Abstract
Purpose
To evaluate the intensity of nocturnal hypoxemia associated with sleepiness in Peruvian men with a diagnosis of obstructive sleep apnea (OSA).
Methods
We carried out a secondary data analysis based on a study which includes patients with OSA who were seen in a private hospital in Lima, Peru from 2006 to 2012. We included male adults who had polysomnographic recordings and who answered the Epworth sleepiness scale (ESE). The intensity of nocturnal hypoxemia (oxygen saturation ≤90 %) was classified in four new categories: 0, <1, 1 to 10 and >10 % total sleep time with nocturnal hypoxemia (NH). When the ESE score was higher than 10, we used the definitions presence or absence of sleepiness. We used Poisson regression models with robust variance to estimate crude and adjusted prevalence ratios (PR) for association between sleepiness and NH.
Results
518 male patients with OSA were evaluated. Four hundred and fifty-two (87 %) patients had NH and 262 (51 %) had sleepiness. Of the 142 (27.4 %) patients who had >10 % total sleep time with NH, 98 (69.0 %) showed sleepiness and had a greater probability of sleepiness prevalence, with a crude PR of 1.82 (95 % CI 1.31–2.53). This association persisted in the multivariate models.
Conclusions
We found an association between NH and sleepiness. Only patients with the major intensity of NH (over 10 % of the total sleep time) had a greater probability of sleepiness. This suggests that sleepiness probably occurs after a chronic process and after overwhelming compensatory mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is the result of episodic upper airway obstruction occurring during sleep. Obstruction can be total (apnea) or partial (hypopnea) and can occur several times during sleep [1]. Apneas or hypopneas last at least 10 s until the patient has an arousal in order to have an effective breath [1, 2]. The OSA prevalence in the general population with sleepiness is 1 to 6 % [2, 3] and OSA prevalence without sleepiness is 15 to 25 % [1, 4, 5]. Sleepiness is related to a decrease of intellectual activity and a greater risk of accidents, such as car accidents, which is six times more frequent in patients with OSA compared to the general population [6].
The causes of sleepiness are multifactorial. For this reason, many studies in patients with OSA have included the link between the numbers of obstructive events (apneas/hypopneas)—which measures the severity of OSA—and encephalographic arousals. However, these studies failed to find a clear association [7–9] between these variables. Therefore, other variables have been sought to explain sleepiness in affected patients such as nocturnal hypoxemia (NH) [10]. Although the molecular mechanisms of NH is associated with sleepiness are not clear, this effect has been found to be mainly due to oxidative damage to wake-active neurons [11] and increased sympathetic activation [12], including inflammatory and oxidative mechanisms [13]. Nonetheless, these studies had limitations because time in hypoxia was short and this may not produce sleepiness [14]. Other studies were able to establish other possible mechanisms such as chronic injury in the cerebral cortex [15] or vascular endothelial injury [16]. Therefore, it is possible that the difficulties of finding associations are due to the inability to establish the necessary period of hypoxia to produce an apparent injury.
NH involves oxygen saturation under or equal to 90 % during sleep and can be evaluated through several characteristics, such as time in NH, low oxygen concentration, and duration of hypoxemia [17–19]. Although there are several publications that consider these variables, few clinical studies have evaluated specifically the average duration of NH associated with sleepiness [7, 20]. Additionally, some exploratory investigations have evaluated the average time in NH [9, 21] but have not used the most adequate statistical procedures such as using parametric methods when the data do not follow a normal distribution [10, 22]. Moreover, previous studies have had analytical limitations possibly because not all the characteristics of NH could be included in the model. Since previous studies had difficulty in identifying whether time to NH is associated with sleepiness, as well as the limited number of studies, we established as our hypothesis that sleepiness is associated only in the advanced stages of NH. For that reason, the objective of this study is to evaluate the association between sleepiness and intensity of NH using a new categorization (four categories) of the NH variable that allows us to explore other characteristics, using appropriate statistical procedures for different scenarios.
Methodology
Study design
We carried out a secondary data analysis based on a study aimed at describing the most frequent diagnoses of patients who voluntarily attended the private hospital Clínica Anglo Americana in Lima, Peru during 2006–2012. Preliminary studies with different objectives have been published with other subgroups of this population [23–25].
Population
Out of 1,033 patients, 621 were diagnosed with OSA through polysomnographic recordings and in accordance with the criteria of the Sleep Spanish Group (Apnea/hypopnea Index equal to or higher than 5).
