Abstract
The present study examined the effects of paradoxical sleep (PS) deprivation on the oxidative stress parameters: lipid peroxidation, superoxide dismutase, glutathione peroxidase, and glutathione in brain regions: cerebral cortex, striatum, hippocampus, thalamus, hypothalamus, and brain stem of adult (8 months) and old (24 months) rats. PS deprivation (96 h) was performed by the classical flower pot technique. PS deprivation did not affect oxidative stress parameters in the striatum of both age groups; and the activity of glutathione peroxidase was not affected in any of the studied brain regions in both age groups. PS deprivation decreased the levels of glutathione only in the hippocampus, thalamus and hypothalamus; the magnitude of decrease was higher in the old than in the adult age group. PS deprivation increased the superoxide dismutase activity in the cerebral cortex and brain stem but reduced it in the hippocampus, thalamus and hypothalamus in both age groups. Increases in the activity were greater in adult animals than in old ones; the decline in the activity was greater in the hippocampus of old animals than in that of the adult ones. Lipid peroxidation was reduced by PS deprivation in the cerebral cortex and brain stem but was elevated in the hypothalamus and thalamus: the magnitude of alteration in the cerebral cortex, brain stem, hippocampus and hypothalamus was higher in adult animals than in old ones. The results showed that oxidative stress was not uniformly affected in all the brain regions. The cerebral cortex and brain stem showed a fall in oxidative stress after PS deprivation; the fall was greater in the adult than in the old animals. However, the oxidative stress was elevated in the hippocampus, thalamus and hypothalamus, and old animals were more severely affected than the adult ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep decreases oxidative stress (Ikeda et al. 2005) as it removes oxidants produced during waking (Reimund 1994). It also involves processes of repair, restoration and detoxification (Horne 1978; Honda et al. 1994), and is composed of two different behavioral states: slow wave sleep, and paradoxical sleep. Although the latter shows elevated neuronal activity at high energy cost (Magistretti 2006), yet it is also antioxidative (D’Almeida et al. 1998).
Experimental sleep deprivation studies are of interest as they provide information about the deleterious effects of loss of sleep. In humans, sleep architecture changes with age (Kryger et al. 2004) and in the elderly lack of adequate sleep is a common symptom. Loss of sleep may induce oxidative stress in the brain (Hippolide et al. 2002). A previous study which measured oxidative stress in the whole brain homogenates from PS-deprived rats reported the absence of oxidative stress in the brain (D’Almeida et al. 1997). A subsequent study with measurements taken from discrete brain regions indicated that PS deprivation might produce brain region specific changes in oxidative stress: decreases were found in glutathione levels in the hippocampus and thalamus (D’Almeida et al. 1998). A total sleep-deprivation induced decrease in superoxide dismutase activity in the hippocampus and brain stem was shown by Ramanathan et al. (2002). Therefore, it is of interest to investigate the effects of PS deprivation on the various parameters of oxidative stress in brain regions.
Both sleep deprivation and ageing disturb brain functions (Spiegel et al. 1999; Urrila et al. 2004; Prinz et al. 2004; Gosselin et al. 2005). Sleep deprivation produces effects similar to those that occur in the course of ageing. For example, in young adult humans (23 years), 36 h sleep deprivation impaired the prefrontal cortex-related psychological functions similar to that which occur during normal healthy ageing in 60–70 years old individuals (Harrison et al. 2000). Systemic inflammation found during ageing or sleep deprivation enhances oxidative stress (Roy et al. 2002; Prinz 2004). Sleep deprivation-induced oxidative stress may thus contribute to the process of ageing. PS deprivation potentiates, attenuates or even reverses some effects of ageing and may affect the brain of adult and old subjects differently: PS deprivation caused a reduction in blood viscosity in aged but not in young rats (Andersen et al. 2004). Ageing relevant studies of sleep deprivation are therefore of interest (Prinz 2004). No study has directly compared the effects of ageing on the brain with those of sleep deprivation (Harrison et al. 2000) and sleep deprivation effects on oxidative stress in discrete brain regions of aged animals have not been studied. Data derived from sleep-deprived aged animals will provide information relevant to the consequences of natural age impairment in sleep that occurs in the elderly humans (Prinz 2004).
