Abstract
Introduction
Sleep disorders are associated with various human pathologies and interfere with biological processes essential for health and quality of life. On the other hand, cancer is one of the most common diseases worldwide with an average of 1,500 deaths per day in the USA. Is there a factor common to both sleep disorders and cancer that serves to link these conditions?
Discussion
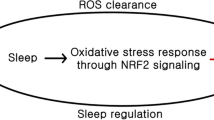
It is a normal process for cellular metabolism to produce reactive oxidant series (ROS). However, when the production of ROS overcomes the antioxidant capacity of the cell to eliminate these products, the resulting state is called oxidative stress. Oxidative DNA damage may participate in ROS-induced carcinogenesis. Moreover, ROS are also produced in the sleep deprivation process. The aim of this article is to review pathways and mechanisms that may point to oxidative stress as a link between sleep deprivation and cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a major consensus that sleep is imperative for life maintenance [1–7]. On average, a third of our lives is spent in sleep [8]. Sleep controls mechanisms on several levels of biological organization, from genes and intracellular pathways to networks of cell populations and to all central neuronal systems, including those that control movement, arousal, autonomic functions, behavior, and cognition [9]. However, over the past several decades, there has been a trend towards a voluntary reduction in sleep time. Studies have shown several behavioral and physiological effects caused by insufficient sleep [10–13]. Reported consequences of insufficient sleep include neurocognitive alterations, psychomotor impairment, adverse mood effects, reduced quality of life, decreased work productivity, deficits in memory and decision making [14–16], cardiovascular diseases [17], increased risk for accidents [18–20], insulin resistance, and increased risk for obesity [21].
Additionally, there appears to be a link between sleep and immune defenses. Studies have indicated that reduced sleep may attenuate subsequent immune function [22, 23], impair the host defense mechanisms, and impact the susceptibility to viral and bacterial pathogens [24, 25]. Investigators have demonstrated that sleep deprivation decreases total cellularity of the bone marrow and peripheral blood concomitantly [26]. Furthermore, there is a decrease in natural killer cell mobilization and slowed recovery in healthy women after a poor night of sleep [27]. Thus, the effect of sleep on various endocrine and cytokine pathways suggests a relationship between sleep and the immune system [28].
Cancer is a major public health problem in the USA and other countries worldwide. Statistics show that one in four deaths in the USA is due to cancer. It is estimated that approximately 569,490 Americans will die from cancer in 2010, which is an average of 1,500 deaths per day [29]. According to the American Cancer Society, breast cancer is the most common cancer diagnosed in US women and the second leading cause of death [30, 31] as is prostate cancer in men [32], following cancer of the lung and colorectum in both genders, accounting for half of total cancer deaths [29]. Oral cancer, globally, is the sixth most common cancer [33] and is a major problem in regions where tobacco habits in the form of chewing and/or smoking are prevalent. The occurrence of oral cancer varies by age, ethnic group, lifestyle, and a country’s level of economic development [34]. There are numerous articles published in MEDLINE/Pubmed databases that address questions focused on cancer and its epidemiology.
Oxidative stress occurs in a cell or tissue when the concentration of reactive oxygen species (ROS) generated exceeds the antioxidant capability of that cell [35]. ROS may interact with and modify the cellular protein, lipids, and DNA, resulting in altered target cell function [36–39). There is considerable evidence that ROS are involved in the pathogenesis of various human diseases [40]. Oxidative DNA damage may participate in ROS-induced carcinogenesis [41]. Moreover, there is evidence that ROS are also involved in the process of sleep deprivation [42–44]. Some investigators theorize that sleep decreases oxidative stress [42, 45] and that sleep is involved in the repair and detoxification process [46, 47]. This theory provides a link between sleep deprivation and carcinogenesis using oxidative stress as the common factor, specifically that sleep deprivation promotes oxidative stress and that oxidative stress may be causally linked to carcinogenesis. The pathways and signals for these associations between sleep deprivation and carcinogenesis remain unclear [3, 48–50].
Material and methods
The concept of this mini-review is to initiate a discussion about the topics of sleep deprivation and carcinogenesis by connecting them to oxidative stress and its signaling pathways. MEDLINE/Pubmed databases of the National Library of Medicine, Bethesda, Maryland, were searched for articles from 1992 to 2012, using the following terms: cancer, carcinogenesis process, sleep deprivation, and oxidative stress. Publications constituting case reports were excluded. Abstracts were reviewed, and relevant papers were identified. All relevant studies were included in this review.
