Abstract
Purpose
Obstructive sleep apnea syndrome (OSAS) is characterized by elevated oxidative stress. Measurement of oxidative stress in saliva seems to be promising in long-term treatment monitoring of OSAS patients. In this study, our aim was to investigate whether short-term continuous positive airway pressure (CPAP) treatment would influence oxidative stress in saliva.
Methods
Patients with diagnosed OSAS (16 women, 28 men) underwent polysomnography during the first night and CPAP treatment during the second night. Saliva samples were taken in the evening and morning on both days. Markers of oxidative stress and antioxidant status were analyzed in saliva.
Results
Evening concentrations of the salivary thiobarbituric acid reacting substances (p < 0.001), advanced glycation end-products (p < 0.001), and advanced oxidation protein products (p < 0.01) were significantly lower than morning values during the diagnostic night. However, salivary concentrations of none of the oxidative stress markers were significantly influenced by the CPAP treatment. No changes in salivary antioxidant status after CPAP therapy were found.
Conclusion
Salivary markers of oxidative stress and antioxidant status do not change significantly after one night treatment with CPAP. On the contrary, after 1 month with CPAP therapy, reduced markers of oxidative stress were reported. Therefore, the future studies should be focused on finding the optimal sampling frequency to clarify the potential of saliva for the monitoring of OSAS treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is a sleep-related breathing disorder with increasing prevalence worldwide [1]. Various studies showed an association between OSAS and a higher risk of cardiovascular diseases, atherosclerosis, and metabolic syndrome [2–4]. OSAS is clinically characterized by repetitive phases of partial (hypopnea) or complete (apnea) upper airflow obstruction. This leads to a lowering of hemoglobin oxygen saturation and sleep cycle fragmentation [5]. These episodes of low oxygenation and reoxygenation mimic ischemic–reperfusion injury leading to formation of free radicals on one side [6] and to shutting the antioxidant enzymes production on the other [7]. The imbalance between free radical overproduction and decreased antioxidant status results in oxidative stress. Both free radicals and the products of oxidative stress interact with cellular components or biomolecules such as proteins, lipids, and DNA, causing their damage and altering their functions. Several studies have clearly shown an association between OSAS and oxidative stress, but the detailed underlying pathomechanism remains unclear [8, 9]. Lavie et al. found increased lipid peroxidation in the plasma of OSAS patients when compared to controls. This difference was diminished by continuous positive airway pressure (CPAP) therapy [10]. Another study showed a higher concentration of protein carbonyls and decreased antioxidant status in these patients [11]. The antioxidant status of OSAS patients was lower when measured as a ratio of oxidized and reduced glutathione in plasma [12]. On the other hand, Kang et al. found neither an association of OSAS with serum malondialdehyde (MDA) nor with DNA damage [13]. The discrepancies in the association between OSAS and oxidative stress might be due to the unknown underlying molecular mechanism and the variety of measured markers of oxidative stress in the particular studies. A palette of measured markers of oxidative stress, as well as antioxidant status, would be more informative in this case, since adequate markers with good clinical predictive value are still missing.
CPAP is currently the standard treatment for OSAS patients, as it has been shown to be effective and safe [14]. CPAP decreased various markers of oxidative stress in a serum of patients with OSAS in several studies [15–17]. However, Svatikova et al. showed no effect of CPAP on oxidative stress [18].
In most of the studies focusing on oxidative stress and OSAS, the markers of oxidative stress were measured in plasma or serum. According to our knowledge, saliva is rarely considered in the measurement of oxidative stress markers in patients with OSAS. Saliva represents an attractive diagnostic fluid, with an easy and noninvasive sample collection, well-established protocols for measuring markers of oxidative stress, and an antioxidant status [19, 20]. In our previous study, CPAP treatment lowered oxidative stress in OSAS patients after 1 and 6 months [21], showing a potential in using saliva as a specimen for monitoring the OSAS treatment effect. The kinetics of the changes of oxidative stress markers are currently not known. Based on the study of Alzoghaibi and Bahammam [22], which showed a significant decrease in lipid peroxidation after a single night with CPAP treatment in plasma, we hypothesized that a CPAP therapy would also decrease the concentration of other oxidative stress markers in saliva. The aim of the current study was to analyze the effect of one night with CPAP on markers of oxidative stress in the saliva of OSAS patients.