Many effects of total sleep deprivation have been found to be similar to those of selective PS deprivation (D’Almeida et al. 1998). In some respects, effects of PS deprivation may differ from those of total sleep deprivation. Specific PS deprivation affects plasticity, and causes specific cognitive impairments (Stickgold et al. 2001; Campbell et al. 2002). Although there is an age stability of PS in late life and maintenance of PS particularly into late life may be a possible mechanism of successful ageing, PS may decrease in the event of neurodegenerative disorders, (Reynolds et al. 1993). Furthermore, PS is important as a vigilance state (Bellingham and Ireland 2002), most obstructive-sleep-apnea events occur during PS; and it is the most antiepileptic state in the sleep-wake cycle (Shouse et al. 1989). The effect of PS deprivation will thus be of particular interest. The present study, therefore, examined and compared the effects of PS deprivation on oxidative stress parameters in the brain regions of adult and old rats with a view to delineate the age-related differences in the effects of PS deprivation.
Materials and methods
Animals
Sixty male Wistar rats of two age groups: 8 (body weight 480.6 ± 32.5 g) and 24 (body weight 572.7 ± 40 g) months were used in this study. These rats were obtained from our University Animal House where they are housed in standard polypropylene cages in temperature-controlled (23 ± 1°C) rooms with a 12:12 h light-dark cycle. Food (commercial rat feed) and water were provided ad libitum. The minimum and maximum life spans of rats in these animals house conditions are 28–30 months. Animals were carefully checked for their health status (Sharma et al. 1993).
Paradoxical sleep deprivation
Rats were deprived of PS by the classical platform (flower pot) procedure (Mendelson et al. 1974; Gulyani and Mallick 1993; D’Almeida et al. 1998; Andersen et al. 2004). Rats of 8 and 24 months of age were each subdivided into two subgroups: One subgroup of animals served as a group of home-cage control rats which remained in their home cages in the same room where PS deprivation was performed on experimental rats (Andersen et al. 2004, 2005). The second subgroup of animals served as experimental rats which were subjected to PS-deprivation. For PS deprivation, the animals were individually placed on a platform of 10 cm diameter (Andersen et al. 2004) in individual containers filled with water up to 1 cm below the platform surface. Whenever these animals pass into a PS phase, they tend to fall off the platform and wake up and then immediately climb up to the platform to sit on the platform. The food pellets and water bottles were provided on the grids located on the top of the containers. The animals stayed in their respective containers for 4 days (i.e. 96 h). In addition to the home-cage controls (as mentioned above), sometimes large platform controls, where the control animals are made to sit on large platform (14 cm diameter) placed in containers filled water up to 1 cm below the platform surface (D’Almeida et al. 1997, 1998) in the same manner as in case of experimental animals, are also used. It has, however been consistently found that control data obtained from large platform controls do not differ from those from home-cage controls (D’Almeida et al. 1998). We did carry out some pilot experiments by using the additional large platform controls, and had found that data derived from these did not differ from those derived from home-cage controls. This is why we only used home-cage controls as done in many previous studies (Andersen et al. 2004). All experimental protocols were approved by the Jawaharlal Nehru University, Institutional Animal Ethics Committee (IAEC).
Biochemical measurements (assays)
All chemicals, reagents used in these experiments were obtained from Sigma Chemical Company (USA).
Biochemical assays were performed to measure lipid peroxidation, superoxide dismutase activity, glutathione peroxidase activity and glutathione levels. In one set of experiments, these parameters were measured in whole brain homogenates, and in the other set, measurements were made in the following discrete brain regions: cerebral cortex, striatum (caudate-putamen), thalamus, hypothalamus, hippocampus and brain stem. The brain regions were located and identified according to the stereotaxic atlas of Paxinos and Watson (1982).
After PS deprivation (performed as described above), all animals (control and experimental) were killed by cervical dislocation and their brains were perfused to remove blood rapidly taken out and placed on ice cold plates. For biochemical analysis on the whole brain tissue, the whole brains were weighed and homogenized; and for biochemical measurements in brain regions, the brains were dissected to obtain the six regions named above. Each region was weighed and homogenized. The PS-deprived animals are known to show body weight loss. In our experiments also, we observed weight loss in PS-deprived animals, which was quantitatively similar to that described in the reported data by various workers (Andersen et al. 2004).
Lipid peroxidation (thiobarbituric acid reactive substances) was measured according to the method of Rehncrona et al. (1980) spectrophotometrically at 532 nm using a Shimadzu UV-260A spectrophotometer. The levels of lipid peroxides were expressed as nmol of malondialdehyde formed g−1 tissue.
