Abstract
The aim of this study was to investigate the relationship between the subjective Epworth Sleepiness Scale (ESS) and objective polysomnographic parameters that was compared in dichotomized groups of obstructive sleep apnea (OSA) patients with and without excessive daytime sleepiness (EDS). A retrospective review of the 96 consecutive snoring patients suspected of OSA due to upper airway problems was performed. ESS score was assessed and EDS was considered present when the ESS score was >10. Polysomnography was performed for each patient. Comparisons of polysomnographic parameters between both groups of patients were performed using Mann–Whitney U-tests. The Pearson’s correlation coefficient was calculated. EDS patients showed significantly greater body mass index (BMI), AHI and ODI, higher percentage of snoring time, a longer time length of SpO2 < 90% and lower minimum SpO2 compared to no EDS patients. There was no statistical difference in other sleep parameters. A positive correlation between the ESS score and BMI, percentage of snoring time, minimum SpO2 and time length of SpO2 < 90% was identified. Despite many controversies, our conclusion is that apnea/hypoxemia has considerable coherence to account for the EDS measured by ESS in OSA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excessive daytime sleepiness (EDS) is one of the most common complaints in obstructive sleep apnea (OSA) patients, the prevalence of which ranges up to 87% [1]. EDS in OSA patients is a known risk factor for various accidents, and can result in poor quality of life [2]. The Epworth Sleepiness Scale (ESS) [3], including eight questions and four-point scales, is the most widely used convenient self-report questionnaire to assess EDS particularly among OSA patients in clinical setting [4]. A cutoff of 10 is generally used.
The mechanisms underlying EDS are unclear. Personality, age, obesity, shortening of the nocturnal sleep and so on has been suggested to affect subjective EDS [5]. Sleep fragmentation [1] and apnea/hypoxemia [6] have been proposed as the most important explanations for EDS in OSA patients. Some authors showed a significant correlation of the ESS with the polysomnographic parameters in OSA patients. However, some other works could not find such evidence. Moreover, only a few reports are found in the literature that studied such correlations in the snoring patients who visited ENT clinic with upper airway problems [7]. It is not possible, of course, at this stage to designate determining polysomnographic parameters of EDS. The aim of this study was to investigate the relationship between the subjective ESS and objective polysomnographic parameters that was compared in dichotomized groups of OSA patients with and without EDS.
Materials and methods
A retrospective review of the charts of 120 consecutive snoring patients with clinical suspicion of OSA between January 2008 and March 2011 was performed. Only patients who visited our ENT clinic with upper airway problems as the possible cause of OSA were included in the study. Data on age, sex and body mass index (BMI) were collected for each patient. Patients suspected of narcolepsy, hypersomnolence, periodic limb movement disorder and obesity hypoventilation syndrome were excluded from the study. Patients with psychiatric or neurological disorders and major systemic co-morbidities were also excluded. The study was approved by the Institutional Review Board to review the data for research purposes retrospectively.
ESS score was assessed for each patient using the validated Korean version of the ESS questionnaire [4]. A well-trained interviewer conducted this survey in the evening before the polysomnography (PSG) study. EDS was considered present when the ESS score was >10, and those with an ESS score of ≤10 were considered to have no EDS.
The diagnosis of OSA was established by a PSG attended overnight (A10 and S7000; Embla, Denver, CO, USA) in the sleep laboratory of our department. Measured parameters included electroencephalography (C4/A1, O2/A1, F4/A1, F3/A2), electro-oculography, electrocardiogram, oronasal airflow either by nasal cannula or thermal sensors, pulse oximetry, thoracoabdominal movements, submental and pretibial electromyography and snoring noises. Sleep stages were scored according to American Academy of Sleep Medicine guidelines [8]. The proportion of time spent in each sleep stage was expressed as a percentage of the total sleep time (TST). An apnea was defined as airflow reduction of >90% for at least 10 s, a hypopnea as any airflow reduction of >30% that lasted for longer than 10 s with a >4% reduction in SpO2. The AHI was defined as the sum of the numbers of apneas and hypopneas per hour of TST.
