were immediately centrifuged, and serums were kept at −80 °C until laboratory tests were performed. Serum Hepcidin 25 (Eastbio Pharm) levels were determined using an enzyme-linked immunosorbent assay (ELISA) method according to the manufacturer’s protocols. The serum iron, ferritin, and unsaturated iron-binding capacity levels were determined with an autoanalyzer Architect C 16000 (Abbott Laboratories, Abbott Park, IL, USA), and the C-reactive protein (CRP) levels were measured by the nephelometric method.
Statistical analysis
Data characterized by a normal distribution were expressed as mean ± standard deviation. The Kolmogorov–Smirnov test was used to determine a normal distribution of continuous variables. The Student’s t test (normal distribution) or the Mann–Whitney test (non-normal distribution) was used for comparing two groups. The Kruskal–Wallis test was used for comparing more than two groups. Categorical data were compared with the χ 2 test or the Fisher’s exact test. Correlations between variables were assessed with the Pearson’s correlation test. All data were analyzed with the Statistical Package for Social Sciences version 11 for Windows® (SPSS® Inc., Chicago, IL, USA). All statistical tests were two-sided, and p values <0.05 were considered statistically significant.
Results
Forty-four newly diagnosed OSAS patients and 49 age-matched non-OSAS control subjects were enrolled in this study. The demographic and clinical characteristics of both study groups are presented in Table 1. There were no differences in age, gender, or smoking between the two groups. The mean age of the OSAS group was 47.4 ± 7.2 years (18–68).
Serum hepcidin levels were significantly higher in OSAS subjects (725.9 ± 362.3 ng/ml) than in non-OSAS subjects (296.3 ± 169.7 ng/ml; p < 0.001).
Serum hepcidin levels in mild (n = 16), moderate (n = 17), and severe (n = 11) OSAS patients were 570.7, 693.6, and 1,001.4 ng/ml, respectively. The differences between each of these values were significant (p = 0.01).
Subjects with PLM disorder had significantly higher hepcidin levels when compared with subjects without PLM disorder (646.0 ± 332.0 ng/ml versus 373.6 ± 318.1 ng/ml; p < 0.001).
Serum iron levels were significantly lower in the OSAS group (p < 0.001). There were no significant differences in the ferritin, total iron-binding capacity (TIBC), or CRP serum levels between the OSAS group and the controls. The laboratory results of the OSAS–non-OSAS and PLM disorder–non-PLM disorder groups are shown in Tables 2 and 3, respectively.
A Pearson correlation analysis indicated a significant correlation between serum hepcidin levels and the apnea–hypopnea index (AHI) (r = 0.453, Fig. 1), oxygen desaturation index 3 % (ODI3%) (r = 0.459, Fig. 2), and PLMI (r = 0.114, Fig. 3) in all of the patients.
There was a significant negative correlation between serum iron levels and AHI (r = −0.169, Fig. 4) and PLMI (r = −0.180, Fig. 5).
There were no significant differences in the hepcidin, iron, ferritin, TIBC, and CRP serum levels or BMI between the male and female patients with OSAS (p > 0.05).
There was a significant negative correlation between serum hepcidin levels and iron levels (r = −0.471).
Discussion
Data from this study indicate that hepcidin serum levels in OSAS patients are higher than those of controls. In addition, there are correlations between hepcidin levels and AHI and PLMI levels.
Previous studies have reported that increased levels of CRP, interleukin-6, other inflammatory markers, and oxidative stress products indicate that both inflammation and oxidative stress play major roles in the pathogenesis of OSAS [20, 21].
Mermigkis et al. investigated possible gender differences in CRP evolution in OSAS patients by measuring CRP levels 3 and 6 months after beginning effective continuous positive airway pressure (CPAP) treatment [22]. Their results suggest that despite effective CPAP treatment, there is a delay in the normalization of CRP levels in females. Women required at least 6 months for their CRP levels to normalize and their cardiovascular risk to decrease. In contrast, CPAP had a protective role in males at an earlier time point. Gender-related hormonal and genetic factors may influence CRP evolution. It has been reported that OSAS patients have elevated serum CRP levels and that these high CRP levels are only related to obesity. In our study, there was no significant difference in CRP levels or BMI between male and female patients with OSAS.
Chronically elevated levels of proinflammatory cytokines are a feature common to both OSAS and anemia related to chronic illness. Because intermittent hypoxia is presumed to stimulate erythropoietin and the release of proinflammatory mediators, the development of anemia may be obscured in OSAS. Iron is an essential trace element, and optimal iron status is known to play a critical role in human performance. The discovery of hepcidin yielded a significant insight into the link between inflammation and systemic iron status [23].