Glutathione peroxidase activity was assayed according to the method of Flohe and Gunzler (1984) by following the NADPH oxidation at 340 nm in the presence of H2O2, reduced glutathione and glutathione reductase using a Shimadzu UV-260A spectrophotometer. The specific activity was expressed as μ mol of NADPH oxidized min−1 mg−1 protein.
Superoxide dismutase (SOD) activity was measured according to the method of Marklund and Marklund (1974). The assay was based on the ability of the enzyme to inhibit auto-oxidation of pyrogallol. Stock solution of pyrogallol was prepared in 10 mM HCl. One unit of the enzyme was defined as the amount of SOD required for producing a half maximal inhibition of autooxidation. Absorbance was measured for 5 min at 420 nm, and expressed as units min−1 mg−1 protein.
Glutathione content (total GSH) of each brain region was measured according to the method of Moron et al. (1979). Assays were done in the presence of 0.3 M Na2HPO4 and 5,5′ dithiobisnitrobenzoic acid (DTNB) (D’Almeida et al. 1998). Absorbance was measured spectrophotometrically at 412 nm (Shimadzu UV-260A spectrophotometer) and expressed as nmol gm−1 tissue.
Statistics
Data were evaluated by using Student’s t-test by comparing values of 8- and 24-month-old paradoxical sleep deprived rat brain regions with those from age-matched controls.
Results
Measurement of changes in oxidative stress parameters in brain regions of PS-deprived rats of adult (8 months) and old (24 months) age groups
Lipid peroxidation
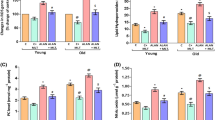
In PS-deprived rats of both age groups, lipid peroxidation levels were reduced in the cortex and brain stem, but were elevated in the hippocampus, thalamus and hypothalamus (Fig. 1) in comparison with controls. PS deprivation did not affect lipid peroxidation levels in the striatum of both adult and old animals.
Effect of paradoxical sleep deprivation on oxidative stress parameters: lipid peroxidation (a), superoxide dismutase activity (b), glutathione peroxidase activity (c), and glutathione levels (d) in various brain regions (Cx—cortex; BS—Brain stem; ST—Striatum; HI—hippocampus; TH—thalamus and HY—hypothalamus) of 8- (A–D) and 24-month-old (A’–D’) rats. Each bar is a mean (+SEM) of 5–6 animals. Significantly different from control group at * P < 0.001, ** P < 0.01, *** P < 0.05 (abbreviations and statistical marks are the same in the subsequent figures)
Percentage comparison of regional changes in the lipid peroxidation levels of adult animals with those of old ones (Fig. 1) showed that in the cortex and brain stem of adult animals, PS-deprivation reduced the lipid peroxidation levels by 38% (P < 0.001) and 56% (P < 0.001) respectively. In the old age group animals, however, the levels were reduced by 20% (P < 0.001) and 21% (P < 0.001) respectively. In the hippocampus, thalamus and hypothalamus of PS-deprived adult animals, the elevation in lipid peroxidation levels was 27% (P < 0.05), 18% (P < 0.05) and 32% (P < 0.05) respectively. In the old age group animals, the increases respectively were 16% (P < 0.05), 22% (P < 0.001) and 25% (P < 0.05).
The data also showed (Fig. 1) that lipid peroxidation was increased with ageing in various brain regions except thalamus irrespective of sleep deprivation condition, and the absolute values of regional lipid peroxidation levels in PS deprived animals were significantly higher in old animals than in adult animals.
Superoxide dismutase (SOD) activity
In PS-deprived rats of both age groups, the activity of SOD was increased in the cortex and brain stem, but was decreased in the hippocampus, thalamus and hypothalamus in comparison with controls (Fig. 1b). The enzyme activity was unaffected in the striatum.
Percentage comparison of changes in the enzyme activity of adult animals (Fig. 1b) with those of old animals showed that: in the cortex and brain stem of adult animals, PS deprivation-induced increases in the enzyme activity were higher (64%, P < 0.001 and 39%, P < 0.05) than those in old animals (40%, P < 0.05 and 11% P < 0.05 respectively). PS deprivation caused a much higher decrease in the enzyme activity of the hippocampus of old animals (32%, P < 0.05) than that of adult animals (6% P < 0.05). PS deprivation-induced decreases in the enzyme activity of the thalamus and hypothalamus was quantitatively similar in the adult (15%, P < 0.05; 22%, P < 0.05) and old (14% P < 0.05; 22% P < 0.05) animals.