The proportion of snoring time was calculated as a percentage of TST. Sleep latency was defined as the period of time between the lights being switched off and the first 30 s of stage 1. Sleep efficiency was defined as the TST divided by total recording time. Arousals were defined as recommended by the American Sleep Disorders Association [9]. Each arousal index was defined as the sum of the number of each arousal per hour of TST. Total arousal index was also calculated. Oxygen desaturation index (ODI) was defined as the sum of the number of oxyhemoglobin desaturation of >4% events per hour of TST. Minimum pulse oxygen saturation (SpO2) and a time length of SpO2 < 90% [time (SpO2 < 90%)] were obtained. We excluded patients with AHI < 5/h (n = 13), sleep efficiency < 70% (n = 11) on the PSG. Thus, 96 patients were enrolled in this study.
All polysomnographic data of the patients were evaluated statistically using the program SPSS for Windows, version 12.0 (SPSS Inc, Chicago, IL, USA). Results are shown as mean ± SD. Comparisons between both groups of patients were performed using Mann–Whitney U-tests. The Pearson’s correlation coefficient was calculated to quantify correlations between the ESS score and the polysomnographic parameters. Statistical significance was defined as p < 0.05.
Results
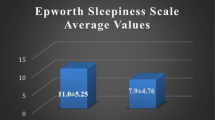
The patients consisted of 81 men (84.4%) and 15 women (15.6%), with a mean age of 41.90 ± 12.49 years and a range of 18–78 years. Table 1 shows the demographic characteristics of the OSA patients with and without EDS. Age distribution was similar in both groups. In contrast, BMI was greater in patients with EDS compared to the no EDS patients. Table 2 shows the polysomnographic data in OSA patients with and without EDS. EDS patients showed significantly greater AHI and ODI, higher percentage of snoring time, a longer time (SpO2 < 90%), and lower minimum SpO2. There was no statistical difference in sleep parameters including TST, sleep latency, sleep efficiency, sleep architecture and arousal indices.
A weak positive correlation between the ESS score and BMI, percentage of snoring time, minimum SpO2 and time (SpO2 < 90%) was identified (Fig. 1). The other remaining parameters including AHI, ODI had no statistical correlation with the ESS score (Table 3).
Discussion
There have been a lot of controversies on the correlation between the ESS and the polysomnographic parameters. The controversies could be due to the following factors. Firstly, the EDS is determined by multiple physiologic or pathologic conditions [7]. Secondly, EDS in OSA patients measured by ESS, a self-assessment method, is not objective but may depend on the subjective personality of the patients [5]. Thus, an assessment of EDS using ESS alone may not truly reflect sleepiness. Multiple sleep latency test (MSLT) may be an objective method to measure EDS, but studies using MSLT to evaluate EDS also have shown discrepant results [1, 2, 10]. Thirdly, any of the previous studies are not homogeneous in subjects or study design, and expectedly, has shown diverse results and different conclusions. In this study, we enrolled specific OSA patients who visited ENT clinic with retropalatal and/or retrolingual airway obstruction, different to earlier studies, and investigated the relationship between the subjective ESS and objective polysomnographic parameters. We used the Korean version of the ESS questionnaire with minor adjustment which was formerly validated to be reliable for screening EDS.
In the current study, compared to previous results, several polysomnographic parameters with significant correlation and differences were found. First of all, EDS patients had more severe apnea/hypopnea and hypoxemia than no EDS patients. Most authors were able to show some relationship between AHI and ESS [5, 6, 10–12]. The ESS score progressively increased as the severity of OSA aggravated [6, 13, 14]. Compared to no EDS patients, EDS patients exhibited a greater AHI [1, 10–12], though not wholly agreed [2]. Recent study showed no correlation between the ESS and the AHI, ODI, and minimum SpO2 in patients with upper airway pathology [7]. Our data also showed no correlation between the ESS and the AHI, ODI in OSA patients, but significant difference in AHI and ODI was found between the patients with and without EDS. The results of the majority of previous studies and this study suggest that though there is no consistent direct correlation, AHI and ODI may be related to ESS and EDS to some degree. And in our opinion, if studied under exquisite condition, a meaningful correlation of ESS with AHI and ODI may be identified.
