Abstract
Background
Obstructive sleep apnea–hypopnea syndrome (OSAHS) is a disease characterized with intermittent hypoxia and sleep fragmentation. Obesity and gender are major risk factors for the onset of OSAHS. Previous studies on obese men with OSAHS have been performed, while few studies on non-obese men with OSAHS have been carried out. The purpose of this study was to explore the clinical characteristics of polysomnography and blood biochemical indexes in non-obese men with OSAHS and to identify the possible influencing factors.
Methods
This retrospective study included patients with OSAHS who underwent polysomnography in our hospital. General clinical data such as overnight polysomnography and biochemical indicators were recorded. The patients were divided into two groups according to the apnea–hypopnea index (AHI): mild to moderate OSAHS and severe OSAHS. The differences in biochemical parameters such as the levels of γ-glutamine transaminase (GGT), triglyceride (TG), glucose (GLU), and sleep structure parameters such as N1, N2, slow-wave sleep (SWS), and rapid eye movement (REM) sleep were compared and analyzed. Spearman correlation analysis and logistic regression were used to identify the risk factors of non-obese men with OSAHS. ROC curves were used to evaluate the predictive ability of SWS and GGT on disease severity.
Results
Of 94 non-obese men with OSAHS, 49 had mild to moderate OSAHS and 45 had severe OSAHS. Our data suggested that the levels of low oxygen saturation (L-SaO2), mean oxygen saturation (M-SaO2), SWS, and GGT were significantly changed in the mild to moderate OSAHS group compared with the severe group (p < 0.05). For patients with OSAHS, the proportion of SWS in the group with severe OSAHS was higher than that in the mild to moderate group (p < 0.05), and the serum GGT enzyme levels were significantly elevated in the severe group compared to the mild to moderate group (p < 0.05). Using logistic regression analyses, our data revealed that both SWS and GGT enzyme levels were independent risk factors for AHI (p < 0.05). In addition, the results of correlation analysis indicated that SWS was related to triglyceride (TG), total cholesterol (TC), apolipoprotein E (APOE), and triglyceride glucose (TyG) index (p < 0.05); GGT was related to TG, TC, APOE, and TyG index (p < 0.05). Furthermore, SWS was independently associated with GGT (p < 0.05). The area under the ROC curve plotted with the combined coefficient of SWS and serum GGT was 0.728, which was predictive of the disease severity.
Conclusions
These results suggest that SWS and GGT are independent associated factors of the severity of the disease. However, TyG index was not an independent associated factor of the severity of disease in non-obese men with OSAHS. In addition, SWS and GGT were negatively correlated. SWS combined with serum GGT may be predictive of the severity of the disease. This study may have added to our understanding of the pathogenesis of OSAHS in non-obese men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive collapses (apnoeas) or near collapses (hypopnoeas) of the upper airway during sleep, resulting in intermittent hypoxemia and increased sympathetic arousal. With symptoms of daytime dysfunction and other neurological impairments caused by the apneas and hypopneas in sleep, the disorder is also known as obstructive sleep apnea-hypopnea syndrome (OSAHS) [1]. Patients with untreated sleep-disordered breathing exhibit an increased risk of hypertension, stroke, heart failure, diabetes, car accidents, depression, and impairment of cognition [2,3,4,5,6,7,8]. Epidemiological studies have indicated that OSAHS is considered to be a gender-related disease, with male:female ratio ranging from 3:1 to 5:1 in the general population and from 8:1 to 10:1 in selected clinical populations [9]. Obesity is a major risk factor for OSAHS [10]. Previous studies have shown that the prevalence of OSAHS ranges from 55 to 90% in the severely obese [11], and only approximately 20% of adults with OSA are non-obese [12]. However, most previous studies on OSAHS have focused on obese subjects, but few studies have been performed on non-obese patients with OSAHS, which may affect the diagnosis and treatment. Therefore, the comorbidity burden is high in non-obese patients with OSA [13, 14].