The data also showed that the SOD activity was lower in the brain regions of old animals than in those of adult animals irrespective of sleep deprivation condition, and the absolute values of the enzyme activity in PS deprived animals were lower in the old age group than in the adult animals.
Glutathione peroxidase (GPx) activity
PS-deprivation did not affect this measure in any of the brain regions examined both in adult and old animals (Fig. 1c). It was, however, evident from the data that old animals had lower glutathione peroxidase activity in their brain regions than adult animals.
Glutathione
In PS-deprived rats of both the age groups (Fig. 1d), glutathione levels were unaffected in the cortex, brain stem and striatum, but were significantly decreased in the hippocampus, thalamus and hypothalamus in comparison to respective controls.
Percentage comparison of changes in the brain regions of the adult age group animals (Fig. 1d) with those of old age group animals showed that: in adult animals, glutathione levels were decreased by 13% in the hippocampus (P < 0.05), 7% (in the thalamus (P < 0.05) and 12% in the hypothalamus; in the old animals, however, the decrease in glutathione levels were greater: 20% in the hippocampus (P < 0.05), 17% in the thalamus, and 17% in the hypothalamus.
The data also make it apparent that glutathione levels were lower in old animals (both control and PS-deprived) than in adult animals (both control and PS-deprived).
Measurement of changes of oxidative stress parameters in the whole brain tissue
The whole brain homogenate from PS-deprived rats of both age groups (Fig. 2A, B, D) did not show changes in lipid peroxidation, SOD activity and glutathione peroxidase activity. The levels of glutathione were, however, found to be decreased in the brain of PS deprived rats of both age groups in comparison to the respective controls (Fig. 2C). The magnitude of the decrease was quantitatively different in the two age groups studied. Percentage comparison of the changes in the two ages groups showed the decrease to be greater in old animals (19% P < 0.01) than in adult ones (8% P < 0.05).
Discussion
It has been hypothesized that sleep loss affects different parts of the brain differently (Babkoff et al. 2005). In a previous PS deprivation study (D’Almeida et al. 1998), it was found that PS deprivation significantly reduced the glutathione levels only in the hypothalamus and thalamus suggesting that PS deprivation had a regionally differential effect on the oxidative stress in the brain. The results of our present study in which glutathione levels were measured in the brain regions of animals of two different age groups revealed that besides the hypothalamus and thalamus, PS deprivation reduced the glutathione levels also in the hippocampus. Our data furthermore showed that in old animals, PS deprivation produced a greater reduction in glutathione levels in these three brain regions in comparison with adult animals indicating that brain regions at older ages were more severely affected by PS deprivation. However, the point that in previous studies, the glutathione levels in the hippocampus were not found to be affected by PS deprivation needs consideration. It is of interest to note that the previous studies used rats of around 3 months of age (D’Almeida et al. 1997, 1998; Hippolide et al. 2002) that were significantly younger than the 8-month age group rats investigated in our study. It would thus appear that at 8 months of age and onwards PS deprivation causes glutathione levels to decrease also in the hippocampus, but at 3 months of age PS deprivation does not affect the glutathione levels in hippocampus. This indicated an age-related effect of PS deprivation on this brain region.
Our data showed that PS deprivation caused a reduction in lipid peroxidation in the cerebral cortex and brain stem of both adult and old animals. However, the reverse (i.e. rise in lipid peroxidation) occurred in the hippocampus, thalamus and hypothalamus. This showed that PS deprivation caused the oxidative stress to fall in the cerebral cortex and brain stem, but caused it to rise in the hippocampus, thalamus and hypothalamus. The reason why lipid peroxidation showed a fall in the said two areas may be the concomitant rise (in these areas) of SOD activity together with no reductions in the activity of both glutathione peroxidase and of glutathione. Similarly, the rise of lipid peroxidation in the hippocampus, thalamus and hypothalamus may be due to the concomitant fall (in these structures) of the activity of both SOD and glutathione. Our results would thus show that PS deprivation elevated the oxidative stress in the hippocampus, thalamus and hypothalamus but decreased it in the cerebral cortex and brain stem while it had no effect on the striatum.