The next important factor is nocturnal hypoxemia, which has been considered in a majority of literature to be a major determinant of EDS in OSA patients [2, 6, 10, 11]. Although denied in a few studies [1, 7], progressive worsening of nocturnal hypoxemia was observed from mild to severe OSA patients with a strong correlation with ESS score [6]. ESS was strongly related with number of oxygen desaturations and lowest oxygen saturation [13]. In this study, a weak positive correlation between the ESS score and the minimum SpO2 and time (SpO2 < 90%) was found. Moreover, nocturnal oxygenation indices were significantly worse [higher ODI, lower minimum SpO2 and longer time (SpO2 < 90%)] in the EDS patients compared to the patients without EDS. Thus, our findings, like others [2, 6, 10, 11], suggest that nocturnal hypoxemia may have a significant role in the pathogenesis of EDS in OSA patients with considerable consistency. Although the exact biological mechanisms are unknown, it was postulated that increased hypoxemia causes severe neural damage of wake-promoting structures, leading to EDS [15].
The loudness degree of snoring assessed subjectively was one of the independent predictors of EDS [1]. In community-dwelling adults, subjective snoring status (never, ever, do not know, all) and frequency were independently associated with EDS determined by ESS scores [16]. No quantification is the limitation of their studies. Our quantified objective data showed significant correlation between percentage of snoring time and ESS score, suggesting dose response in the relation between percentage of snoring time and EDS. The mechanism underlying this relation is uncertain, but it may be somewhat natural that more respiratory events will be expected to occur during the longer snoring time. BMI was also one of the three major determinants of the ESS score [6]. Our study showed that BMI was also significantly greater in the EDS patients than in the no-EDS patients, suggesting that obesity may have an effect on the severity of OSA in accordance with previous studies [5, 6, 10, 12, 13], contrary to other studies [11].
Interestingly and unexpectedly, in our study, sleep fragmentation, disruption of sleep structure and sleep drive showed no correlation with ESS score. EDS among OSA patients may be associated with a high frequency of recurrent arousals, which appears to disrupt the continuity of sleep, leading to fragmented sleep [1, 14]. A weak positive correlation of the ESS with the arousal index was found [7]. A significant increase in arousal index was also observed in EDS patients compared to the no-EDS patients [1, 10, 11, 14]. But our study and other previous studies showed neither direct correlation nor statistical difference between the two groups [2, 12].
Disruption of sleep structure was found in more severe OSA patients as evidenced by remarkable increase in light sleep [10] and decreased slow-wave sleep [6, 17], suggesting low quality of sleep. But, unpredictably, a decrease in light sleep and an increase in SWS in the EDS patients compared to no EDS patients was reported [11]. An increase in REM sleep in EDS patients were rarely reported for OSA [13]. Along with these inconsistencies of previous result, our study in accordance with previous works [2, 12], showed almost similar sleep macro-architecture in the two groups with and without EDS.
EDS may be directly affected by individual differences in the demand for sleep. OSA patients with EDS slept longer [10, 11, 13] after shorter sleep latency [2, 10, 11], and more efficiently [1, 2, 10, 11, 13] than those without EDS. The previous authors suggested that these findings are probably not a cause but a consequence of EDS, and that patients with EDS are sleepy throughout 24-h [2]. But we found no differences in the sleep drive between the two groups with and without EDS. Taking all the above-mentioned results into consideration, fragmentation, disruption and drive of sleep may not primarily explain the presence of EDS.
