Abstract
Purpose
Obstructive sleep apnea (OSA) is associated with oxidative stress that is involved in the pathogenesis of cardiovascular and metabolic complications. The concentrations of salivary markers of oxidative stress in patients with OSA increase considerably during the night. The dynamics is not affected by continuous positive airway pressure (CPAP) in mild to moderate OSA. The aim of this study was to analyze the short-term effects of CPAP on salivary oxidative stress markers in patients with severe OSA.
Methods
Salivary samples were collected from 24 patients with apnea-hypopnea index higher than 30 during the first (diagnostic) night, who were treated by CPAP during the second (therapeutic) night.
Results
The salivary markers of oxidative stress (TBARS, AGEs, and AOPP) were higher in the morning after the diagnostic night when compared to the evening concentrations (p < 0.01 for TBARS and p < 0.05 for AGEs and AOPP). Treatment by CPAP significantly decreased the morning concentrations of TBARS, AOPP (p < 0.01 for both), and AGEs (p < 0.05). Also, TBARS and AGEs positively correlated with apnea-hypopnea index (r = 0.48 and 0.49, respectively; p < 0.05). Antioxidant statuss was not affected.
Conclusion
Severe OSA is associated with increased levels of saliva markers for lipid peroxidation, protein oxidative damage, and carbonyl damage. Even short-term CPAP partially prevents oxidative and carbonyl stress during the night and this can be monitored non-invasively using saliva.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular disease and mortality [1]. Oxidative stress has been hypothesized as one of the underlying pathomechanisms that might explain the link between OSA and hypertension, endothelial dysfunction, and atherosclerosis leading to ischemic heart disease [2]. The true molecular mechanisms leading to oxidative stress in OSA are unclear, but intermittent hypoxia and reoxygenation likely play a role in inducing oxidative damage, which disrupts the physiological role of reactive oxygen species in homeostasis.
Oxidative stress is defined as an imbalance between the production of free radicals and the antioxidant status of cells and tissues [3]. This imbalance can be caused by the overproduction of free radicals or by insufficient antioxidant activity. Free radicals interact with the cellular components and large molecules such as proteins, lipids, and nucleic acids, causing damage and alterations to their functions. In OSA, there is higher lipid peroxidation in plasma when compared to control group of patients [4]. Higher protein carbonyls and decreased antioxidant status were also found in plasma of OSA patients [5]. Numerous markers of oxidative stress with different inter- or intra-individual variability are used to quantify lipid peroxidation, protein oxidation, and total antioxidant capacity. We have established several salivary markers in our laboratory. Our previous studies analyzed thiobarbituric acid reacting substances (TBARS), advanced oxidation protein products (AOPP), advanced glycation end products (AGEs), and total antioxidant capacity (TAC) in saliva of patients with periodontitis [6], oral premalignant lesions [7], or multiple sclerosis [8]. These markers were measured in OSA studies previously [9]. A variety of measured markers would be more appropriate, since the adequate markers with good predictive value are still not available. In our study, a widely used indicator of lipid peroxidation—TBARS—was used. Protein damage was measured using AOPP, and damage to carbonyl substances was assessed via measurement of AGEs. Lastly, the ability of an organism to prevent further damage by free radicals was expressed as TAC. The markers of oxidative stress in saliva might reflect the systemic level of oxidative stress but might be affected also by local intraoral processes [10].
Saliva is a promising diagnostic fluid with several important advantages over blood [11]. The sampling of saliva is easy and non-invasive and can be repeated in patients of any age group. The diagnostic use of saliva is based on its biochemical components including nucleic acids, proteins, and small metabolites. However, these components could be affected by several host-derived factors as well as by oral bacteria [12]. Several biomarkers have already been analyzed in the saliva of patients with OSA including the stress hormone cortisol [13], sex steroid hormones [14], and proinflammatory cytokines [15]. In one study, the authors searched in saliva for biomarkers of cardiovascular complications using mass spectrometry [16].
Continuous positive airway pressure (CPAP) is the primary treatment for patients with OSA [17]. We have previously shown that from a long-term perspective CPAP decreases oxidative stress markers in plasma and partially also in saliva [9]. On the contrary, salivary markers of oxidative stress were not affected by one night with CPAP in patients with mostly mild or moderate OSA [18]. Whether CPAP might have rapid effects on oxidative stress in patients with severe forms of OSA is currently unknown.
The aim of our study was to analyze the short-term effects of CPAP on oxidative stress markers in saliva.