PS deprivation elevates the activity of Na+,K+-ATPase (i.e. sodium pump) in brain synaptosomal fractions (Gulyani and Mallick 1993; Adya and Mallick 2000). The increase is mediated by PS deprivation-induced elevation in norepinephrine’s action Pal et al. (2005) on α1-receptors (Gulyani and Mallick 1995; Mallick et al. 2002). Increased norepinephrinergic activity reduces neuronal membrane lipid peroxidation (Schaefer et al. 1974; Mallick et al. 2002). Therefore, the PS-deprivation-induced decline in lipid peroxidation observed in some brain regions in the present study may be related to PS-deprivation induced elevation in brain norepinephrinergic activity. Furthermore, a decrease in lipid peroxidation is also associated with elevation in Na+,K+-ATPase activity (Kaur et al. 1998, 2001; Singh et al. 2006; Mattson 1998). Thus, PS-deprivation-induced increase in Na+,K+-ATPase activity (Gulyani and Mallick 1993) may have been due to PS-deprivation-induced fall in lipid peroxidation observed in the present study.
Concerning the age-related differences in PS deprivation-induced changes of lipid peroxidation and SOD, it is evident from the results that the cerebral cortex and brain stem of adult animals showed a greater decrease in lipid peroxidation than old animals. The increase in SOD activity in these two regions also was greater in adult animals than in old ones, showing thereby that in adult animals PS deprivation produced a greater reduction of oxidative stress in these brain regions than in old animals. This is, therefore, interesting to note that in the cerebral cortex and brain stem of old animals, PS deprivation produced a reduction in oxidative stress to a lesser degree than in adult animals. It is also worth noting that the basic levels of various parameters (i.e. lipid peroxidation, SOD) differed highly between adult and old animals due to ageing-related changes. This might give the impression that the absolute differences due to PS deprivation in the two age groups are not very much different from each other. It would however be evident from the data that the magnitudes of PS-deprivation-induced absolute differences in adult and in old animals determined by comparing the experimental values with the respective basic levels were highly significantly different.
Concerning the differences in the PS deprivation-induced elevation in the oxidative stress in the three brain regions: hippocampus, thalamus and hypothalamus of old and young animals, it is evident from the data that a consistent age-related effect was seen only with respect to the parameter glutathione which was more severely affected in 24-month-old rats than in 8 months old rats. The other two parameters (lipid peroxidation and SOD) did not show change in a consistent age-dependent manner. For example, in the hippocampus, PS deprivation induced an increase in lipid peroxidation by a higher degree in young animals than in older ones, but PS deprivation induced a decrease in SOD activity by a higher degree in old ones than in young ones. In the thalamus, however, both these parameters showed the same magnitude of (PS deprivation-induced) changes in the two age groups. In the hypothalamus, the PS deprivation-induced rise in lipid peroxidation was greater in adults than in aged ones and the same trend was seen in the fall of SOD activity.
Previous studies by D’Almeida et al. (1997) performed on the whole brain homogenate showed that PS deprivation did not affect lipid peroxidation, and SOD in the brain. We also obtained the same results when these parameters were assayed in the whole brain homogenate. Our data derived from the measurements made in discrete brain regions, however, showed that PS deprivation caused the lipid peroxidation to fall in some brain regions while in some other brain regions it caused the to rise. Similarly, the SOD activity was elevated in some regions whereas the activity was decreased in other regions. Thus, since in some regions these parameters rise while in other regions they simultaneously fall (i.e. the rise and fall occurring together), the measurements made in homogenates derived from the whole brain are most likely to show the parameters unaltered. Thus our data provide an explanation for the finding of the absence of oxidative stress following PS deprivation obtained in experiments performed on the whole brain homogenate. Recently, Kalonia and Kumar (2006) measured lipid peroxidation in the mice whole brain homogenate and showed that 48 h of total sleep deprivation (using disc over water method of insomnia (Shinomiya et al. 2003) caused lipid peroxidation to rise by 88%. This is of interest since PS deprivation fails to cause a detectable rise in lipid peroxidation (in whole brain homogenates) whereas total sleep deprivation produces an increase in lipid peroxidation.
