Abstract
Introduction
Obstructive sleep apnea (OSA) in children has been associated with systemic inflammation and oxidative stress. Limited evidence indicates that pediatric OSA is associated with oxidative stress and inflammation in the airway.
Objective
The objective of this study is to assess the hypothesis that levels of oxidative stress and inflammatory markers in the exhaled breath condensate (EBC) of children with OSA are higher than those of control subjects.
Methods
Participants were children with OSA and control subjects who underwent overnight polysomnography. Morning levels of hydrogen peroxide (H2O2) and sum of nitrite and nitrate (NO x ) in EBC of participants were measured.
Results
Twelve subjects with moderate-to-severe OSA (mean age ± standard deviation: 6.3 ± 1.7 years; apnea–hypopnea index—AHI, 13.6 ± 10.1 episodes/h), 22 subjects with mild OSA (6.7 ± 2.1 years; AHI, 2.8 ± 1 episodes/h) and 16 control participants (7.7 ± 2.4 years; AHI, 0.6 ± 0.3 episodes/h) were recruited. Children with moderate-to severe OSA had higher log-transformed H2O2 concentrations in EBC compared to subjects with mild OSA, or to control participants: 0.4 ± 1.1 versus −0.9 ± 1.3 (p = 0.015), or versus −1.2 ± 1.2 (p = 0.003), respectively. AHI and % sleep time with oxygen saturation of hemoglobin <95% were significant predictors of log-transformed H2O2 after adjustment by age and body mass index z score (p < 0.05). No significant differences were demonstrated between the three study groups in terms of EBC NO x levels.
Conclusions
Children with moderate-to-severe OSA have increased H2O2 levels in morning EBC, an indirect index of altered redox status in the respiratory tract.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past two decades, obstructive sleep apnea (OSA) in childhood has been recognized as a disorder related to metabolic, cardiovascular, and neurocognitive morbidity [1–3]. Increased upper airway resistance and pharyngeal collapsibility, mostly in children with adenotonsillar hypertrophy or obesity, result in intermittent pharyngeal airway collapse with concomitant decline in airflow, hypoxemia and hypercapnia, and brief arousals from sleep [4]. Repetitive cycles of hypoxia and reoxygenation during periods of airway obstruction and resumption of ventilation, promote systemic oxidative stress and inflammation [1, 5, 6]. Oxidative stress is defined as the predominance of oxidant-producing systems over antioxidant mechanisms resulting in excessive generation of reactive oxygen species (reactive oxygen metabolites) which predispose to morbidity from the cardiovascular and central nervous systems [7].
However, pediatric OSA is not merely an imbalance between mechanical forces accompanied by systemic maladaptive responses. Accumulating evidence indicates that similar to asthma in childhood, pediatric OSA is related to enhanced oxidative stress and inflammation in the airway [8–11]. In particular, augmented activity of leukotrienes in both the airway and the pharyngeal lymphoid tissue of sleep apneic children has been demonstrated by several studies [10, 12, 13] and a single investigation has also documented raised levels of 8-isoprostane in the exhaled breath condensate (EBC) [11].
The assessment of inflammatory and oxidant stress biomarkers in EBC is a growing research tool for the diagnosis and management of chronic respiratory disorders in adults and children [14–16]. The method is based on the assumption that concentrations of substances in EBC reflect the composition of the fluid layer which covers the bronchoalveolar epithelium [17]. The aim of the present study was to expand the limited available pediatric evidence which supports the relationship between OSA and oxidative stress or inflammation in the airway. It was hypothesized that levels of hydrogen peroxide (H2O2) and the sum of nitrites and nitrates (NO x ) (oxidative and inflammatory cell markers) in the EBC of children with OSA: (a) differ from those of control subjects and (b) are positively related to increasing OSA severity.
Patients and Methods
Participants
Consecutive children referred to the Sleep Disorders Laboratory for polysomnography due to OSA symptoms, with an age range of 4–14 years and an apnea–hypopnea index (AHI) > 1 episode/h were eligible for recruitment. Consecutive subjects of a similar age range, without history of habitual snoring, who were referred for polysomnography due to sleep problems (nightmares, restless sleep, enuresis) and had AHI ≤ 1 episode/h, participated as controls. Exclusion criteria for both patients and controls were: (a) symptoms or signs of acute respiratory tract infection; (b) a diagnosis of allergic rhinitis, asthma, or cystic fibrosis; (c) neuromuscular disorders or craniofacial abnormalities; and (d) current use of cysteinyl leukotriene receptor inhibitors, antihistamines, or nasal, inhaled or systemic corticosteroids. The study was approved by the Institutional Review Board of the Larissa University Hospital and informed consent for participation was obtained from parents.
Clinical evaluation and polysomnography
A detailed history was received from parents and physical examination was performed. Weight and standing height were measured and body mass index z score was calculated [18]. Obesity was defined as BMI z score >1.645 [19]. Size of tonsils was graded from 1+ to 4+ by direct inspection of the oropharynx [20]. Tonsils were considered enlarged if their size was greater than 2+.
Participants underwent overnight polysomnography in the Sleep Disorders Laboratory. The following signals were recorded: electroencephalogram (C3/M2, F4/M1, O1/M2, O2/M1); right and left oculogram; submental and tibial electromyogram; body position; electrocardiogram; thoracic and abdominal wall motion; oronasal airflow (three-pronged thermistor and nasal pressure transducer); and oxygen saturation of hemoglobin (SpO2). Arousals, sleep stages, and respiratory events were scored and polysomnography indices were defined according to the recent American Academy of Sleep Medicine Manual [21].