Methods
Ethical approval
Written informed consent was obtained from all participating patients. The study conformed to the standards set by the Declaration of Helsinki, except for registration in a database. All the procedures were approved by the Ethics committee of the University Hospital Bratislava, Comenius University, Bratislava, Slovakia.
Patients
A convenience sample of patients referred by a pneumologist to a sleep center for suspicion of OSA was enrolled in the study. The severity of OSA was confirmed during the first night by polysomnography (Alice 4 or Alice 5, Respironics, Inc., Murrysville, PA USA) in the sleep center. The apnea-hypopnea index (AHI) was calculated as the number of apneas and hypopneas per total sleep time. Only patients with an AHI of more than 30 were included in the study. The study was performed in the sleep laboratory of the University Hospital, Bratislava, Slovakia. The design of the observation was similar to our previous study [18]. Briefly, patients spent two nights in the sleep laboratory with four sampling times. Whole unstimulated saliva was collected in the evening and morning for two consecutive days. The first night was without CPAP (no CPAP), and the second night was with CPAP. Prior to a visit to the sleep laboratory, none of the patients had experience with CPAP.
Biochemical analyses
For the assessment of markers of oxidative stress, saliva samples were used. Saliva samples were obtained from the patients by spitting into sterile 15 ml tubes in the evening (8 p.m.) and in the morning (8 a.m.) for the two consecutive days spent in the sleep laboratory. The patients were instructed to spit 3 ml of liquid saliva (no bubbles or foam). Sampling was performed 1 h after tooth-brushing, and patients were instructed to avoid eating for at least 1 h before sampling. Four patients had samples with visible blood contamination and were excluded from the analysis. Overall, 5 females and 19 males with severe obstructive sleep apnea were analyzed. Samples were then centrifuged at 4000g for 10 min at 4 °C. Afterwards, the supernatants were frozen at − 80 °C until biochemical analysis was performed.
The multimode spectrofluorometer Saphire II Tecan (Grödig, Austria) was used for all measurements. To examine lipid peroxidation, thiobarbituric acid-reacting substances were assessed according to the protocol of Behuliak et al. [19]. First, thiobarbituric and acetic acid was added to saliva samples. Colored complexes formed after incubation at 94 °C for 45 min. Afterwards, the samples were cooled to 4 °C, and n-butanol was added to enable phase separation after vigorous shaking and centrifugation at 2000g for 10 min. The upper phase was then carefully removed and measured at 553 nm emission and 515 nm excitation wavelengths. Using 1,1,3,3-tetramethoxypropane, the calibration curve for the quantification of TBARS contents was constructed.
For the analysis of protein oxidation, advanced oxidation protein products were measured using a spectrophotometric protocol: 200 μl of saliva samples were mixed with glacial acetic acid for 2 min. The concentration of AOPP in the samples was expressed as μmol l−1 of chloramine-T equivalents. Specific absorbance was measured at 340 nm [20].
Advanced glycation end products, as markers of carbonyl stress, were measured as follows: 20 µl of sample were diluted in 180 μl of phosphate buffer solution (pH = 7.2), and the specific absorbance of prepared reaction mixture was immediately measured at 440 nm emission and 370 nm excitation wavelengths, respectively [21]. The concentration of AGEs in the samples was expressed in arbitrary unit per μl (AU μl−1).
The total antioxidant capacity of saliva was assessed in the presence of acetate buffer (pH = 5.8) and the initial absorbance was measured at 660 nm, as described previously [22]. Following the addition of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) to the acetate buffer with hydrogen peroxide, and incubation at room temperature for 5 min, the absorbance at 660 nm was recorded again. The difference compared to the initial values was calculated. Trolox, a water-soluble derivative of tocopherol, was used for the calibration curve.
Statistical analysis
Statistical analysis was performed using GraphPad Prism vs. 6.01 (La Jolla, CA, USA). The data were analyzed using two-way ANOVA (one factor being the time of day, and the second factor being the treatment) with subsequent Bonferroni post hoc tests. The differences between the variables describing the sleep characteristics were analyzed using a paired t test. The Pearson correlation test was used to evaluate linear associations between quantitative variables. A p value < 0.05 was considered statistically significant. Data are presented as mean ± standard deviation (SD)where appropriate.
Results
The basic characteristics of patients as well as their sleep structure are shown in Table 1. Data of both genders were combined and analyzed together. There was a significant difference in AHI, as well as in minimal and average oxygen saturation during the CPAP night when compared to the night with no CPAP treatment. While AHI decreased with CPAP, oxygen saturation increased with CPAP (Table 1). Moreover, the rapid eye movement (REM) phase and N3 phase were significantly prolonged with CPAP, whereas N2 was significantly shortened. However, no differences in wakefulness and N1 stage were observed between the groups (Table 1).