We think that, ESS, owing to its subjectivity, may not be reliable to show direct correlation with polysomnographic parameters. This fact along with the retrospective study design is the limitation to weaken the scientific power of this study. But, in the context that ESS was related to some parameters of polysomnographic evaluation more or less in all literatures reviewed, ESS could be used as an indicator of EDS, if the ESS scores are obtained by comprehensive interviews with the patients. The differences for each significant parameter that previous studies and ours obtained between the two groups have not been or will not be entirely consistent in the field of research on EDS in OSA patients. Despite all the controversies, our conclusion is that apnea/hypoxemia has considerable coherence to account for the EDS measured by ESS in OSA patients.
References
Seneviratne U, Puvanendran K (2004) Excessive daytime sleepiness in obstructive sleep apnea: prevalence, severity, and predictors. Sleep Med 5:339–343
Mediano O, Barceló A, De la Peña M, Gozal D, Agustí A, Barbé F (2007) Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J 30:110–113
Briones B, Adams N, Strauss M, Rosenberg C, Whalen C, Carskadon M, Roebuck T, Winters M, Redline S (1996) Relationship between sleepiness and general health status. Sleep 19:583–588
Cho YW, Lee JH, Son HK, Lee SH, Shin C, Johns MW (2011) The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath 15:377–384
Hayashida K, Inoue Y, Chiba S, Yagi T, Urashima M, Honda Y, Itoh H (2007) Factors influencing subjective sleepiness in patients with obstructive sleep apnea syndrome. Psychiatry Clin Neurosci 61:558–563
Chen R, Xiong KP, Lian YX, Huang JY, Zhao MY, Li JX, Liu CF (2011) Daytime sleepiness and its determining factors in Chinese obstructive sleep apnea patients. Sleep Breath 15:129–135
Bausmer U, Gouveris H, Selivanova O, Goepel B, Mann W (2010) Correlation of the Epworth Sleepiness Scale with respiratory sleep parameters in patients with sleep-related breathing disorders and upper airway pathology. Eur Arch Otorhinolaryngol 267:1645–1648
Iber C, Ancoli-Israel S, Chesson AL, Quan SF (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st edn. American Academy of Sleep Medicine, Westchester
DA AS (1992) EEG arousals: scoring rules and examples: a preliminary report from the sleep disorders atlas task force of the American sleep disorders association. Sleep 15:173–184
Sun Y, Ning Y, Huang L, Lei F, Li Z, Zhou G, Tang X (2011) Polysomnographic characteristics of daytime sleepiness in obstructive sleep apnea syndrome. Sleep Breath (Epub ahead of print)
Roure N, Gomez S, Mediano O, Duran J, Peña Mde L, Capote F, Teran J, Masa JF, Alonso ML, Corral J, Sánchez-Armengod A, Martinez C, Barceló A, Gozal D, Marín JM, Barbé F (2008) Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med 9:727–731
Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto FJ (2005) Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep 28:472–477
Jiménez-Correa U, Haro R, González-Robles RO, Velázquez-Moctezuma J (2011) How is the Epworth Sleepiness Scale related with subjective sleep quality and polysomnographic features in patients with sleep-disordered breathing? Sleep Breath 15:513–518
Goncalves MA, Paiva T, Ramos E, Guilleminault C (2004) Obstructive sleep apnea syndrome, sleepiness, and quality of life. Chest 125:2091–2096
Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A (2004) Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 15:194–201
Gottlieb DJ, Yao Q, Redline S, Ali T, Mahowald MW (2000) Does snoring predict sleepiness independently of apnea and hypopnea frequency? Am J Respir Crit Care Med 162:1512–1517
Lim LL, Tham KW, Fook-Chong SM (2008) Obstructive sleep apnoea in Singapore: polysomnography data from a tertiary sleep disorders unit. Ann Acad Med Singapore 37:629–636
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.J., Kang, H.W. & Lee, L.H. The relationship between the Epworth Sleepiness Scale and polysomnographic parameters in obstructive sleep apnea patients. Eur Arch Otorhinolaryngol 269, 1143–1147 (2012). https://doi.org/10.1007/s00405-011-1808-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-011-1808-3