It may sometimes be argued that results derived from sleep deprivation studies could be attributed to the stress caused by sleep deprivation procedure (or sleep loss) rather than to sleep deprivation phenomenon. The sleep deprivation procedure used in the present study involves some degree of stress for the animals (Andersen et al. 2005). Thus, the PS-deprived animals would experience an elevation in stress-related hormones (such as cortisol, adenocorticotropin) which are known to rise following sleep deprivation both in humans (Spiegel et al. 1999) and rats (Andersen et al. 2005). Sleep loss is thus likely to increase the severity of age-related changes which may be more pronounced in aged subjects (Spiegel et al. 1999). Sleep loss is known to potentiate some effects of ageing in old animals but has no effect on the same in young animals (Andersen et al. 2004). It can, thus be argued that all observed changes in the present study could have resulted from the effect of stress hormones. It will, however, be worthwhile to point out that changes produced by various types of stress do not necessarily match with those occurring after sleep deprivation. For example, there are significant differences between the effects of sleep deprivation and those of stress: shock and immobilization stress changes a glutathione level in the cortex but sleep deprivation does not (see D’Almeida et al. 1998); homocysteine levels increase after stress, but they decrease after sleep deprivation (Andersen et al. 2005). According to Andersen et al. (2004), the stress associated with sleep deprivation could be an important factor but does not by itself account for all the effects produced by sleep deprivation.
Sleep deprivation (loss of sleep) produces effects similar to those that occur during ageing (Spiegel et al. 1999; Prinz 2004). Sleep deprivation produces heterogeneous physiological effects in aged animals (Andersen et al. 2004), and it intensifies some ageing-related effects. For example, in aged animals PS deprivation potentiated the effects of ageing on high-density lipoproteins, low-density lipoproteins, and vitamin B12 in the blood (Andersen et al. 2004). Our present data on the brain clearly showed that PS deprivation produced: an elevation in lipid peroxidation; a decrease in superoxide dismutase activity and glutathione levels in certain brain regions indicating an accentuation of age-related oxidative stress effects. These results would also show that sleep deprivation causes changes in brain regions similar to those that occur in the course of ageing. At the same time it is also apparent from our results that PS deprivation more severely affected some old brain regions than the adult brain regions. For example, the decrease in glutathione levels was greater in the hippocampus, thalamus, and hypothalamus of old rats than in those of adult rats, indicating that PS deprivation intensified certain ageing-related effects.
Our finding that PS deprivation augments oxidative stress in several brain regions will be consistent with the notion that sleep deprivation augments the process of ageing or produces changes similar to ageing because oxidative stress contributes to the process of ageing. Furthermore, the enhancement of oxidative stress by sleep deprivation reflects the notion that sleep is antioxidative. In this regard it is noteworthy that brain oxidation (i.e. lower levels of oxidative stress that does not produce oxidative damage) which occur during wakefulness promote sleep induction probably by release of sleep-inducing neuromodulators (Ikeda et al. 2005). There is an accumulation of reactive oxygen species, nitric oxide, and increased glutamatergic activity during wakefulness. In the preoptic area of the hypothalamus which is a sleep regulatory center, there is an increased glutamatergic tone during wakefulness (Azuma et al. 1996). During sleep excitatory neuronal circuits may be inhibited to counteract reactive oxygen species-mediated toxicity (Ikeda et al. 2005). For example, oxidized glutathione which was found to enhance sleep (Honda et al. 1994) may promote sleep by inhibition of glutamatergic neurotransmission (Inoue et al. 1995). These results show how sleep may decrease oxidative stress and perform the function of neuronal detoxification at cellular level (Honda et al. 1994).
Sleep deprivation also has been shown to be a clinically effective antidepressant treatment as it improves the mood of depressed patients (Bjorvatn et al. 2002). Selective PS deprivation has also been found to have a marked antidepressant effect since people suffering from depression seem to have an overabundance of paradoxical sleep (Reynolds et al. 1993). These findings indicate that sleep deprivation can also have beneficial effects although why sleep deprivation should be beneficial is not clear. Our data showing that PS deprivation causes a reduction in the oxidative stress in the cerebral cortex and brain stem would seem to be an example of a beneficial biochemical effect of sleep deprivation. Reduction in oxidative stress in the cerebral cortex and brain stem could be associated with psychological benefits. However, the beneficial effect was confined to only two regions and other brain regions did not show this effect. The brain stem, hypothalamus and thalamus are some of the brain regions known to be intimately involved with sleep and its regulation (Ikeda et al. 2005). The latter two regions did not show PS-induced reduction in oxidative stress. It is known that oxidative stress impairs brain electrical activity. Thus, reduction of oxidative stress in brain stem and cortex would have beneficial effect on their electrical activity (Sharma et al. 1993). The augmentation of brain stem electrical activity may further potentiate cerebral cortex activity. Thus improved electrical activity (thus physiological activity) resulting from lowered oxidative stress could be the basis of a beneficial effect of sleep deprivation in depression.