Results
Oxidative stress network in cancer and sleep deprivation
Oxidative stress is defined as an imbalance between production of free radicals and reactive metabolites, the so-called ROS as well as reactive nitrogen species (RNS), both products of normal cellular metabolism. ROS and RNS are well recognized for playing a dual role as both deleterious and beneficial species, since they can be either harmful or beneficial to living systems [36]. Beneficial effects of ROS occur at low/moderate concentrations and involve physiological cellular responses to anoxia. Examples of this beneficial effect are defense against infectious agents and utility in a number of cellular signaling systems [51–53]. The harmful effects of free radicals are termed oxidative stress and nitrosative stress. For the purposes of this review, we will limit discussion to the oxidative stress process. Such oxidative stress occurs in biological systems when there is an overproduction of ROS with a relative deficiency of enzymatic and nonenzymatic antioxidants. This imbalance leads to damage of important biomolecules and whole cells with potential impact on the entire organism [54]. ROS evoke many intracellular events such as proliferation, gene activation, cell-cycle arrest, and apoptosis [55]. Excess ROS can damage cellular lipids, proteins, or DNA, inhibiting their normal function. Among the major biologically relevant free radical species in cells and biofluids are a one-electron product of oxygen reduction, the superoxide anion radical and its dismutation product. This product, hydrogen peroxide, activates nuclear factor- < kappa > B. Other oxidants induce protein tyrosine phosphorylation in immune cells [56, 57]. Because of this, oxidative stress has been implicated in a number of human diseases as well as in the aging process. Cells of the immune systems produce ROS during the oxidative burst triggered during inflammatory processes [58, 59]. Under these conditions, immune cells may react together to produce significant amounts of a much more oxidatively active molecule, the peroxynitrite anion (ONOO–). Peroxynitrite is a highly potent oxidizing agent that can cause DNA fragmentation and lipid oxidation [60].
Recent studies have shown an important role for ROS in tumor development [39, 61]. Under sustained environmental stress, ROS are produced over a long period, causing significant damage to cell structures and functions. Such damage may induce somatic mutations resulting in cancer [62]. Studies have shown interaction of ROS with all three stages of the mutation process: initiation, progression, and promotion [63–65]. ROS are known not only to attack DNA but additional cellular components including proteins and lipids leaving behind reactive species that can, in turn, couple to DNA bases. The most extensively studied DNA lesion is the formation of 8-hydroxyguanine [8-OH-G], the major pre-mutagenic lesion generated from ROS [66]. This lesion is crucial because it is easily formed, becomes mutagenic, and is a potential biomarker of carcinogenesis. Such DNA mutation is a critical step in the carcinogenesis process. Elevated levels of oxidative DNA lesions have been noted in various tumors, though the exact role DNA lesions play in carcinogenesis is not clear. DNA lesions have been linked with the initiation process of cancer [67]. ROS can also contribute to abnormal gene expression, blockage of cell-to-cell communication, and modification of second-messenger systems in the promotion stage, thus resulting in an increase in cell proliferation or a decrease in apoptosis of the initiated cell population [64]. Additionally, oxidative damage to protein-coding or noncoding RNA may potentially cause errors in protein synthesis or dysregulation of gene expression. Studies have shown that these mechanisms are present in various human diseases, especially chronic degeneration in neurons [68–71]. Accurate and reliable measurement of oxidative damage in the carcinogenesis process is relevant to understand the evolution of oxidative stress and distribution of ROS-induced damage in several pathologies.