Polysomnography (PSG) is an essential method to diagnose and evaluate this disease, and PSG provides detailed information about sleep architecture, duration, and quality. SWS is a category of non-rapid eye movement (NREM) sleep and clinical studies have shown that increased depth of NREM sleep may improve OSA [15]. Energy conservation [16], immune regulation [17], memory consolidation [18], and other neuroendocrine changes also occur during SWS, and include increased growth hormone release, insulin sensitivity, and decreased adrenocorticotropic hormone, [19] as well as sympathetic nervous system activity [20].
Previous studies have also found that serum biochemical indicators were also related to the severity of OSAHS, such as the levels of glucose, triglyceride(TG), andγ-glutamine transamina(GGT) enzyme. TyG index is calculated as Ln (fasting triglycerides (mg/dl) × fasting blood glucose (mg/dl)/2). Previous studies have revealed that the TyG index is a reliable marker of insulin resistance (IR) [21,22,23,24]. Kang et al. [25] have indicated that the TyG index was an independent predictor of increased OSA risk. And previous studies have suggested that intermittent hypoxia, oxidative stress, sympathetic activation, elevated serum GGT, and GGT are related to the severity of this disease [26].
As OSAHS seems to occur more commonly in men, previous studies on obese men with OSAHS have been carried out, but few studies have been performed on non-obese men. Therefore, this study included non-obese men with OSAHS as the study population, and the clinical characteristics of polysomnography and blood biochemical indexes in these patients were examined, and possible influencing factors were explored.
Methods
Population sampling
This observational study included adults who underwent polysomnography (PSG) at the Second Hospital of Chongqing Medical University from February 2013 to June 2022. The study was approved by the ethics committee of our hospital (2022 Colum Review No. (144)). Inclusion criteria were as follows: participants with a body mass index (BMI) < 28 kg/m2, BMI was calculated as weight in kilograms divided by height by in meters squared. Exclusion criteria were: (1) Under the age of 18; (2) History of upper airway surgery, oral appliances, or continuous positive airway pressure therapy; (3) Active infection within the last 4 weeks; (4) Other sleep disorders (severe insomnia, restless legs syndrome or narcolepsy); (5) Alcohol abuse or use of drugs that can cause liver damage;(6) Acute ischemic stroke. OSAHS [27] was classified as mild to moderate (5 times/h ≤ AHI < 30 times/h) and severe (AHI ≥ 30 times/h) according to the diagnostic criteria.
Polysomnography
Sleep recording was performed in all individuals using ambulatory polysomnography (Philips, Alice 5 and NicoletOne), during which synchronized eye movements, oral and nasal airflow, chest and abdominal activity, oxygen saturation, electroencephalogram, and electrocardiogram were evaluated. The monitoring results were automatically analyzed by Alice software and then interpreted by a specialist. Sleep apnea was defined as the absence or significant reduction of oronasal airflow during sleep, with a reduction of 90% or more compared to baseline time and a duration of 10 s or longer. Hypopnea [28] was defined as follows: (1) 30% or greater reduction in oronasal airflow from baseline level during sleep. This is accompanied by a decrease in oxygen saturation of 4% or more for 10 s or longer. Or (2) 50% or greater reduction in oronasal airflow from baseline level accompanied by 3% or greater decrease in oxygen saturation for 10 s or longer. AHI, minimum saturated carbon saturation (L-SaO2), and mean oxygen saturation (M-SaO2) were recorded. TST was recorded as the total number of minutes of any form of sleep. Sleep efficiency was determined by calculating the ratio of TST to time spent in bed. N1, N2, SWS (stage N3), and duration of rapid eye movement (REM) sleep were also recorded.