We included the records of male adults who answered the Epworth scale (ESE) questionnaire and had a recording with the oxygen saturation during the sleep. Those with a history of stroke, transitory ischemic attack, myocardial infarction, surgery for the treatment of respiratory disorders during sleep or with a second diagnosis of sleep disorder were excluded from the analysis.
For the polysomnography, Easy II® (Cadwell II Inc.) and EB Neuro B.E. light equipment were employed. Two protocols were used for polysomnographic recordings: all-night (conventional) or split-night. Although differences have been identified in the selection of patients for a type of polysomnographic recording, no variations have been reported in oximeter measurements [26] or apnea/hypopnea indexes [27]. For this reason, both types of recordings were included. However, since a new NH classification was used, the variable “type-of-polysomnographic-recording” was included in the analysis to control for confounding.
Variables
Sleepiness
Subjective sleepiness was evaluated with the ESE adapted to Peruvian adults which has an alfa Cronbach of 0.79 and an intraclass correlation coefficient of 0.84 [28]. The ESE has eight questions which test the probability of falling asleep in different daily life situations. The answers are designed on a 4-point Likert scale (0 to 3). The final scores range from 0 to 24 points. The original version helps to adequately identify people with sleepiness when the ESE score is higher than 10 by using the definitions presence or absence of sleepiness [29].
Nocturnal hypoxemia
During the polysomnography, two variables exploring NH were independently recorded as follows: (1) the percentage of total sleep time with oxygen saturation ≤90 % (T90) and (2), the maximum arterial oxygen desaturation during sleep (MOD).
The T90 is a quantitative variable in a discrete scale which presents the NH values from 0 to 100 % of the total sleep time (TST). The MOD variable only identifies the lowest level of nocturnal hypoxemia. In theory, patients with 0 % in the T90 variable would not have NH; however, by exploring the MOD variable, some of these patients were found to have an MOD reaching hypoxemia levels. Therefore, by integrating T90 and MOD, the NH <1 % category of the TST is created. This integration is valid only for values <1 % of the TST in NH.
Since NH does not have a normal distribution, it is not advisable to use parametric statistics [22]. Because an NH classification could not be found, the distribution was classified based on the 25th percentile, which, in prior studies, corresponds to NH values higher than 10 % of the TST [9, 17].
Based on the above, the intensity of the NH was classified in four new categories to facilitate clinical interpretation:
-
Without NH: If it does not have NH in the T90 and MOD variables;
-
NH <1 %: If MOD indicates NH but T90 indicates 0;
-
NH 1–10 %: based on the T90 value, and
-
NH greater than 10 %: If T90 was greater than 10.
Other covariates
The covariates included in the description and analysis were: age (years), weight (kilogram), body mass index (BMI: kilogram per square metre), cervical perimeter (centimetre), classification of blood pressure by the Seventh Report of the Joint National Committee [30], history of coronary disease, diabetes mellitus, rhinitis, dyslipidemia, and type of polysomnographic recording of all-night or split-night.
Statistical analysis
The categorical variables are described in absolute frequencies and percentages, and the numerical variables are described in means with standard deviations. The association between sleepiness and numerical data was evaluated with Student’s t test. In order to consider differences as significant, the Bonferroni correction was applied to 11 comparisons; therefore, only p values lower than 0.005 were considered to be significant [31]. Furthermore, a chi-square test for trend was used to evaluate whether the percentage of patients with sleepiness increases as NH time gets longer using a p < 0.05 value as significant.
We performed Poisson regression models with robust variance to estimate crude and adjusted prevalence ratios (PR) and determined the 95 % confidence interval (CI) for the association between sleepiness and nocturnal hypoxemia. We used PR instead of the odds ratio because the proportion of patients with sleepiness was greater than 20 %. Poisson regression was used to calculate the PR due to convergence problems in log-binomial regression [32].
We used four multivariate regression models. The first model included age and BMI because in previous studies they were considered as potential confounding variables (“model created with theoretical confounding variables”). In the second model, the variables that were significant in the bivariate analysis (at a level of p < 0.005) were included (“model created with significant variables”). The third model included only the variables that improved the crude model according to the Akaike information criterion [33] (“built with the variables improving the crude model”). Finally, the fourth model only included patients with an all-night polysomnographic recording. The statistical power was set at 85 % to detect 50 % increases in the sleepiness ratio with respect to the category of reference, with an alpha level of 0.05, assuming that the proportion of patients with sleepiness would be 40 %. This analysis was calculated with PASS 11 Trial version (NCCS, LLC, UT, USA). All other analyses were performed with STATA 12.1 (STATA Corp, College Station, Texas, USA).