Material and methods
Patients
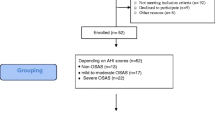
Sixteen women and 28 men, nonsmokers, were enrolled in this study. All patients underwent polysomnography (Alice 4 or Alice 5, Respironics, Inc., Murrysville, USA) to confirm the OSA and to determine its severity. OSA was scored when there was a presence of continued respiratory effort during the hypoxic event. The apnea/hypopnea index (AHI) was calculated as the number of apneas and hypopneas per hour of sleep for the total sleep time. Patients with AHI of more than 5 were further analyzed. The study was performed in the sleep laboratory of the University Hospital, Bratislava, Slovakia. As outlined in Fig. 1, patients spent two nights in the sleep laboratory and underwent a total of four sample collections. Whole, unstimulated saliva samples were obtained in 15 ml tubes without any additives during two consecutive days spent at the hospital. Morning sampling was conducted before eating or tooth-brushing, at 8.00 a.m. Evening sampling was conducted at least 60 min after the last meal and before tooth-brushing, at 8.00 p.m. The first night was considered as a diagnostic night with polysomnography (legend for the figures for the diagnostic night is Evening/Morning before treatment). The second night was considered as the treatment night with the CPAP (evening and morning with CPAP in the figures). After collection, the saliva samples were centrifuged at 1,000×g for 10 min at 4 °C and the supernatants were stored frozen at −20 °C (Fig. 1). All participants signed an informed consent form. The study was approved by the Ethics committee of the University Hospital, Comenius University, Bratislava, Slovakia.
Biochemical analyses
All markers of oxidative stress were assessed according to previously published protocols [21, 23]. For assessment of thiobarbituric acid reacting substances (TBARS), 20 μl of saliva was mixed with the same volume of thiobarbituric acid, acetic acid, and 30 μl of distilled water. Samples were briefly mixed and incubated for 45 min at 95 °C in a Master cycler (Eppendorf, Hamburg, Germany). After derivatization with n-butanol, the upper organic phase was transferred to a new plate. Fluorescence was measured at an emission wavelength of 553 nm and excitation wavelength of 515 nm. The calibration curve was constructed using 1,1,3,3-tetramethoxypropane (intra- and inter-assay variability 8–10 % and 10 to 14 %, respectively). Advanced glycation end-products (AGEs), as markers of carbonyl stress, were measured after dilution of saliva in phosphate-buffered saline (pH = 7.2). AGEs-specific fluorescence was measured using excitation and emission wavelengths of 370 and 430 nm, respectively. AGE-modified bovine serum albumin was used as a standard for the calibration curve (intra- and inter-assay variability 5–9 % and 7–10 %, respectively). Protein oxidation was determined by the measurement of advanced oxidation protein products (AOPP). Two hundred microliters of samples was mixed with 20 μl of glacial acetic acid. The absorbance at 340 nm was measured. Chloramine T with potassium iodine was used to construct the calibration curve (intra-assay variability 3–7 % and inter-assay variability 2–10 %). The antioxidant status of saliva samples were assessed as ferric-reducing ability of saliva (FRAS) and total antioxidant capacity (TAC). An initial absorbance of freshly prepared FRAS reagent (a mixture of acetate buffer, tripyridyl-s-triazine, FeCl3·6H2O, and distilled water pre-warmed to 37 °C) was measured at 593 nm. Afterwards, samples and standards (prepared using FeSO4·7H2O) were added and the specific absorbance was measured again. The difference to the initial values was calculated and compared to standards (intra- and inter-assay variability 0–5 % and 7–10 %, respectively). TAC in saliva was measured in the presence of acetate buffer (pH = 5.8). The initial absorbance at 660 nm was recorded. Following the addition of ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)) in acetate buffer with hydrogen peroxide, the absorbance at 660 nm was recorded again. The difference to the initial values was calculated and compared to the calibration curve constructed using Trolox (intra- and inter-assay variability 1–6 % and 5–15 %, respectively).
All measurements were done on the Saphire II instrument (Tecan, Grödig, Austria). All chemicals were purchased from Sigma-Aldrich (Steinheim, Germany).
Statistical analysis
Obtained data regarding the time course were analyzed with repeated-measures ANOVA and Tukey post hoc test using GraphPad v.6.01 statistical software (GraphPad Software, San Diego, CA, USA). The changes between the two conditions were calculated as the difference between the morning versus evening values of the respective nights. The differences between genders were analyzed by an unpaired t test. The changes between the two conditions were analyzed by a paired t test. The Pearson correlation test was used to evaluate the association between AHI and markers of oxidative stress. p values less than 0.05 were considered significant. Results are expressed as mean + standard error of the mean (SEM).