Biochemical measurements
Fasting venous blood was collected from all participants at 7 am, then the levels of serum GGT enzyme levels and serum total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (APOA1), apolipoprotein B (APOB), and apolipoprotein E (APOE) were measured using the autoanalyzer (H-7600, Hitachi, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using SPSS software (version: 22.0, IBM Corp., Armonk, USA), and a two-sided p-value < 0.05 was considered significant. The data conforming to the normal distribution shall be averaged; the data were presented as mean ± standard deviation. If they did not conform to the normal distribution, the data were presented as the median (interquartile interval). The data has been inspected by Shapiro Wilk according to the inspection level α = 0.05, p > 0.05, and it was considered that the data conform to the normal distribution. For the comparison of two groups, two independent t-test was used for data that conform to a normal distribution, and Mann Whitney U test was used for data that do not conform to the normal distribution, and Kruskal Wallis H (K) test was used for continuous variables with non-normal distribution. Chi-square tests or Fisher exact tests were used for variable classification. Correlation between two variables: Pearson correlation analysis was used for continuous variables with normal distribution, otherwise Spearman correlation analysis was used. Independent risk factors for disease severity in non-obese men with OSAHS were analyzed by logistic regression. The ROC curve was used to evaluate the predictive ability of SWS and GGT on disease severity.
Results
Overall baseline characteristics
Of 94 patients with OSAHS 49 had mild to moderate disease and 45 had severe disease. The average age was 46.6 ± 11.0 years. There were 27 cases with hypertension, 8 cases with diabetes, 4 cases with coronary heart disease, 4 cases with arrhythmia, and 36 cases with hyperlipidemia. The baseline characteristics of the cohort are summarized in Table 1.
Differences of clinical characteristics in non-obese men with OSAHS with various disease severity
Our data suggested that there were significant differences in L-SaO2, M-SaO2, SWS, and GGT between the mild to moderate and severe OSAHS group (p < 0.05). The proportion of SWS in the severe OSAHS group was significantly reduced compared to the mild to moderate group (p < 0.05). The biochemical index revealed that for patients with OSAHS, the serum GGT levels were remarkably increased as the severity of OSAHS enhanced, and the differences were statistically significant (p < 0.05). The results were presented in Table 2.
Logistic regression models for disease severity in non-obese men with OSAHS
In logistic regression analyses, increased GGT enzyme and SWS were independent risk factors for disease severity after adjusting for other confounding parameters (OR = 1.014, p = 0.007; OR = 1.071, p = 0.002). The results were presented in Table 3.
The association between other variables and SWS, GGT, and AHI
Our data indicated that SWS was negatively correlated with TC, TG, APOE, and TyG index (r = − 0.211, p = 0.041; r = − 0.263, p = 0.010; r = − 0.239, p = 0.020; r = − 0.218, p = 0.035), respectively; GGT was positively correlated with TC, TG, APOE, and TyG index (r = 0.229, p = 0.027; r = 0.345, p = 0.001; r = 0.299, p = 0.003; r = 0.376, p = 0.000). The result was presented in Table 4.
Correlation between SWS and serum GGT in non-obese men with OSAHS
SWS was correlated with the serum GGT levels. After the age was adjusted, smoking, alcohol consumption, BMI, comorbidities, and SWS were negatively correlated with serum GGT (r = 0.420, p < 0.001). The results are presented in Fig. 1.
The predictive value of SWS and serum GGT on disease severity
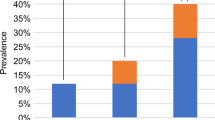
ROC curve revealed the area under SWS (AUC = 0.647; p < 0.05) and GGT curve (AUC = 0.661; p < 0.01). The area under SWS combined GGT curve was also presented (AUC = 0.728; p < 0.05). The predictive efficacy of SWS + GGT was better than SWS/GGT alone. The results were presented in Fig. 2.
Discussion
Previous studies have suggested that BMI is an independent predictor of enhanced OSA risk [10]. However, no significant difference was observed in the TyG index between severe and mild to moderate OSAHS in this study. The reason could be most of the previous studies focus on obese men with OSAHS, but little was known about non-obese men. Secretion of free fatty acids (FFA) is increased in obesity, and adipocytokines secreted by adipose tissue may damage the insulin signaling [29, 30]. A recent study has indicated that OSA combined with SWS exhibits greater effects on insulin resistance (IR) [31]. However, the roles of SWS and IR in OSAHS remain unclear.