Ethical considerations
We used a de-identified database in order to account for confidentiality; therefore, the study did not pose any risk to patients. The primary study was approved by the Bioethical Committee of the Clínica Anglo Americana and the Comité de Ética en Investigación Biomédica of the Hospital Nacional Dos de Mayo (Cod. 034-2012-CEIB), Lima (Peru). The secondary analysis was approved by the Comité de Ética of the Universidad Peruana Cayetano Heredia (Cod. SIDISI: 60356), Lima (Peru).
Results
Five hundred and eighteen male patients with OSA were evaluated. The average age was 48.6 ± 11.7 years, mean BMI of 30.2 ± 4.5 kg/m2 and mean neck perimeter of 43.0 ± 3.5 cm (Table 1).
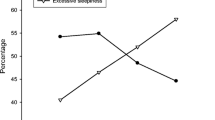
Sleepiness was present in 262 (50.6 %) patients with OSA and 452 (87.2 %) patients had NH. Among the 66 (12.7 %) patients who did not have NH, 25 (37.8 %) had sleepiness. Among those who had NH, in 142 (27.4 %) patients, it lasted 10 % more of the TST and 98 (69.0 %) showed sleepiness (Fig. 1). The percentage of patients with sleepiness increased in each NH category, thus representing a significant trend (p < 0.001).
The analysis showed a strong association between sleepiness and NH (p < 0.001). When analysing patients with sleepiness compared to patients without it, in addition to NH, the analysis showed a significant association with weight, BMI, cervical perimeter, diabetes mellitus and the type of polysomnographic recording (p < 0.05). However, with the Bonferroni correction for multiples variables, diabetes mellitus (0.013) and type of polysomnographic recording (0.006) were no longer significant (Table 2).
Patients with NH greater than 10 % of the TST were associated with a greater probability of sleepiness, with a crude PR of 1.82 (95 % CI 1.31–2.53). A rising trend in PR is observed as NH time increases in all models; however, these differences were not significant. This relationship persisted for the four multivariate models, although the PR in the NH category over 10 % of the TST decreased 8 % in the model created with theoretical confounding variables, 10 % in the model created with significant variables, 13 % in the model built with the variables improving the crude model, and increased 5 % when including only patients with an all-night polysomnographic recording (Table 3).
Discussion
In this study, we found an independent association between sleepiness and nocturnal hypoxemia. Our results are consistent with the work performed in animal models [11, 34] and the reports in the clinical population [7, 9, 10, 17]. However, two aspects deserve to be highlighted: First, sleepiness is present even in patients without NH (around 38 %). Second, there is a higher probability of sleepiness shown by patients with NH values over 10 % of total sleep time. This type of association had not been clearly identified in previous clinical studies because of the low correlation found between NH and sleepiness [17, 20, 21], in part due to the non-normal distribution of the variables; the behaviour of the hypoxia is not similar in all levels to produce sleepiness, necessitating a reclassification. Our study helped to correct this methodological error.
Studies in mice models exploring the association between long-term intermittent hypoxemia and sleepiness have identified a neural protection process against oxidative stress produced by NH [11, 35]. At the clinical level, NH is not acute in patients with OSA but is associated with the collapse of upper respiratory airways and becomes progressive as the person starts to gain weight, get older or where pharyngeal neuromuscular control is affected [36]. These studies have suggested that there might be protective mechanisms in early stages which are vulnerable to higher levels of NH or in chronic processes. This hypothesis requires further study, but our results suggest this possibility, since the analysis shows that the proportion of patients with sleepiness increases according to greater time in NH.
Using a reclassification of the variable NH helps to improve three analytical aspects: (1) the categories and results generated are easy to understand, (2) the statistical analyses are less complicated to apply and there is no need to transform the quantitative variable and (3) the integration with the maximum desaturation variable is permitted such that a group which has not been previously analysed can be included in the model. Although there are no similar studies for comparison, our results are consistent with previous publications that suggested a non-linear relationship between NH levels and the development of sleepiness [17, 18, 20, 21]; although the magnitude of the association is markedly different. For example, in the study by Saldías et al. [18], which dichotomized the time in NH as higher than or lower than 15 % of the TST, an odds ratio of 4.5 was found. However, our study finds an association of smaller magnitude because it uses PR as a conservative estimate.