Salivary TBARS concentration, as a marker of lipid peroxidation, showed a significant effect of daytime (F = 12.57; p < 0.01), treatment (F = 10.68; p < 0.01), and interaction: daytime × treatment (F = 6.39; p < 0.05). The morning values of TBARS were significantly lower after CPAP treatment with mean concentration of 0.39 ± 0.27 μmol l−1 when compared to the morning without CPAP treatment with mean concentration of 0.68 ± 0.6 μmol l−1 (Fig. 1a; p < 0.02).
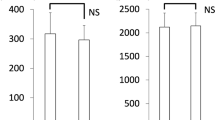
Similar to TBARS, salivary AOPP and AGEs showed a significant effect of daytime (F = 11.45; p < 0.01 for AOPP and F = 16.81; p < 0.001 for AGEs) and treatment (F = 7.29; p < 0.05 for AOPP; F = 4.65; p < 0.05 for AGEs). There was no significant interaction: daytime × treatment in AOPP or AGEs. The concentrations of AOPP and AGEs were significantly lower in the morning after CPAP treatment with the mean concentrations 82.36 ± 49.11 μmol l−1 for AOPP and 8.46 ± 5.51 AU μl−1 for AGEs when compared to the morning without CPAP treatment with the mean concentrations of 110.9 ± 61.42 μmol l−1 for AOPP and 11.64 ± 6.99 AU μl−1 for AGEs (Fig. 1b, c, respectively; p < 0.01 for AOPP and p < 0.05 for AGEs). The antioxidant capacity of saliva measured as TAC was affected neither by daytime (F = 0.03; Fig. 1d) nor treatment (F = 2.60; Fig. 1d).
Of all oxidative stress markers measured, only TBARS and AGEs morning values without CPAP significantly positively correlated with AHI (Fig. 2a, b; r = 0.48 and 0.49, respectively; p < 0.05 for both). In addition, AHI negatively correlated with the CPAP-no CPAP morning difference in salivary TBARS and AGEs (Fig. 2c, d; r = − 0.53 and − 0.56 respectively; p < 0.01 for both).
Correlation of a salivary TBARS and b salivary AGEs versus apnea-hypopnea index (AHI) and the correlation of c salivary TBARS difference and d salivary AGEs difference versus AHI. Salivary differences were calculated as differences between morning values after CPAP and morning values with no CPAP. r correlation coefficient
Discussion
CPAP is the gold standard in the treatment of OSA [23]. In our study, even the first night with CPAP significantly reduced AHI, RDI, and ODI variables. Minimal and average oxygen saturations were improved with an increase in REM phase.
Our main result shows that in patients with a severe form of OSA, lipid peroxidation, as expressed by measurement of TBARS, the CPAP mitigates evening to morning rise of TBARS. This is consistent with prior studies that show increased reactive oxygen species in OSA. The observed decrease in TBARS with CPAP has been documented long ago [4]. Even the short-term effects of one night with CPAP on lipid peroxidation have already been described [24]. However, most of the published data rely on analysis of serum or plasma. In the present study, we have measured all of the markers in saliva. The non-invasive sampling of saliva, the possibility to repeat the sampling several times even within a short time frame, and especially the possibility to sample saliva at home without the need for a physician or a nurse, make it a very interesting diagnostic fluid in the monitoring of OSA or treatment effects [25]. Of course, such applications are only possible after the development of dedicated point of care tests and require additional testing in clinical studies. In this study, the change in TBARS was not accompanied by an increase in TAC. The induced changes can be possibly attributed to a lower production of reactive oxygen species rather than to an increased antioxidant activity. However, TBARS may be affected by other mechanisms. It is possible that CPAP reduces the concentration of free fatty acids in plasma and, thus, there is less substrate for lipid peroxidation [26]. In our study, CPAP reduced the number of hypoxic phases—AHI decreased by 80%. Lower oxidative stress could, thus, be expected.
AOPP and AGEs are well established markers of oxidative and carbonyl stress that were found to be higher in serum of OSA patients [5]. In our study, these markers displayed similar daily dynamics to TBARS. The morning concentrations of TBARS, AOPP, and AGEs were higher when compared to the evening values. Although it is not possible to confirm diurnal variation in salivary makers of oxidative stress from these results, other studies have shown variations of salivary markers of oxidative and carbonyl stress during the day. These include our previous study showing that TBARS concentrations were highest in the morning [27]. Salivary AOPP also displayed diurnal variation with the highest concentration in the early afternoon [28]. On the other hand, TAC showed no daytime variability similarly to our recent study [29]. The found daytime dynamics in AOPP and AGEs concentrations seem to be only partially related to the CPAP treatment. There was a significant decrease in the morning rise of both AGEs and AOPP after CPAP when compared to morning values without CPAP, but the interaction between the daytime and treatment was not significant, suggesting that CPAP did not change the time-related decrease from evening to morning.