Results
Basic characteristics of patients are shown in Table 1. No gender differences were found in any of the measured parameters, except of BMI (t = 2.253, p < 0.05). Therefore, data from both genders were combined and further analyzed.
Salivary TBARS concentration as a marker of lipid peroxidation was significantly higher by 100 % in the morning, in comparison with the evening of the diagnostic night (Fig. 2a, F = 6.690, p < 0.001), showing a similar trend in the evening with CPAP (Fig. 2a, F = 6.690, p = 0.06). Similarly, salivary AGEs were higher by 107 % in the morning when compared to the evening of the diagnostic night (Fig. 2b, F = 9.764, p < 0.001), with the same trend on the treatment night with CPAP (Fig. 2b, F = 9.764, p = 0.088). Salivary AOPP showed similar trends as TBARS and AGEs, evening vs. morning without treatment, and evening vs. morning with CPAP (Fig. 2c, F = 7.656, p < 0.01 and p = 0.058, respectively).
Salivary TBARS (a) were higher in the morning in comparison to the evening before treatment (evening without treatment vs. morning without treatment, F = 6.6690, p < 0.001). Salivary AGEs (b) were higher in the morning when compared to the evening in both the night before treatment and the night with CPAP (F = 9.764, p < 0.001). Salivary AOPP (c) showed similar inclination as TBARS and AGEs (F = 7.656, p < 0.01). All three parameters decreased until the next sampling, i.e., the evening before treatment, to baseline values (morning before treatment vs. evening with CPAP, p < 0.05). One night treatment with continuous positive airway pressure did not affect oxidative stress markers in saliva (morning before treatment vs. morning with CPAP). *p < 0.05, **p < 0.01, and ***p < 0.001. TBARS thiobarbituric acid reacting substance, AGEs advanced glycation end-products, AOPP advanced oxidation protein products
CPAP had no effect on TBARS and AGEs. The concentrations of these markers after one night with CPAP were comparable to the concentrations after a night without CPAP (Fig. 2a–c; morning without treatment vs. morning with CPAP, p = 0.96 for TBARS, p = 0.73 for AGES). CPAP partially decreased salivary AOPP by 44 %, without reaching statistical significance (Fig. 2c, morning without treatment vs. morning with CPAP, p = 0.35). The antioxidant status assessed by TAC and FRAS showed no dynamic changes during the study, except FRAS during the night without treatment (Fig. 3b, F = 4.386, evening vs. morning without treatment, p < 0.05).
Total antioxidant capacity in saliva (a) showed no significant changes during the diagnostic and treatment night, and ferric-reducing ability of saliva (b) showed a higher ability of saliva to reduce ferric ions in the morning when compared to the evening, however only during the diagnostic night (evening before treatment vs. morning before treatment). **p < 0.01, TAC total antioxidant capacity, FRAS ferric reducing ability of saliva
The difference between the increase of individual oxidative stress markers and antioxidant status markers during the control evening to morning and evening to morning with CPAP is shown in Fig. 4a–e. None of the parameters showed significant differences between the control and treatment night.
The subgroup analysis performed next did not reveal any new information. When the patients were divided by severity into subgroups of mild (AHI 5–15), moderate (AHI 15.1–30), and severe (AHI > 30.1) OSAS, no significant changes were found in oxidative stress markers and antioxidant status in the morning with CPAP when compared to the morning without treatment (data not shown). Nevertheless, the subgroup analysis copied the trends of the whole group analysis.
The Pearson correlation test showed no significant association between salivary markers of oxidative stress and antioxidant status with AHI (Fig. 5a–e, r = 0.01231, p = 0.94 for TBARS; r = −0.01315, p = 0.94 for AGEs; r = 0.02905, p = 0.91 for AOPP; r = 0.0819, p = 0.62 for TAC; and r = 0.05329, p = 0.74 for FRAS).