Reimund [42] hypothesized that free radicals or ROS produced during waking are removed during sleep. Reimund postulated that sleep has an anti-oxidative function [42, 45]. Similarly, Ikeda et al. [45] showed that sleep decreases oxidative stress. Maintenance of steady state concentrations of ROS is essential for adequate functioning of aerobic organisms. However, in order to protect cells from the deleterious effects from ROS, a variety of systems has evolved [38]. Mammalian cells possess enzymatic antioxidant defenses to cope with ROS, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx). SOD catalyzes the reaction of the superoxide anion to hydrogen peroxide and catalase. GPx catalyzes the breakdown of peroxides [38]. Reduced glutathione is a potent scavenger of free radicals and is also a substrate for glutathione-S-transferase, an enzyme responsible for a number of detoxification reactions within the cell [38]. Experimental studies have been performed to support the assertion that prolonged wakefulness may cause oxidative damage. Ramanathan and Siegel (2011) showed that sleep loss under sustained hypoxia leads to increased nitric oxide production in the rat hippocampus and increased total glutathione levels in the rat neocortex, brainstem, and cerebellum, protecting against oxidative stress. However, other investigators have described studies which measured oxidative stress in whole rat-brain homogenates under conditions of sleep deprivation, reporting the absence of oxidative stress in the brain [37, 72]. Apparently, the brain is capable of responding to stress by changing the activity of antioxidant enzymes, inducing heat shock proteins and upregulating uncoupling proteins, thus facilitating recovery from the oxidative damage [73, 74].
On the other hand, some investigators have demonstrated that sleep loss may induce oxidative stress in the brain and cause regional changes [43, 44, 47, 75]. It has been demonstrated that sleep loss produces effects similar to those that occur during aging [76–78]. Chang et al. (2008) reported that sleep deprivation significantly decreases hepatic phosphatidylcholine (the most prominent component of all plasma lipoprotein expressions) and sharply increases the oxidative stress in the hepatocytes. It has been demonstrated that phosphatidylcholine concentration may be reduced in many experimental-induced pathologies where oxidative stress is a contributing factor [79–81].
Obstructive sleep apnea (OSA) is a sleep-related respiratory disorder, which is characterized by repetitive episodes of complete (apnea) or partial (hypopnea) obstruction of airflow in the upper airway during sleep. It has been well documented that OSA plays an important role in several complications such as obesity, type 2 diabetes, metabolic syndrome, cardiovascular, and neurophysiological diseases [82–88]. Studies have also shown that hypoxia has been associated with various stages of tumor formation and progression [89–91]. Recently, studies have demonstrated the influence of sleep loss in cancer progression [50, 92]. The hypoxia caused by sleep disturbances may be key to increased levels of ROS. Hypoxia-inducible factor-1 (HIF-1) is a master regulator of O2 homeostasis that controls multiple physiological processes by regulating the expression of hundreds of genes [93]. The over-expression of HIF-1 is correlated to proangiogenic mediators, such as vascular endothelial growth factor in tumor cells as well as apoptosis, glycolysis, and cell-cycle control mediators. These functions are central to the survival and expansion of malignant cell populations in an oxygen-deficient environment [94–97]. A recent study has demonstrated that the more severe the obstructive sleep apnea and sleep fragmentation is, the more severe the oxidative stress state becomes [98].
Oxidative stress appears to be an important factor in various human diseases. This review clearly implicates the role of ROS in various phases of the carcinogenesis process and the promotion of ROS in states of sleep loss. Our hypothesis suggests that oxidative stress may be a crucial factor in both processes. The causal effects between sleep deprivation and carcinogenesis remain to be elucidated.