In order to facilitate the comparison of our results with future studies, we have made some adjustments in several multivariate regression analyses, selected in different ways. This is important, since potential confounding variables, such as the polysomnographic record protocol, BMI, age and others are often included in multivariate regressions. Our results show that there is little variation in the PR found in different models. Even when the size of the sample is reduced to 40 %, confidence intervals continue to be similar, thus explaining its consistency. This model could be helpful for generalisation or comparison with other scenarios.
This study has limitations. We were restricted to a secondary data analysis; therefore, data were not collected for all potential confounding variables including sleep quality, depressive symptoms, or hypoxic profile during the day. Additionally, since our sample was a clinical population with special care, bias was introduced during the selection of patients with OSA and without sleepiness, who account for 75 % of patients with OSA in studies of the general population. However, it is reasonable to expect that these patients have fewer comorbidities, and therefore, lower NH levels. Therefore, if these patients had been included, our group of reference (patients without NH or sleepiness) could have had more cases so the magnitude of the association could have been greater. This suggests that our results may be conservative.
Furthermore, only men were included because women have a higher probability of sleepiness due to reasons that have not been evaluated in this study and could interact with the association between NH and sleepiness. Additionally, the proportion of women included in the primary study was 10 %, which would have made the statistical analysis difficult. Finally, the reclassification used also has disadvantages, such as not using all the information generated, and including in just one category a wide group of patients who may not share the same outcome.
Sleepiness may show to be independent from NH in the early stages, and after an induction process leading to high levels of NH, it could contribute to its presentation. This aspect should be considered in future studies that induce hypoxia and evaluate the association with sleepiness. Therefore, we recommend diligence in the study of the chronic effect of NH and the initial mechanisms of susceptibility to sleepiness, which could be independent from NH, through the use of longitudinal studies. We also suggest analysing carefully the association between sleepiness and NH or other polysomnographic parameters with similar statistical characteristics in their distributions as they may lead to inadequate interferences [22].
In conclusion, in the population studied, there is an independent association between nocturnal hypoxemia and sleepiness. Only patients with NH over 10 % of the TST have a greater probability of sleepiness. This result is consistent when an adjustment is made through potential confounding variables in different scenarios. Furthermore, sleepiness is present even in patients without NH. This suggests that sleepiness would probably occur after a chronic process and after overwhelming compensatory mechanisms.
References
Grupo Español de Sueño (2005) Definición y concepto, fisiopatología, clínica y exploración del SAHS. Arch Bronconeumol 41(Supl 4):12–29
Kapur VK (2010) Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respir Care 55(9):1155–1167
Punjabi NM (2008) The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5(2):136–143
Hernandez Garcia C (2009) Sleep apnea-hypopnea syndrome without excessive daytime sleepiness. Arch Bronconeumol 45(5):240–244
Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165(9):1217–1239
Terán-Santos J, Jimenez-Gomez A, Cordero-Guevara J, Burgos–Santander CG (1999) The association between sleep apnea and the risk of traffic accidents. N Engl J Med 340:847–851
Jimenez-Correa U, Haro R, Gonzalez-Robles RO, Velazquez-Moctezuma J (2011) How is the Epworth Sleepiness Scale related with subjective sleep quality and polysomnographic features in patients with sleep-disordered breathing? Sleep Breath 15(3):513–518
Roure N, Gomez S, Mediano O, Duran J, Pena Mde L, Capote F, Teran J, Masa JF, Alonso ML, Corral J, Sanchez-Armengod A, Martinez C, Barcelo A, Gozal D, Marin JM, Barbe F (2008) Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med 9(7):727–731
Sun Y, Ning Y, Huang L, Lei F, Li Z, Zhou G, Tang X (2012) Polysomnographic characteristics of daytime sleepiness in obstructive sleep apnea syndrome. Sleep Breath 16(2):375–381
Eiseman NA, Westover MB, Mietus JE, Thomas RJ, Bianchi MT (2012) Classification algorithms for predicting sleepiness and sleep apnea severity. J Sleep Res 21(1):101–112
Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC (2005) NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med 172(7):921–929