Pearson correlation test for markers of oxidative stress and apnea:hypopnea index (AHI): a TBARS for lipid peroxidation; b AGEs for carbonyl stress; c AOPP for protein damage; d TAC for water and fat soluble antioxidants; e FRAS for fat soluble antioxidants. No significant correlation was found between AHI and individual markers
Discussion
Several previously published studies showed that CPAP decreases oxidative stress markers in the plasma of OSAS patients [24, 25]. Our previous study showed that this effect might also be seen in saliva, at least after 1 and 6 months of CPAP treatment. Acute effects on oxidative stress in serum or plasma were found even after one night with CPAP [16, 22]. However, not all results are that clear [18]. The present study was designed to prove whether salivary markers of oxidative stress are affected by a single night with CPAP. The results of the present study showed no significant effect of CPAP treatment on measured markers of oxidative stress after one night. In line with our previous results, antioxidant status in saliva seems not to be affected by CPAP, either by one night, or by 6 months of treatment. The only significant result was the FRAS increase from evening to morning during the diagnostic night. We cannot fully explain this, particularly as the next collections showed no change. However, one possible explanation could be the fact that patients came to the hospital for planned admission in the afternoon, during the rush hour, after their regular work ended, so we could not control what they ate or did before. Also, admission to hospital represents a stressful stimuli, which could also lead to decreased baseline evening FRAS levels [26] when compared to the rest of the hospital stay in a controlled environment.
Our previous results in a long-term study found a significant change of AGEs in saliva after 1 month of CPAP treatment [21]. In this study, this result was not found. On the contrary, it was AOPP that seemed to be most influenced after one night of CPAP treatment, although not reaching any level of significance (p = 0.3107, Fig. 4c). Currently, we cannot determine why there was such a change. However, the main difference between the previous long-term and the current short-term study was in the environmental settings. In the long-term study, the saliva was collected at home, whereas in the current study patients were in a controlled hospital environment with a standard diet. There are some external factors that might influence oxidative stress in saliva, such as physical activity prior to sampling [27], or diet composition, vitamin supplementation [28], etc. Since it is not possible to exclude all external parameters influencing oxidative stress at home, this might contribute to the differences observed. Indeed, the question of the reliability of salivary markers of oxidative stress measurement should also be addressed. Generally, all the markers measured by our group are considered as nonspecific; therefore, they might be changed in various diseases, starting with oral diseases such as periodontitis [29], oral premalignancies [30, 31], dental caries [32], through to systemic diseases such as diabetes mellitus [33]. Principally, there are two potential ways to cope with this non-specificity—either finding a new, and more specific marker for a particular disease, or trying to use a set of markers, instead of one. Moreover, one of our previous studies showed high intra- and inter-individual variability pointing out that identification of variability sources should allow the use of oxidative stress markers for individual treatment or disease monitoring. In current study design settings, these sources of variability have been avoided as much as we know. The time of sampling was the same in all subjects [34]: before tooth-brushing [35], either before a meal or at least 60 min after a meal [23]. The whole saliva was collected, since salivary collection systems may influence the concentration of oxidative stress markers [19]. These are all known factors contributing to individual variability, as well as smoking (smokers excluded), or acutely ill patients who were not included in the study. To our knowledge, this is the second study regarding OSAS and oxidative stress in saliva, and the first to assess such short-term dynamics of salivary markers of oxidative stress, at least in patients with CPAP. It is known that oxidative stress plays a role in OSAS and its complications, when the markers are measured in plasma [36]. But it is unknown what happens with redox status in saliva during OSAS. The saliva is of special interest, since its collection is easy and noninvasive compared to blood taking. It is especially useful for longer monitoring of disease progress or treatment effects. Saliva collection can be easily performed even at home, and in patients that are less collaborative, such are children or elderly. Therefore further research regarding its use should be conducted.
In comparison to studies measuring oxidative stress markers in plasma, our study was not only different due to the use of saliva. Alzoghaibi and Bahammam [22] found a decrease in plasma malondialdehyde after one night of CPAP. The study group consisted solely of severe OSAS patients (AHI >30). In our patients, the mean AHI was 30.8, ranging from 5 to 66.6 thus including mild, moderate, and severe groups of OSAS patients [37]. Although the origin of oxidative stress markers in saliva is still unknown, their association to some pathologies was proved for several diseases such as periodontitis [38] and diabetes mellitus [33]. Whether salivary markers of oxidative stress might be useful in sleep apnea and its treatment remains to be seen, particularly since the correlation analysis revealed no association between markers of oxidative stress or antioxidant status in saliva and the severity of OSAS quantified as AHI. This is in line with some published studies [12, 18], but in contrast with others [39, 40]. In all these studies, oxidative stress was assessed in plasma.