References
Andersen M, Bignotto M, Machado R, Tufik S (2002) Does paradoxical sleep deprivation and cocaine induce penile erection and ejaculation in old rats? Addict Biol 7:285–290
Andersen M, Bignotto M, Tufik S (2003) Cocaine-induced genital reflexes during paradoxical sleep deprivation and recovery. Physiol Behav 78:255–259
Blask D, Dauchy R, Sauer L (2005) Putting cancer to sleep at night—the neuroendocrine/ circadian melatonin signal. Endocrine 27:179–188
Drummond S, Brown G (2001) The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology 25:S68–S73
Gunzman-Marin R, Suntsova N, Methippara M (2005) Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Nuerosci 22:2111–2116
Leibowitz S, Lopes M, Andersen M, Kushida C (2006) Sleep deprivation and sleepiness caused by sleep loss. Sleep Med Clin 1:31–45
Cirelli C, Tononi G (2008) Is sleep essential? PLoS Biol 6:e216
Sejnowski T, Destexhe A (2000) Why do we sleep? Brain Res 886:208–223
Pace-Schott E, Hobson J (2002) The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nature 3:591–605
Banks S, Dinges D (2007) Behavioral and physiological consequences of sleep restriction. J CLin Sleep Med 3:519–28
Andersen M, Bignotto M, Tufik S (2003) The effect of apomorphine on genital reflexes in male rats deprived of paradoxical sleep. Physiol Behav 80:211–215
Biswas S, Mishra P, Mallick N (2006) Increased apoptosis in rat brain after rapid eye movement sleep loss. Neuroscience 142:315–331
Graves L, Heller E, Pack A, Abel T (2003) Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem 10:168–176
Killgore W, Balking T, Wesensten N (2006) Impaired decision making following 49 hours of sleep deprivation. J Sleep Res 15:7–13
Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI (1997) Cumulative sleepiness, mood disturbance and psychomotor vigilance performance decrements during sleep restricted to 4–5 hours per night. Sleep 20:267–277
Sateia M (2003) Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med 24:249–259
Shamsuzzaman A, Gersh B, Somers V (2003) Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 290:1906–1914
Masa J, Rubio M, Findley L (2000) Habitually sleepy drivers have a high frequency of automobile crashes associated with respiratory disorders during sleep. Am J Respir Crit Care Med 162:1407–1412
Ingre M, Akerstedt T, Peters B, Anund A, Kecklund G, Pickles A (2006) Subjective sleepiness and accident risk avoiding the ecological fallacy. J Sleep Res 15:142–148
Connor J, Norton R, Ameratunga S, Robinson E, Civil I, Dunn R, Bailey J, Jackson R (2002) Driver sleepiness and risk of serious injury to car occupants: population based case control study. BMJ 324:1125
Buscemi D, Kumar A, Nugent R, Nugent K (2007) Short sleep times predict obesity in internal medicine clinic patients. J Clin Sleep Med 3:681–688
Irwin M, Mascovich A, Gillin J, Willoughby R, Pike J, Smith T (1994) Partial sleep deprivation reduces natural killer cell activity in humans. Psychom Med 56:493–498
Irwin M, McClintick K, Costlow C, Fortner M, White J, Gillin C (1996) Partial night sleep deprivation reduces natural killer and cellular immune response in humans. FASEB J 10:643–653
Benca R, Quintas J (1997) Sleep and host defenses: a review. Sleep 20:1027–1037
Dinges D, Douglas S, Hamarman S, Zaugg L, Kapoor S (1995) Sleep deprivation and human immune function. Adv Neuroimmunol 5:97–110
Guariniello L, Vicari P, Lee K, Ad O, Tufik S (2012) Bone marrow and peripheral white blood cells number is affected by sleep deprivation in a murine experimental model. J Cell Physiol 227:361–366
Wright C, Erblich J, Valdimarsdottir H, Bovbjerg D (2007) Poor sleep the night before an experimental stressor predicts reduced NK cell mobilization and slowed recovery in healthy women. Brain Behav Immun 21:358–363
Bryant P, Trinder J, Curtis N (2004) Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol 4:457–467
Jemal DA, Siegel R, Xu J, Ward E (2010) Cancer statistics. CA Cancer J Clin 60:277–300
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics. CA Cancer J Clin 59:225–249
Smith R, Cokkinides V, Brooks D, Saslow D, Brawley O (2010) Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 60:99–119
Majumdar S, Buckles E, Estrada J, Koochekpour S (2011) Aberrant DNA methylation and prostate cancer. Curr Genom 12:486–505
Parkin D, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics. CA Cancer J Clin 55:74–108
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun M (2007) Cancer statistics. CA Cancer J Clin 57:43–66
Sies H (1991) Oxidative stress: introduction. In: Sies H (ed) In Oxidants and antioxidants. Academic, San Diego, pp 15–22