Previous studies have suggested that the proportion of SWS was reduced as the severity of OSAHS increases. However, our results indicated that the proportion of SWS in the severe OSAHS group was higher than that in the mild to moderate OSAHS group. The reason could be that as the disease progresses, other SWS-associated factors lead to the increase of AHI. Consistent with the findings of Sánchez-Armengol A et al. [26], our data revealed that the serum GGT enzyme levels were increased as the severity of OSAHS increased. Furthermore, logistic regression analyses indicated that SWS and GGT enzyme levels were independently associated factors of AHI. Thus, SWS might be associated with GGT levels in non-obese men with OSAHS.
In addition, previous study has suggested that IR was related to GGT in non-obese men with OSAHS, which may be caused by increased TG synthesis [32]. Consistent with these findings, this study revealed that the TyG index was associated with GGT. In addition, Huang et al. [31] have indicated that SWS is involved in insulin resistance. Feng et al. [33] have suggested that the proportion of SWS is negatively correlated with glucose and triglyceride (TG) levels. Xu et al. [34] have revealed that the apnea–hypopnea index (AHI) during NREM sleep (AHINREM) is closely correlated with blood lipid composition such as TC, TG, and APOB. Moreover, Bikov et al. [35] suggested that AHINREM is related to TG levels. Consistent with previous results, this study further indicated that SWS was related to TG, TC, TyG index, and APOE. In addition, previous studies have revealed that GGT is related to IR and TG in non-obese patients with OSAHS. In this study, our results suggested that GGT was also related to TG, TC, TyG index, and APOE. As GGT and SWS exhibit similar physiological functions, both of them can reflect the severity of OSAHS [15, 26] and are involved in the regulation of lipid metabolism [31,32,33,34,35]. Therefore, we hypothesized that SWS could be related to GGT through lipid metabolism and IR. However, further investigations are required to confirm this.
Furthermore, linear analyses indicated that SWS was correlated with serum GGT. The potential underlying mechanisms may involve the synthesis of TC, TG, APOE, and TyG index. First, chronic intermittent hypoxia and activation of the pituitary-adrenocortical (HPA) axis lead to elevated levels of adrenocorticotropic hormone and cortisol, further inducing lipolysis [36]. Second, a lack of SWS could cause a reduction of growth hormone at night, and the deficiency of growth hormone may lead to lipid metabolism disorders, further resulting in insulin resistance [37, 38]. In addition, intermittent hypoxia and sleep fragmentation could lead to systemic inflammation, resulting in the disruption of lipid homeostasis [39]. Defects in the ApoE gene may also lead to the retention of chylomicrons, resulting in the increase of TG [40]. Third, triglyceride deposition decreasing fluidity and increasing permeability of hepatocyte membrane may lead to elevated serum GGT. Due to the relationship between SWS and GGT, SWS combined diagnosis may be helpful to predict the progress of this disease. Furthermore, ROC curve analyses indicated that SWS combined with GGT enzyme exhibit a higher predictive value for severe non-obese men with OSAHS.
There are several limitations in the current study. First, the sample size is relatively small, which may cause selection bias. Second, this study is single-centered potentially limiting the ability to generalize the results.
Conclusions
A higher percentage of SWS and serum GGT enzyme levels were found in non-obese men with severe OSAHS, and SWS and GGT enzyme levels were independent associated factors of disease severity. However, the TyG index was not an independent associated factor. In addition, SWS was correlated with serum GGT enzyme levels. A reduced percentage of SWS combined with increased GGT enzyme levels may exhibit a stronger association with increased disease severity in non-obese men with OSAHS.
Data availability
Data will be made available on reasonable request.