The present study is a follow-up study to our previously published negative findings [18]. In both studies, the morning concentrations of salivary markers of oxidative stress were higher than in the evening. The difference between these studies lies in the observed effects of CPAP. In unselected patients with OSA consisting mostly of patients with a mild or moderate form of OSA, CPAP did not induce any significant changes in salivary markers of oxidative stress. In the previous publication, we postulated that the results might be different in patients with severe OSA. The severity of OSA as quantified by AHI is clearly positively associated with oxidative stress and related oxidative damage [30]. In patients with severe OSA, we also found positive correlations of salivary TBARS and AGEs with the severity of OSA. The CPAP-noCPAP difference between two mornings showed a negative correlation with the severity of OSA suggesting that patients with a severe OSA benefit more from CPAP than patients with mild to moderate forms.
The present study has several important limitations. The observed clinical parameters still remain relatively high despite CPAP treatment. This might be explained by the fact that it was the first experience of the patients with CPAP. Further improvements after adjusting CPAP parameters might have occurred later. Oxidative stress markers were only measured in saliva and cannot be correlated with likely changes in plasma or serum as blood samples were not collected in this study. The number of subjects involved is low, but the study focused on severe cases, and patients with mild or moderate OSA were excluded. Salivary markers of oxidative stress can be affected by other pathologies including periodontitis, multiple sclerosis, and oral premalignant lesions [7, 8, 10]. These pathologies were not assessed in our study group. A correction factor usable for the normalization of salivary concentrations similarly to creatinine in urine would be needed. The CPAP treatment might have local effects on salivary flow and, thus, on the concentration of biomarkers that do not reflect systemic oxidative stress. The design of the study did not allow us to randomize the order of nights without and with CPAP. The patients first underwent the diagnostic night and then the treatment night, which might also be seen as a limitation.
In conclusion, our study shows that in severe OSA, one night with CPAP is enough to reduce the morning concentrations of salivary TBARS. Oxidative stress seems to be accepted as a mediator of the negative consequences of OSA. However, whether the antioxidant effects of CPAP are responsible for the metabolic changes induced by the treatment remains unclear. Whether antioxidants can further improve the effects of CPAP is currently unknown and should be the focus of follow-up studies in both, experimental animals and patients.
References
Lavie P, Lavie L (2008) Cardiovascular morbidity and mortality in obstructive sleep apnea. Curr Pharm Des 14:3466–3473
Jurkovicova I, Celec P, Mucska I, Hodosy J (2003) On the origin of cardiovascular complications of sleep apnea syndrome by the means of molecular interactions. Bratisl Lek Listy 104:167–173
Sies H, Berndt C, Jones DP (2017) Oxidative stress. Annu Rev Biochem 86:715–748
Lavie L, Vishnevsky A, Lavie P (2004) Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27:123–128
Mancuso M, Bonanni E, LoGerfo A, Orsucci D, Maestri M, Chico L, DiCoscio E, Fabbrini M, Siciliano G, Murri L (2012) Oxidative stress biomarkers in patients with untreated obstructive sleep apnea syndrome. Sleep Med 13:632–636
Banasova L, Kamodyova N, Jansakova K, Tothova L, Stanko P, Turna J, Celec P (2015) Salivary DNA and markers of oxidative stress in patients with chronic periodontitis. Clin Oral Investig 19:201–207
Vlkova B, Stanko P, Minarik G, Tothova L, Szemes T, Banasova L, Novotnakova D, Hodosy J, Celec P (2012) Salivary markers of oxidative stress in patients with oral premalignant lesions. Arch Oral Biol 57:1651–1656
Karlik M, Valkovic P, Hancinova V, Krizova L, Tothova L, Celec P (2015) Markers of oxidative stress in plasma and saliva in patients with multiple sclerosis. Clin Biochem 48:24–28
Celec P, Hodosy J, Behuliak M, Palffy R, Gardlik R, Halcak L, Mucska I (2012) Oxidative and carbonyl stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath 16:393–398
Tothova L, Kamodyova N, Cervenka T, Celec P (2015) Salivary markers of oxidative stress in oral diseases. Front Cell Infect Microbiol 5:73
Kaufman E, Lamster IB (2002) The diagnostic applications of saliva--a review. Crit Rev Oral Biol Med 13:197–212