Heinonen IH, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos M, Oikonen V, Nuutila P, Knuuti J, Boushel R, Kalliokoski KK (2010) Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am J Physiol Regul Integr Comp Physiol 299(1):R72–R79. doi:10.1152/ajpregu.00056.2010
Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI (2009) Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep 32(4):447–470
Colt HG, Haas H, Rich GB (1991) Hypoxemia vs sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea. Chest 100(6):1542–1548
Beebe DW, Gozal D (2002) Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 11(1):1–16
Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M (2003) Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107(8):1129–1134
Lee SJ, Kang HW, Lee LH (2012) The relationship between the Epworth Sleepiness Scale and polysomnographic parameters in obstructive sleep apnea patients. Eur Arch Otorhinolaryngol 269(4):1143–1147
Saldias PF, Jorquera AJ, Diaz PO (2010) Predictive value of clinical features and nocturnal oximetry for the detection of obstructive sleep apnea syndrome. Rev Med Chil 138(8):941–950
Foster GE, Poulin MJ, Hanly PJ (2007) Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Exp Physiol 92:51–65
Bausmer U, Gouveris H, Selivanova O, Goepel B, Mann W (2010) Correlation of the Epworth Sleepiness Scale with respiratory sleep parameters in patients with sleep-related breathing disorders and upper airway pathology. Eur Arch Otorhinolaryngol 267:1645–1648
Mediano O, Barcelo A, de la Pena M, Gozal D, Agusti A, Barbe F (2007) Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J 30(1):110–113
Pértega Díaz S, Pita Fernández S (2006) Métodos no paramétricos para la comparación de dos muestras. Cad Aten Primaria 13:109–113
Rey de Castro J, Rosales-Mayor E (2011) Diferencias clínicas y polisomnográficas entre obesos y no obesos con síndrome de apneas-hipopneas del sueño. Rev Peru Med Exp Salud Publ 28(4):595–601
Rey de Castro J, Rosales-Mayor E (2013) Depressive symptoms in patients with obstructive sleep apnea/hypopnea syndrome. Sleep Breath 17(2):615–620
Rey de Castro J, Rosales-Mayor E, Ferreyra-Pereyra J (2011) Using a generic measure of quality of life in patients with obstructive sleep apnea. Sleep Breath 15(4):729–735
Khawaja I, Olson E, van der Walt C, Bukartyk J, Somers V, Dierkhising R, Morgenthaler T (2010) Diagnostic accuracy of split-night polysomnograms. J Clin Sleep Med 6(4):357–362
Sanders M, Black J, Costantino J, Kern N, Studnicki K, Coates J (1991) Diagnosis of sleep-disordered breathing by half-night polysomnography. Am Rev Respir Dis 144(6):1256–1261
Rosales-Mayor E, Rey de Castro J, Huayanay L, Zagaceta K (2012) Validation and modification of the Epworth Sleepiness Scale in Peruvian population. Sleep Breath 16(1):59–69
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14(6):540–545
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ (2003) Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42(6):1206–1252
Lesack K, Naugler C (2011) An open-source software program for performing Bonferroni and related corrections for multiple comparisons. J Pathol Inf 2:52
Barros AJ, Hirakata VN (2003) Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 3:21
Kitagawa G, Konishi S (2010) Bias and variance reduction techniques for bootstrap information criteria. Ann Inst Stat Math 62:209–234
Almendros I, Farre R, Planas AM, Torres M, Bonsignore MR, Navajas D, Montserrat JM (2011) Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep 34(8):1127–1133
Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A (2004) Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27(2):194–201
Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL (2008) Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc 5(2):185–192
Acknowledgments
Thanks to Dr. Kim Hoffman for her recommendations on the final version of this manuscript. This study was developed as part of the activities of the Masters in Clinical Epidemiology offered jointly by the Cayetano Heredia University and the US Naval Medical Research Unit Six (NAMRU-6), Peru. This Master of Science (MSc) programme is sponsored by 2D43 TW007393 “Peru Epidemiology Research Training Consortium”, awarded to NAMRU-6 by the Fogarty International Center of the US National Institutes of Health. The first author (CHS) prepared this paper to meet the graduation requirements of this programme.
Conflict of interest
The authors indicate that there are no financial conflicts of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huamaní, C., Rey de Castro, J. & Mezones-Holguín, E. Sleepiness and nocturnal hypoxemia in Peruvian men with obstructive sleep apnea. Sleep Breath 18, 467–473 (2014). https://doi.org/10.1007/s11325-013-0907-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-013-0907-3