References
White DP (1995) Sleep-related breathing disorder.2. Pathophysiology of obstructive sleep apnoea. Thorax 50(7):797–804. https://doi.org/10.1136/thx.50.7.797
Peppard PE, Young T, Palta M et al (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342(19):1378–1384. https://doi.org/10.1056/NEJM200005113421901
Gottlieb DJ, Yenokyan G, Newman AB et al (2010) Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 122(4):352–360. https://doi.org/10.1161/CIRCULATIONAHA.109.901801
Yaggi HK, Concato J, Kernan WN et al (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353(19):2034–2041. https://doi.org/10.1056/NEJMoa043104
Redline S, Yenokyan G, Gottlieb DJ et al (2010) Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 182(2):269–277. https://doi.org/10.1164/rccm.200911-1746OC
Kendzerska T, Gershon AS, Hawker G et al (2014) Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med 190(2):218–225. https://doi.org/10.1164/rccm.201312-2209OC
Peppard PE, Szklo-Coxe M, Hla KM et al (2006) Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 166(16):1709–1715. https://doi.org/10.1001/archinte.166.16.1709
Turner K, Zambrelli E, Lavolpe S et al (2019) Obstructive sleep apnea: neurocognitive and behavioral functions before and after treatment. Funct Neurol 34(2):71–78
Wimms A, Woehrle H, Ketheeswaran S et al (2016) Obstructive sleep apnea in women: specific issues and interventions. Biomed Res Int 2016:1764837. https://doi.org/10.1155/2016/1764837
Young T, Skatrud J, Peppard PE (2004) Risk factors for obstructive sleep apnea in adults. JAMA 291(16):2013–2016. https://doi.org/10.1001/jama.291.16.2013
Frey WC, Pilcher J (2003) Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg 13(5):676–683. https://doi.org/10.1381/096089203322509228
Heinzer R, Vat S, Marques-Vidal P et al (2015) Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 3(4):310–318. https://doi.org/10.1016/S2213-2600(15)00043-0
Akahoshi T, Uematsu A, Akashiba T et al (2010) Obstructive sleep apnoea is associated with risk factors comprising the metabolic syndrome. Respirology (Carlton, Vic.) 15(7):1122–1126. https://doi.org/10.1111/j.1440-1843.2010.01818.x
Gündüz C, Basoglu OK, Hedner J et al (2018) Obstructive sleep apnoea independently predicts lipid levels: data from the European Sleep Apnea Database. Respirology (Carlton, Vic.) 23(12):1180–1189. https://doi.org/10.1111/resp.13372
Ratnavadivel R, Chau N, Stadler D et al (2009) Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med 5(6):519–524
Léger D, Debellemaniere E, Rabat A et al (2018) Slow-wave sleep: from the cell to the clinic. Sleep Med Rev 41:113–132. https://doi.org/10.1016/j.smrv.2018.01.008
Raison CL, Rye DB, Woolwine BJ et al (2010) Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiat 68(10):942–949. https://doi.org/10.1016/j.biopsych.2010.04.019
Diekelmann S, Born J (2010) The memory function of sleep. Nat Rev Neurosci 11(2):114–126. https://doi.org/10.1038/nrn2762
Tasali E, Leproult R, Ehrmann DA et al (2008) Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 105(3):1044–1049. https://doi.org/10.1073/pnas.0706446105
Somers VK, Dyken ME, Mark AL et al (1993) Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328(5):303–307. https://doi.org/10.1056/NEJM199302043280502
Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L et al (2020) Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol 2020:4678526. https://doi.org/10.1155/2020/4678526
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F (2008) The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 6(4):299–304. https://doi.org/10.1089/met.2008.0034
Vasques AC, Novaes FS, de Oliveira MS et al (2011) TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract 93(3):e98–e100. https://doi.org/10.1016/j.diabres.2011.05.030