Valko M, Izakovic M, Mazur M, Rhodes C, Telser J (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:37–56
D'Almeida V, Hippolide D, Azzalis A, Lobo L, Junqueira V, Tufik S (1997) Absence of oxidative stress following paradoxical sleep deprivation in rats. Neurosci Lett 235:25–28
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Oxford, Clarendon Press
Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320:661–664
Halliwell B and Gutteridge J (1989) Free radicals in Biology and Medicine.(Editor (eds.). Claredon
Breimer L (1990) Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog 3:188–197
Reimund E (1994) The free radical theory of sleep. Med Hypotheses 43:231–233
Hipólide D, D'Almeida V, Raymond R, Tufik S, Nobrega J (2002) Sleep deprivation does not affect indices of necrosis or apoptosis in rat brain. Int J Neurosci 112:155–166
D'Almeida V, Lobo L, deliveira A, Nobrega J, Tukik S (1998) Sleep deprivation induces brain region-specific decrease in glutathione levels. Neuroreport 9:2853–2856
Ikeda M, Ikeda-Sagara M, Okada T, Clement P, Urade Y, Nagai T, Sugiyama T, Yoshioka T, Honda K, Inoué S (2005) Brain oxidation is an initial process in sleep induction. Neuroscience 130:1029–1040
Horne J (1978) A review of the biological effects of total sleep deprivation in man. Biol Psychol 7:55–102
Honda K, Kamoda Y, Inoue S (1994) Oxidized glutathione regulates physiological sleep in unrestrained rats. Brain Res 636:253–258
Blask D, Dauchy R, Sauer L, Krause J (2004) Melatonin uptake and growth prevention in rat hepatoma 7288 CTC in response to dietary melatonin: melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxydecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 25:951–960
Blask D, Sauer L, Dauchy R (2002) Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem 2:113–132
Almendros I, Montserrat JM, Ramírez J, Torres M, Duran-Cantolla J, Navajas D, Farré R (2012) Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J 39:215–217
Kovacic P, Jacintho J (2001) Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem 8:773–796
Ridnour L, Isenberg J, Espey M, Thomas D, Roberts DD, Wink D (2005) Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA 102:13147–13152
Valko M, Morris H, Mazur M, Rapta P, Bilton R (2001) Oxygen free radical generating mechanisms in the colon: do the semiquinones of Vitamin K play a role in the aetiology of colon cancer? Biochim Biophys Acta 1527:161–166
Durackova Z (2010) Some current insights into oxidative stress. Physiol Res 59:459–469
Nakamura H, Nakamura K, Yodoi J (1997) Redox regulation of cellular activation. Annu Rev Immunol 15:351–369
Gorbunov N, Elsayed N, Kisin E, Kozlov A, Kagan V (1997) Air blast-induced pulmonary oxidative stress: interplay among hemoglobin, antioxidants, and lipid peroxidation. Am J Physiol 272:L320–334
Nakamura K, Hori T, Yodoi J (1996) Alternative binding of p56 and phosphatidylinositol 3-kinase in T cells by sulfhydryl oxidation: implication of aberrant signaling due to oxidative stress in T lymphocytes. Mol Immunol 33:855–865
Victor V, Rocha M, Fuente MD (2004) Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol 4:327–347
Fuente MD, Hernanz A, Vallejo M (2005) The immune system in the oxidative stress conditions of aging and hypertension: favorable effects of antioxidants and physical exercise. Antioxid Redox Signal 9–10:1356–1366
Carr A, McCall M, Frei B (2000) Oxidation of LDL by myeloperoxidase and reactive nitrogen species-reaction pathways and antioxidant protection. Arterioscl Thromb Vasc Biol 20:1716–1723
Kumar B, Koul S, Khandrika L, Meacham R, Koul H (2008) Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 68:1777–1785
Fang J, Seki T, Maeda H (2009) Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev 61:290–302
Schulte-Herman R, Timmermann-Trosiener I, Barthel G, Bursch W (1990) DNA synthesis, apoptosis and phenotypic expression as determinants of growth of altered foci in rat liver durinhg phenobarbital promotion. Cancer Res 50:5127–5135
Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, Stevenson DE, Walborg EF Jr (1998) The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect 106:289–295
Ames B, Gold L (1992) Animal cancer tests and cancer prevention. J Natl Cancer Inst 12:125–132
Arai T, Kelly V, Minowa O, Noda T, Nishimura S (2002) High accumulation of oxidative DNA damage, 8-hydroxyguanine, in Mmh/Ogg1 deficient mice by chronic oxidative stress. Carcinogenesis 12:2005–2010
Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE (1999) Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci USA 96:13300–13305