Duru et al. reported decreased levels of hepcidin in the serum of patients with chronic obstructive pulmonary disease and suggested that hepcidin may play a role in preventing hypoxemia [24]. Similarly, we found a negative correlation between hypoxemia and hepcidin.
Khan et al. reported that a group of patients (n = 10) with severe OSAS had no anemia and normal hepcidin levels [25]. In our study, we determined that patients with OSAS had increased serum hepcidin levels and that these levels were further increased in patients with severe OSAS. We hypothesize that this increase may be positively correlated with inflammation.
Clardy et al. hypothesized that the pathophysiology of PLM is associated with abnormalities in the iron metabolism pathway and iron storage and transferase as well as abnormalities in the ferritin subunits, hepcidin, and transferrin receptor expression [26]. The relationship between hepcidin, iron status, and PLMD in patients with OSAS is not known. In our study, hepcidin was increased in patients with PLMD, and hepcidin and iron levels were associated with a significantly high risk of PLMD.
The synthesis of hepcidin is increased by both inflammation and infection. Patients with sepsis, inflammatory bowel disease, myeloma, burns, and C-reactive protein (CRP) levels >10 mg/dL exhibit significantly elevated hepcidin levels [27]. Macrophages are stimulated during the inflammatory process, and the degree of stimulation depends on the severity of inflammation.
Activated macrophages release a network of cytokines. Among them is interleukin-6 (IL-6), which is one of the primary inducers of hepcidin expression. An increase in hepcidin levels can cause hypoferremia. In our study, there was a significant negative correlation between serum hepcidin levels and iron levels. Because OSAS is an inflammatory disease, we hypothesize that hypoferremia can induce PLMD in patients with OSAS. Because hepcidin plays a central role in iron homeostasis, it is possible to consider its use as a novel treatment. Although hepcidin is not presently used for the treatment of anemia, we can use hepcidin level as a diagnostic tool to determine the severity of both anemia and OSAS.
Iron-related diseases, such as PLMD, are common and clinically important. Recent research has clarified the pathogenesis of hypoferremia and of PLMD that is related with hypoferremia. This study and future studies with larger series may allow for the development of new tests and new treatments.
Sımakajornboon et al. assessed potential relationships between serum iron and ferritin levels and the severity of PLMS in a pediatric population and evaluated their response to supplemental iron therapy [28]. They determined that in children, the presence of PLMD is frequently associated with low serum iron and a tendency toward low serum ferritin levels. In addition, iron therapy was associated with clinical improvement in most of these patients [28].
Similarly, we also determined that PLMD is frequently associated with low serum iron, low serum ferritin, and high serum hepcidin levels in patients with OSAS.
There are potential limitations of the current study. First, the sample size was relatively small. The general applicability of our results to those with OSAS requires larger studies in order to establish the roles of PLMD and hepcidin and to confirm hepcidin and iron levels in patients with OSAS and those in other subgroups. Second, our study did not contain the follow-up records of subjects after treatment.
Since high hepcidin levels contribute to inflammation, the evaluation of this peptide in OSAS may have the potential to be useful as a diagnostic or prognostic indicator of the disease. Thus, screening hepcidin levels in OSAS patients may be useful for evaluating the prognosis of OSAS. To address this hypothesis, further prospective studies are needed in order to evaluate the role of hepcidin in patients with OSAS [29]. These prospective studies must have larger series to determine its clinical utility and cost-effectiveness.
O’Brien et al. hypothesized that OSA patients may have increased PLM during sleep and have more daytime sleepiness due to lower body iron stores, as reflected by serum ferritin levels, which cause downstream effects on dopaminergic transmission [30]. They showed that lower minimum oxygen saturation and increased sleep stage shifts predicted increased ferritin levels. The results of their study, which were powered to detect small to moderate effect sizes, strongly suggest that OSAS does not cause lower serum ferritin levels, which, in turn, cannot explain PLMD or daytime sleepiness in their patients.
In our study, other markers of iron metabolism (e.g., TIBC and ferritin levels) in the OSAS group were different from those of the control group, although these differences were not significant due to the small sample size.
To our knowledge, an investigation regarding the association between hepcidin and iron status and PLMD in patients with OSAS has not been published. In this study, we demonstrated that there is a significant increase in hepcidin levels in patients with OSAS when compared to healthy controls. We believe that hepcidin levels in patients with OSAS may increase due to inflammation, which results in a reduction in iron levels. Low iron levels and high hepcidin levels may also play a role in the pathogenesis of PLMD.