There are several limitations of this study. Oxidative stress was assessed only in saliva. It is thus not possible to compare saliva and plasma concentrations. Collecting saliva four times within 2 days is much easier than blood collection. The higher compliance and the number of involved patients were considered more important than the information gathered from parallel plasma measurements. In one of our previous studies on healthy volunteers, no correlation between salivary and plasma levels of oxidative stress markers was found [23]. Another limitation was the broad spectrum of patients with variable severity of OSAS. Focusing on the severe OSAS might bring positive results in the future. The present study group was too small for reliable subgroup analyses and, in addition, no correlation of oxidative stress with AHI was found. Finally, only one night with CPAP was determined. Extending the saliva collection to several more days after leaving the hospital could be more informative. This was unfortunately not possible with the current patient group. These limitations could be addressed in following studies.
In conclusion, no significant changes of salivary markers of oxidative stress markers after one night with CPAP treatment were observed. As CPAP treatment decreases oxidative stress markers in saliva after 1 and 6 months, it is currently unclear how fast these changes are. Further follow-up studies are needed to find appropriate sampling frequency before saliva can be used as a potential tool for the monitoring of the treatment effects in OSAS.
References
Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM (2013) Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulm Med 13:10
Lavie P, Herer P, Hoffstein V (2000) Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 320:479–482
Parish JM, Adam T, Facchiano L (2007) Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med 3:467–472
Mehra R, Redline S (2008) Sleep apnea: a proinflammatory disorder that coaggregates with obesity. J Allergy Clin Immunol 121:1096–1102
Gharibeh T, Mehra R (2010) Obstructive sleep apnea syndrome: natural history, diagnosis, and emerging treatment options. Nat Sci Sleep 2:233–255
Jurkovicova I, Celec P, Mucska I, Hodosy J (2003) On the origin of cardiovascular complications of sleep apnea syndrome by the means of molecular interactions. Bratisl Lek Listy 104:167–173
Wang Y, Zhang SX, Gozal D (2010) Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol 174:307–316
Lavie L (2003) Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7:35–51
Zhang J, Veasey S (2012) Making sense of oxidative stress in obstructive sleep apnea: mediator or distracter? Front Neurol 3:179
Lavie L, Vishnevsky A, Lavie P (2004) Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27:123–128
Mancuso M, Bonanni E, LoGerfo A, Orsucci D, Maestri M, Chico L, DiCoscio E, Fabbrini M, Siciliano G, Murri L (2012) Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep Med 13:632–636
Ntalapascha M, Makris D, Kyparos A, Tsilioni I, Kostikas K, Gourgoulianis K, Kouretas D, Zakynthinos E (2013) Oxidative stress in patients with obstructive sleep apnea syndrome. Sleep Breath 17:549–555
Kang IG, JungKim ST JH, Kim ST (2013) The effect of obstructive sleep apnea on DNA damage and oxidative stress. Clin Exp Otorhinolaryngol 6:68–72
Baessler A, Nadeem R, Harvey M, Madbouly E, Younus A, Sajid H, Naseem J, Asif ABawaadam H (2013) Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers—a meta-analysis. J Inflamm (Lond) 10:13
Minoguchi K, Yokoe T, Tanaka A, Ohta S, Hirano T, Yoshino G, O'Donnell CP, Adachi M (2006) Association between lipid peroxidation and inflammation in obstructive sleep apnoea. Eur Respir J 28:378–385
Christou K, Kostikas K, Pastaka C, Tanou K, Antoniadou I, Gourgoulianis KI (2009) Nasal continuous positive airway pressure treatment reduces systemic oxidative stress in patients with severe obstructive sleep apnea syndrome. Sleep Med 10:87–94
Singh TD, Patial K, Vijayan VK, Ravi K (2009) Oxidative stress and obstructive sleep apnoea syndrome. Indian J Chest Dis Allied Sci 51:217–224
Svatikova A, Wolk R, Lerman LO, Juncos LA, Greene EL, McConnell JP, Somers VK (2005) Oxidative stress in obstructive sleep apnoea. Eur Heart J 26:2435–2439
Kamodyova N, Celec P (2011) Salivary markers of oxidative stress and Salivette collection systems. Clin Chem Lab Med 49:1887–1890