Numonura A, Moreira P, Takeda A, Smith M, Perry G (2007) Oxidative RNA damage and neurodegeneration. Curr Med Chem 14:2968–2975
Gandhi S, Abramov A (2012) Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev 2012:428010
Casetta I, Govoni V, Granieri E (2005) Oxidative stress, antioxidants and neurodegenerative diseases. Curr Pharm Des 11:2033–2052
Behl C, Moosmann B (2002) Oxidative nerve cell death in Alzheimer's disease and stroke: antioxidants as neuroprotective compounds. Biol Chem 383:521–536
Gopalakrishnan A, Ji L, Cirelli C (2004) Sleep deprivation and cellular responses to oxidative stress. Sleep 27:27–35
Cirelli C (2006) Cellular consequences of sleep deprivation in the brain. Sleep Med Rev 10:307–321
Cirelli C (2006) Sleep disruption, oxidative stress, and aging: new insights from fruit flies. Proc Natl Acad Sci U S A 103:13901–13902
Singh R, Kiloung J, Singh S, Sharma D (2007) Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats. Bioderontology 9:153–162
Spiegel K, Leprout R, Cauter EV (1999) Impact of sleep debt on metabolic and endocrine function. Lancet 354:1435–1439
Prinz P (2004) Age impairments in sleep, metabolic and immune functions. Exp Gerontol 39:1739–1743
Andersen M, Martins P, D'Almeida V, Santos R, Bignotto M, Tufik S (2004) Effects of paradoxical sleep deprivation on blood parameters associated with cardiovascular risk in aged rats. Exp Gerontol 39:817–824
Omoi N, Arai M, Saito M, Takatsu H, Shibata A, Fukuzawa K (2006) Influence of oxidative stress on fusion of pre-synaptic plasma membranes of the rat brain with phosphatidylcholine liposomes and protective effect of vitamin E. J Nutr Sci Vitaminol 52:248–255
Petursdottir A, Farr S, Morley J, Banks W, Skuladottir G (2006) Lipid peroxidation in brain during aging in the senescence-accelerated mouse (SAM). Neurobiol Aging 28:1170–1178
Tsuda T, Yoshimura H, Hamasaki N (2006) Effect of phosphatidylcholine, phosphatidylethanolamine and lysophosphatidylcholine on the activated factor X-prothrombin system. Blood Coagul Fibrinolysis 17:465–469
Hicks D (2011) Obstructive sleep apnoea: its link with diabetes. Nurs Times 107:31–32
Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi H (2009) Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med 122:1122–1127
Bardwell W, Ancoli-Israel S, Berry C, Dimsdale J (2001) Neuropsychological effects for one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: a placebo-controlled study. Psychol Med 63:579–584
Fogel R, Malhotra A, White D (2004) Sleep 2 Pathophysiology of obstructive sleep apnea/hypopnea syndrome. Thorax 59:159–163
Young T, Peppard P, Gottlieb D (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165:1217–1239
Lavie L (2003) Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7:35–51
Bradley T, Floras J (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373:82–93
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E (1998) Role of HIF-1a in hypoxiamediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485–490
Knowles H, Harris A (2001) Hypoxia and oxidative stress in breast cancer. Hypoxia and tumourigenesis. Breast Cancer Res 3:318–322
Pugh C, Gleadle J, Maxwell P (2001) Hypoxia and oxidative stress in breast cancer. Hypoxia signalling pathways. Breast Cancer Res 3:313–7
Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R (2012) Sleep-disordered breathing and cancer mortality results from the Wisconsin Sleep Cohort Study. Am J Resp Crit C Med 186:190–194
Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ (2009) Genome-wide association of hypoxia-inducible factor (HIF)-1a and HIF-2a DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem 284:16767–16775
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumours: a review. Cancer Res 49:6449–6465
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–395
Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS (2000) Hypoxia-inducible factor-1α is a positive factor in solid tumour growth. Cancer Res 60:4010–4015
Franco CM, Lima AM, Ataíde L Jr, Lins OG, Castro CM, Bezerra AA, de Oliveira MF, Oliveira JR (2012) Obstructive sleep apnea severity correlates with cellular and plasma oxidative stress parameters and affective symptoms. J Mol Neurosci 47:300–310
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noguti, J., Andersen, M.L., Cirelli, C. et al. Oxidative stress, cancer, and sleep deprivation: is there a logical link in this association?. Sleep Breath 17, 905–910 (2013). https://doi.org/10.1007/s11325-012-0797-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-012-0797-9