Guerrero-Romero F, Villalobos-Molina R, Jim’enez-Flores JR et al (2016) Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch Med Res 47(5):382–387. https://doi.org/10.1016/j.arcmed.2016.08.012
Kang HH, Kim SW, Lee SH (2020) Association between triglyceride glucose index and obstructive sleep apnea risk in Korean adults: a cross-sectional cohort study. Lipids Health Dis 19(1):182. https://doi.org/10.1186/s12944-020-01358-9
Sánchez-Armengol A, Villalobos-López P, Caballero-Eraso C et al (2015) Gamma glutamyl transferase and oxidative stress in obstructive sleep apnea: a study in 1744 patients. Sleep Breath 19(3):883–890. https://doi.org/10.1007/s11325-014-1115-5
Ruehland WR, Rochford PD, O’Donoghue FJ et al (2009) The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 32(2):150–7. https://doi.org/10.1093/sleep/32.2.150
Berry RB, Budhiraja R, Gottlieb DJ et al (2012) American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8(5):597–619. https://doi.org/10.5664/jcsm.2172
Dresner A, Laurent D, Marcucci M et al (1999) Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Investig 103(2):253–259. https://doi.org/10.1172/JCI5001
Xu H, Barnes GT, Yang Q et al (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Investig 112(12):1821–1830. https://doi.org/10.1172/JCI19451
Huang W, Liu Y, Wang X et al (2021) Effect of interaction between slow wave sleep and obstructive sleep apnea on insulin resistance: a large-scale study. Nat Sci Sleep 13:739–749. https://doi.org/10.2147/NSS.S311130
Qi JC, Huang JC, Lin QC et al (2016) Relationship between obstructive sleep apnea and nonalcoholic fatty liver disease in nonobese adults. Sleep Breath 20(2):529–35. https://doi.org/10.1007/s11325-015-1232-9
Feng N, Yang J, Xu H et al (2021) The associations between sleep architecture and metabolic parameters in patients with obstructive sleep apnea: a hospital-based cohort study. Front Neurol 12:606031. https://doi.org/10.3389/fneur.2021.606031
Xu H, Xia Y, Li X et al (2020) Association between obstructive sleep apnea and lipid metabolism during REM and NREM sleep. J Clin Sleep Med 16(4):475–482. https://doi.org/10.5664/jcsm.8242
Bikov A, Lazar Z, Horvath P et al (2019) Association between serum lipid profile and obstructive respiratory events during REM and Non-REM sleep. Lung 197(4):443–450. https://doi.org/10.1007/s00408-019-00195-7
Jun JC, Shin MK, Yao Q et al (2012) Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab 303(3):E377-88. https://doi.org/10.1152/ajpendo.00641.2011
Martínez-Cerón E, Casitas R, Galera R et al (2021) Contribution of sleep characteristics to the association between obstructive sleep apnea and dyslipidemia. Sleep Med 84:63–72. https://doi.org/10.1016/j.sleep.2021.05.012
Van Cauter E, Latta F, Nedeltcheva A et al (2004) Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res 14(Suppl A):S10–S17. https://doi.org/10.1016/j.ghir.2004.03.006
Adedayo AM, Olafiranye O, Smith D et al (2014) Obstructive sleep apnea and dyslipidemia: evidence and underlying mechanism. Sleep Breath 18(1):13–18. https://doi.org/10.1007/s11325-012-0760-9
Liu X, Lin Q, Fan K et al (2021) The effects of genetic polymorphisms of APOE on circulating lipid levels in middle-aged and elderly chinese Fujian Han population: toward age- and sex-personalized management. Lipids Health Dis 20(1):158. https://doi.org/10.1186/s12944-021-01587-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (2022 Colum Review No. (144)).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, Q., Li, H., Gan, X. et al. Relationship between slow-wave sleep and serum γ-glutamine transaminase in non-obese men with obstructive sleep apnea–hypopnea syndrome. Sleep Breath 27, 1717–1724 (2023). https://doi.org/10.1007/s11325-022-02775-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-022-02775-z