Zhang Y, Sun J, Lin CC, Abemayor E, Wang MB, Wong DT (2016) The emerging landscape of salivary diagnostics. Periodontol 70:38–52
Raff H, Ettema SL, Eastwood DC, Woodson BT (2011) Salivary cortisol in obstructive sleep apnea: the effect of CPAP. Endocrine 40:137–139
Ghiciuc CM, Dima-Cozma LC, Bercea RM, Lupusoru CE, Mihaescu T, Cozma S, Patacchioli FR (2016) Imbalance in the diurnal salivary testosterone/cortisol ratio in men with severe obstructive sleep apnea: an observational study. Braz J Otorhinolaryngol 82:529–535
Nizam N, Basoglu OK, Tasbakan MS, Nalbantsoy A, Buduneli N (2014) Salivary cytokines and the association between obstructive sleep apnea syndrome and periodontal disease. J Periodontol 85:e251–e258
Zheng H, Li R, Zhang J, Zhou S, Ma Q, Zhou Y, Chen F, Lin J (2014) Salivary biomarkers indicate obstructive sleep apnea patients with cardiovascular diseases. Sci Rep 4:7046
Levy P, Kohler M, McNicholas WT, Barbe F, McEvoy RD, Somers VK, Lavie L, Pepin JL (2015) Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 1:15015
Tothova L, Hodosy J, Mucska I, Celec P (2014) Salivary markers of oxidative stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath 18:563–570
Behuliak M, Palffy R, Gardlik R, Hodosy J, Halcak L, Celec P (2009) Variability of thiobarbituric acid reacting substances in saliva. Dis Markers 26:49–53
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49:1304–1313
Munch G, Keis R, Wessels A, Riederer P, Bahner U, Heidland A, Niwa T, Lemke HD, Schinzel R (1997) Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur J Clin Chem Clin Biochem 35:669–677
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37:277–285
Cao MT, Sternbach JM, Guilleminault C (2017) Continuous positive airway pressure therapy in obstuctive sleep apnea: benefits and alternatives. Expert Rev Respir Med 11:259–272
Kanimozhi S, Balaji C, Saravanan A, Ravi K (2015) Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diagn Res 9:CC07–CC10
Malon RS, Sadir S (2014) Saliva-based biosensors: noninvasive monitoring tool for clinical diagnostics. Biomed Res Int 2014:962903
Chopra S, Rathore A, Younas H, Pham LV, Gu C, Beselman A, Kim IY, Wolfe RR, Perin J, Polotsky VY, Jun JC (2017) Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab 102:3172–3181
Kamodyova N, Tothova L, Celec P (2013) Salivary markers of oxidative stress and antioxidant status: influence of external factors. Dis Markers 34:313–321
Su H, Gornitsky M, Geng G, Velly AM, Chertkow H, Schipper HM (2008) Diurnal variations in salivary protein carbonyl levels in normal and cognitively impaired human subjects. Age (Dordr) 30:1–9
Hodosy J, Celec P (2005) Daytime of sampling, tooth-brushing and ascorbic acid influence salivary thiobarbituric acid reacting substances--a potential clinical marker of gingival status. Dis Markers 21:203–207
Eisele HJ, Markart P, Schulz R (2015) Obstructive sleep apnea, oxidative stress, and cardiovascular disease: evidence from human studies. Oxidative Med Cell Longev 2015:608438
Funding
This contribution is the result of the project implementation, “Development of the Centre of Excellence for Exploitation of Informational Biomacromolecules for Improvement of Quality of Life” project supported by the Research and Development Operational Programe funded by the ERDF (Contract No. ITMS 26240120027).
Author information
Authors and Affiliations
Contributions
LT - first draft of the manuscript, responsible for the material and method part of the manuscript, interpretation of the oxidative stress markers data and designing the biochemical part of the study.
PC - design of the study, contribution mainly to the result part and discussion of the manuscript.
IM - design of the study and salivary collections, responsible for patients evaluation and polysomnography, informing patients, general conception of the whole study.
JH - ethical committee approval, responsible for introduction part and overall finalizing of the manuscript, data evaluation, and figure plotting.
All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained for each patient. The study was approved by the local ethics committee (Ethical committee of the University Hospital Bratislava, Slovakia).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tóthová, Ľ., Celec, P., Mucska, I. et al. Short-term effects of continuous positive airway pressure on oxidative stress in severe sleep apnea. Sleep Breath 23, 857–863 (2019). https://doi.org/10.1007/s11325-018-01777-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-01777-0