References
Adya HVA, Mallick BN (2000) Uncompetitive stimulation of rat brain Na–K ATPase activity by rapid eye movement sleep deprivation. Neurochem Int 36:249–253
Andersen ML, Martins PJF, D’Almeida V, Santos RF, Bignotto M, Tufik S (2004) Effects of paradoxical sleep deprivation on blood parameters associated with cardiovascular risk in aged rats. Exp Gerontol 39:817–824
Andersen ML, Martins PJF, D’Almeida V, Bignotto M, Tufik S (2005) Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res 14:83–90
Azuma S, Kodama T, Honda K, Inoue S (1996) State dependent changes of extracellular glutamate in the medial preoptic area of freely behaving rats. Neurosci Lett 214:179–182
Babkoff H, Zukerman G, Fostick L, Ben-Artzi E (2005) Effect of diurnal rhythm and 24h of sleep deprivation on dichotic temporal order judgment. J Sleep Res 14:7–15
Bellingham MC, Ireland MF (2002) Contribution of cholinergic system to state-dependent modulation of respiratory control. Respir Physiol Neurobiol 131:135–144
Bjorvatn B, Gronli J, Hamre F, Sorensen E, Fiske E, Bjorkum AA, Portas CM, Ursin R (2002) Effects of sleep deprivation on extracellular serotonin in hippocampus and frontal cortex of the rat. Neuroscience 113:323–330
Campbell IG, Guinan MJ, Horowitz JM (2002) Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol 88:1073–1076
D’Almeida V, Hippolide DC, Azzalis LA, Lobo LL, Junqueira VBC, Tufik S (1997) Absence of oxidative stress following paradoxical sleep deprivation in rats. Neurosci Lett 235:25–28
D’Almeida V, Lobo LL, Hippolide DC, de Oliveira AC, Nobrega JN, Tufik S (1998) Sleep deprivation induces brain region-specific decrease in glutathione levels. Neuroreport 9:2853–2856
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. In: Packer P (ed) Methods of enzymology, vol 105. Academic Press, New York, pp 114–121
Gosselin A, Koninck JD, Campbell KB (2005) Total sleep deprivation and novelty processing: implications for frontal lobe functioning. Clin Neurophysiol 116:211–222
Gulyani S, Mallick BN (1993) Effect of rapid eye movement sleep deprivation on rat brain Na+,K+ ATPase activity. J Sleep Res 2:45–50
Gulyani S, Mallick BN (1995) Possible mechanism of REM sleep deprivation induced increase in Na–K ATPase activity. Neuroscience 64:255–260
Harrison Y, Horne JA, Rothwell A (2000) Prefrontal neurophysiological effects of sleep deprivation in young adults—a model for healthy ageing. Sleep 23:1067–1073
Hippolide DC, D’Almeida V, Raymond R, Tufik S, Nobrega JN (2002) Sleep deprivation does not affect indices of necrosis or apoptosis in rat brain. Int J Neurosci 112:155–166
Honda K, Kamoda Y, Inoue S (1994) Oxidized glutathione regulates physiological sleep in unrestrained rats. Brain Res 636:253–258
Horne JA (1978) A review of the biological effects of total sleep deprivation in man. Biol Psychol 7:55–102
Ikeda M, Ikeda-Sagara M, Okada T, Clement P, Urade Y, Nagai T, Sugiyama T, Yoshioka T, Honda K, Inoue S (2005) Brain oxidation is an initial process in sleep induction. Neuroscience 130:1029–1040
Inoue S, Honda K, Komoda Y (1995) Sleep as neuronal detoxification and restitution. Behav Brain Res 69:91–96
Kalonia H, Kumar A (2006) Protective effect of BR-16A (polyherbal preparation) on the behavioral and biochemical alterations in sleep disturbed mice (grid suspended over water method). Ann Indian Acad Neurosci 13:94–98
Kaur J, Sharma D, Singh R (1998) Regional effects of ageing on Na+,K+-ATPase activity in rat brain and correlation with multiple unit action potentials and lipid peroxidation. Indian J Biochem Biophys 35:364–371
Kaur J, Sharma D, Singh R (2001) Acetyl-l-carnitine enhances Na+,K+-ATPAse glutathione-s-transferase and multiple unit activity and reduces lipid peroxidation and lipofuscin concentration in aged rat brain regions. Neurosci Lett 301:1–4
Kryger M, Monjan A, Bilwise D, Ancoli-Israel S (2004) Sleep, health, and aging—bridging the gap between science and clinical practice. Geriatrics 59:24–30
Magistretti PJ (2006) Neuron-glia metabolic coupling and plasticity. J Exp Biol 209:2304–2311