Celecova V, Kamodyova N, Tothova L, Kudela M, Celec P (2013) Salivary markers of oxidative stress are related to age and oral health in adult non-smokers. J Oral Pathol Med 42:263–266
Celec P, Hodosy J, Behuliak M, Palffy R, Gardlik R, Halcak L, Mucska I (2012) Oxidative and carbonyl stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath 16:393–398
Alzoghaibi MA, Bahammam AS (2012) The effect of one night of continuous positive airway pressure therapy on oxidative stress and antioxidant defense in hypertensive patients with severe obstructive sleep apnea. Sleep Breath 16:499–504
Tothova L, Ostatnikova D, Sebekova K, Celec P, Hodosy J (2013) Sex differences of oxidative stress markers in young healthy subjects are marker-specific in plasma but not in saliva. Ann Hum Biol 40:175–180
Del Ben M, Fabiani M, Loffredo L, Polimeni L, Carnevale R, Baratta F, Brunori M, Albanese F, Augelletti T, Violi F, Angelico F (2012) Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm Med 12:36
Karamanli H, Ozol D, Ugur KS, Yildirim Z, Armutcu F, Bozkurt BYigitoglu R (2012) Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath. doi: 10.1007/s11325-012-0761-8c
Poljsak B (2011) Strategies for reducing or preventing the generation of oxidative stress. Oxid Med Cell Longev 2011:194586. doi:10.1155/2011/194586
Sasaki S, Matsuura T, Takahashi R, Iwasa T, Watanabe H, Shirai K, Nakamoto H, Goto S, Akita S, Kobayashi Y (2013) Effects of regular exercise on neutrophil functions, oxidative stress parameters and antibody responses against 4-hydroxy-2-nonenal adducts in middle aged humans. Exerc Immunol Rev 19:60–71
Bjelakovic G, Nikolova D, Gluud C (2013) Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin a, and vitamin e singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One 8:e74558
Pollanen MT, Paino A, AIhalin R (2013) Environmental stimuli shape biofilm formation and the virulence of periodontal pathogens. Int J Mol Sci 14:17221–17237
Shpitzer T, Hamzany Y, Bahar G, Feinmesser R, Savulescu D, Borovoi I, Gavish M, Nagler RM (2009) Salivary analysis of oral cancer biomarkers. Br J Cancer 101:1194–1198
Vlkova B, Stanko P, Minarik G, Tothova L, Szemes T, Banasova L, Novotnakova D, Hodosy J, Celec P (2012) Salivary markers of oxidative stress in patients with oral premalignant lesions. Arch Oral Biol 57:1651–1656
Hegde AM, Rai K, Padmanabhan V (2009) Total antioxidant capacity of saliva and its relation with early childhood caries and rampant caries. J Clin Pediatr Dent 33:231–234
Su H, Velly AM, Salah MH, Benarroch M, Trifiro M, Schipper HM, Gornitsky M (2012) Altered redox homeostasis in human diabetes saliva. J Oral Pathol Med 41:235–241
Behuliak M, Palffy R, Gardlik R, Hodosy J, Halcak L, Celec P (2009) Variability of thiobarbituric acid reacting substances in saliva. Dis Markers 26:49–53
Kamodyova N, Tothova L, Celec P (2013) Salivary markers of oxidative stress and antioxidant status: influence of external factors. Dis Markers 34:313–321
Quintero M, Gonzalez-Martin MD, Vega-Agapito V, Gonzalez C, Obeso A, Farre R, Agapito T, Yubero S (2013) The effects of intermittent hypoxia on redox status, NFkappaB activation, and plasma lipid levels are dependent on the lowest oxygen saturation. Free Radic Biol Med 65:1143–1154
Force TRoaAAoSMT (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22:667–689
Pendyala G, Thomas B, Joshi SR (2013) Evaluation of total antioxidant capacity of saliva in type 2 diabetic patients with and without periodontal disease: a case–control study. N Am J Med Sci 5:51–57
Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ (2002) Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest 122:1162–1167
Franco CM, Lima AM, Ataide L Jr, Lins OG, Castro CM, Bezerra AA, de Oliveira MF, Oliveira JR (2012) Obstructive sleep apnea severity correlates with cellular and plasma oxidative stress parameters and affective symptoms. J Mol Neurosci 47:300–310
Acknowledgments
This study was funded by the Slovak Ministry of Education, Science, Research and Sport—grant number VEGA 1/0674/12. We would like to thank the anonymous reviewers for their constructive approach.
Conflict of interest
No conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tóthová, L., Hodosy, J., Mucska, I. et al. Salivary markers of oxidative stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath 18, 563–570 (2014). https://doi.org/10.1007/s11325-013-0919-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-013-0919-z