References
Chervin RD (2001) Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med 164:1454–1458
Al-Alawi A, Mulgrew A, Tench E, Ryan CF (2006) Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med 2:281–287
Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, Lamm CI, Tracy SL, Rosenberg RS, American Academy of Sleep Medicine (2012) The treatment of restless legs syndrome and periodic limb movement disorder in adults–an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 35:1039–1062
Grim K, Lee B, Sung AY, Kotagal S (2013) Treatment of childhood-onset restless legs syndrome and periodic limb movement disorder using intravenous iron sucrose. Sleep Med 14:1100–1104
Lin CH, Wu VC, Li WY, Sy HN, Wu SL, Chang CC, Chiu PF, Lion HH, Lin CY, Chang HW, Lin SY, Wu KD, Chen YM, Wu RM (2014) Restless legs syndrome in end-stage renal disease: a multicenter study in Taiwan. Eur J Neurol 21:492–498
Manconi M, Govoni V, De Vito A, Economou NT, Cesnik E, Casetta I, Mollica G, Ferini-Strambi L, Granieri E (2004) Restless legs syndrome and pregnancy. Neurology 63:1065
Ekbom KA (1969) Restless legs syndrome. Neurology 10:868–873
Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ (2013) The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol 88:261–264
Lee CS, Lee SD, Kang SH, Park HY, Yoon IY (2014) Comparison of the efficacies of oral iron and pramipexole for the treatment of restless legs syndrome patients with low serum ferritin. Eur J Neurol 21:260–266
Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC (2002) Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood 100:3776–8371
Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S (2002) The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 110:1037–1044
Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101:2461–2463
Bergamaschi G, Villani L (2009) Serum hepcidin: a novel diagnostic tool in disorders of iron metabolism. Haematologica 94:1631–1633
Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M (2008) Immunoassay for human serum hepcidin. Blood 112:4292–4297
Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S (2006) Suppression of hepcidin during anemia requires erythropoietic activity. Blood 108:3730–3735
Ganz T (2006) Molecular pathogenesis of anemia of chronic disease. Pediatr Blood Cancer 46:554–557
Jairam A, Das R, Aggarwal PK, Kohli HS, Gupta KL, Sakhuja V (2010) Iron status, inflammation and hepcidin in ESRD patients: the confounding role of intravenous iron therapy. Indian J Nephrol 20:125–131
Berry RB, Budhiraja R, Gottlieb DJ (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med 8:597–619
Ferri R, Fulda S, Manconi M (2013) Night-tonight variability of periodic leg movements during sleep in restless legs syndrome and periodic limb movement disorder: comparison between the periodicity index and the PLMS index. Sleep Med 14:293–296
Celec P, Hodosy J, Behuliak M (2012) Oxidative and carbonyl stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath 16:393–398
Suzuki YJ, Jain V, Park AM, Day RM (2006) Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med 40:1683–1692
Mermigkis C, Bouloukaki I, Mermigkis D, Kallergis E, Mavroudi E, Varouchakis G, Tzortzaki E, Siafakas N, Schiza SE (2012) CRP evolution pattern in CPAP-treated obstructive sleep apnea patients. Does gender play a role? Sleep Breath 16:813–819
Stomberg EG, McClung JP (2012) Inflammation and diminished iron status: mechanisms and functional outcomes. Curr Opin Clin Nutr Metab Care 15:605–613
Duru S, Bilgin E, Ardiç S (2012) Hepcidin: a useful marker in chronic obstructive pulmonary disease. Ann Thorac Med 7:31–35
Khan A, Appel D (2009) Serum hepcidin levels and anemia among patients with severe obstructive sleep apnea: a prospective pilot study. Chest 136(4_MeetingAbstracts):69S-c-70S
Clardy SL, Earley CJ, Allen RP (2006) Ferritin subunits in CSF are decreased in restless legs syndrome. J Lab Clin Med 147:67–73
D’Angelo G (2013) Role of hepcidin in the pathophysiology and diagnosis of anemia. Blood Res 48:10–15
Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM (2003) Periodic limb movements in sleep and iron status in children. Sleep 26:735–738
Kanbay A, Hasanoglu HC (2007) A new prognostic marker for obstructive sleep apnea: hepcidin. Med Hypotheses 69:1381–1382
O’Brien LM, Koo J, Fan L, Owusu JT, Chotinaiwattarakul W, Felt BT, Chervin RD (2009) Iron stores, periodic leg movements, and sleepiness in obstructive sleep apnea. J Clin Sleep Med 5:525–531
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abakay, O., Abakay, A., Palanci, Y. et al. Relationship between hepcidin levels and periodic limb movement disorder in patients with obstructive sleep apnea syndrome. Sleep Breath 19, 459–466 (2015). https://doi.org/10.1007/s11325-014-1028-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-014-1028-3