Mallick BN, Majumdar S, Faisal M, Yadav V, Madan V, Pal D (2002) Role of norepinephrine in the regulation of rapid eye movement sleep. J Biosci 27:539–551
Marklund S, Marklund G (1974) Involvement of the super oxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem 47:469–472
Mattson MP (1998) Modification of ion homeostasis by lipid peroxidation: roles in neuronal degeneration and adaptive plasticity. Trends Neurosci 21:53–57
Mendelson WB, Guttrie RD, Frederick G, Wyatt RJ (1974) The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav 2:553–556
Moron MA, Dipierre IW, Mannervick B (1979) Levels of glutathione, glutathione reductase and glutathione transferase activities in rat lung and liver. Biochem Biophy Acta 582:67–78
Pal D, Madan V, Mallick BN (2005) Neural mechanism of rapid eye movement sleep generation: cessation of locus coeruleus neurons is a necessity. Acta Physiol Sin 57:401–413
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, New York
Prinz PN (2004) Age impairments in sleep, metabolic and immune functions. Exp Gerontol 39:1739–1743
Ramanathan L, Gulyani S, Nienhuis R, Siegel JM (2002) Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brain stem. Neuroreport 13:1387–1390
Rehncrona S, Smith DS, Akesson B (1980) Peroxidative changes in brain cortical fatty acids and phospholipids as characterized during Fe2+ and ascorbic acid stimulated peroxidation in vitro. J Neurochem 34:1630–1638
Reimund E (1994) The free radical theory of sleep. Med Hypotheses 43:231–233
Reynolds CF, Hoch CC, Buysse DJ, Monk TH, Houck PR, Kupfer DJ (1993) REM sleep in successful, usual and pathological aging: the Pittsburg experience 1980–1993. J Sleep Res 2:203–210
Roy AK, Oh T, Rivera O, Mubiru J, Song CS, Chatterjee B (2002) Impact of transcriptional regulation on aging and senescence. Ageing Res Rev 1:367–380
Schaefer A, Seregi A, Komlos M (1974) Ascorbic acid-like effect of the soluble fraction of rat brain on adenosine triphosphatases and its relation to catecholamines and chelating agents. Biochem Pharmacol 23:2257–2271
Sharma D, Maurya AK, Singh R (1993) Age-related decline in multiple unit action potentials of CA3 region of rat hippocampus: correlation with lipid peroxidation and lipofuscin concentration and the effect of centrophenoxine. Neurobiol Aging 14:319–330
Shinomiya K, Shigemoto Y, Okuma C, Mio M, Kamei C (2003) Effects of short-acting hypnotics on sleep latency in rats placed on grid suspended over water. Eur J Pharmacol 460:139–144
Shouse MN, Siegel JM, Wu MF, Szymusiak R, Morrison AR (1989) Mechanism of seizure suppression during rapid-eye-movement (REM) sleep in cats. Brain Res 505:271–282
Singh R, Sharma D, Singh S, Kaur J (2006) Biochemical correlates of electrophysiological ageing of the brain. In: Mathur R (ed) Pain updated: mechanisms and effects. Anamaya Publishers, New Delhi, pp 116–125
Spiegel K, Leproult R, Van Cauter E (1999) Impact of sleep debt on metabolic and endocrine function. Lancet 354:1435–1439
Stickgold R, Hobson JA, Fosse R, Fosse M (2001) Sleep, learning and dreams: off-line memory processing. Science 294:1052–1057
Urrila AS, Hakkarainen A, Heikkinen S, Vuori K, Stenberg D, Hakkinen A-M, Lundbom N, Porkka-Heiskanen T (2004) Stimulus-induced brain lactate: effect of aging and prolonged wakefulness. J Sleep Res 13:111–119
Acknowledgments
We gratefully thank Prof. BN Mallick who offered many suggestions during the course of this work, critically read the manuscript, and provided several constructive suggestions. JK thanks JNU/UGC for the award of a Junior Research Fellowship. SS is grateful to CSIR (New Delhi) for the award of a Research Associateship and to Prof GN Verma for part of this work carried out during the tenure of her Research Associateship at Lucknow University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, R., Kiloung, J., Singh, S. et al. Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats. Biogerontology 9, 153–162 (2008). https://doi.org/10.1007/s10522-008-9124-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-008-9124-z