Outcome measures: H2O2 and NO x levels in the EBC
All children provided EBC samples between 07.00 and 08.00 in the morning after polysomnography. EBC was collected orally using the Ecoscreen condenser system with a mouthpiece (Viasys; Wurzburg, Germany) according to American Thoracic Society/European Respiratory Society Task Force recommendations [17]. Sample collection time was set at 10 min aiming to collect 1.5 ml of condensate. During the procedure, children were not crying, laughing or coughing. Samples were placed in plastic sterile tubes and were immediately stored at −80°C. Measurements of H2O2 and NO x were carried out within one month after the EBC sample collection.
Levels of H2O2 were determined in undiluted and non-concentrated EBC samples and in triplicate for each sample. A colorimetric assay based on the horseradish peroxidase-catalyzed oxidation of tetramethylbenzidine was used [22]. Briefly, 100 μL 3,3′,5,5′-tetramethylbenzidine and 10 μL horseradish peroxidase (Sigma Chemicals; St Louis, MO) were reacted with 100 μL of the EBC sample for 20 min at room temperature. Subsequently, the mixture was acidified to pH 1 with 10 μL sulphuric acid. The reaction product was measured spectrophotometrically at 450 nm using a microplate reader (BioTek Instruments; Winooski, VT). The lowest detection level of the assay was 0.1 μM.
EBC contains both nitrate (NO −3 ) and nitrite (NO −2 ). For the measurement of NO x (sum of nitrate and nitrite), each EBC sample was analyzed in triplicate. Initially, aliquots with EBC were incubated for 30 min at 37°C with nitrate reductase (10 mU) and nicotinamide adenine dinucleotide phosphohydrogenase (NADPH; 100 μM) to convert all nitrate contained in the EBC to nitrite. Subsequently, total nitrite concentration was assayed by the Griess reaction which converts nitrite into a deep purple azo compound [23]. Absorbance of the Griess reaction product was measured at 540 nm by a spectrophotometric plate reader. The lowest detection limit of the method was 2 μΜ.
Data analysis
To test our hypothesis three groups of participants were formed: (a) control subjects without snoring (AHI ≤ 1 episode/h); (b) children with mild OSA (AHI >1 and ≤ 5 episodes/h); and (c) subjects with moderate-to-severe OSA (AHI > 5 episodes/h). The three study groups were compared in terms of subjects’ characteristics, polysomnography indices, and H2O2 or NO x levels. H2O2 and NO x concentrations were log-transformed (natural logarithm) to approach a normal distribution.
One-way analysis of variance followed by post-hoc tests for pair comparisons (Bonferroni’s) was used for continuous variables, and χ 2 test (Yate’s correction) for categorical characteristics. Multiple linear regression analysis was applied to assess whether polysomnography indices (AHI or % sleep time with SpO2 < 95%), age or BMI z score were significant predictors of the oxidative stress and inflammatory markers levels in the EBC. Both AHI and% sleep time with SpO2 < 95% were normally distributed (Kolmogorov–Smirnov test; p > 0.05).
Results
Subjects’ characteristics and polysomnography findings
During the study period, a total of 50 children were offered participation to the study and parents of all subjects provided informed consent. The three groups of participants were similar in age, BMI z score and ratios of female-to-male gender and obese-to-nonobese subjects (p > 0.05; Table 1). Participants with mild OSA had significantly higher frequency of tonsillar hypertrophy relative to controls (p < 0.05) (Table 1). As expected, subjects with moderate-to-severe OSA had significantly worse polysomnography indices in comparison to children with mild OSA or control individuals (p < 0.01) (Table 1).
EBC levels of H2O2
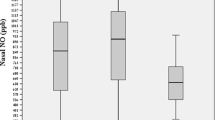
The three study groups were significantly different regarding log-transformed levels of H2O2 (p = 0.003; Table 1 and Fig. 1). Subjects with moderate-to-severe OSA had significantly higher log-transformed EBC concentrations of H2O2 compared to those with mild OSA (p = 0.015), or relative to controls (p = 0.003). The untransformed values of H2O2 for children with moderate-to-severe OSA, mild OSA and controls were: 2.4 ± 2, 0.8 ± 0.9 and 0.6 ± 0.6 μΜ, respectively. In linear regression analysis, both AHI and % sleep time with SpO2 < 95% were significant predictors of log-transformed H2O2 EBC levels after adjustment for age and BMI z score (p < 0.05; Table 2).
Log-transformed (natural logarithm) hydrogen peroxide (H2O2) levels in the exhaled breath condensate of 50 children without and with obstructive sleep apnea (OSA). Subjects with moderate-to-severe OSA (apnea–hypopnea index >5 episodes/h) had significantly higher H2O2 concentrations compared to children with mild OSA (apnea–hypopnea index >1 and ≤5 episodes/h; p = 0.015) or compared to children without OSA (apnea–hypopnea index ≤ 1 episode/h; p = 0.003)
EBC levels of NO x
Children with moderate-to-severe OSA did not differ significantly in log-transformed EBC concentrations of NO x when compared to those with mild OSA (p = 0.06), or to controls (p = 1.00; Table 1). The untransformed values of NO x for children with moderate-to-severe OSA, mild OSA and for controls were: 17.5 ± 20.2, 8.8 ± 11.8, and 12.6 ± 10 μΜ, respectively. When multivariable analysis was carried out, neither AHI nor % sleep time with SpO2<95% had significant effects on log-transformed NO x EBC levels despite adjustment for age and BMI z score (p > 0.05). Log-transformed NO x EBC levels were not associated significantly with BMI z score (p > 0.05).
Discussion
In the present investigation, higher H2O2 EBC levels were demonstrated in children with OSA compared to control participants. This finding is indicative of increased oxidative stress in the airway of sleep apneic children. In addition, a positive correlation between % sleep time with SpO2 < 95% and H2O2 concentrations was identified, implying an association of nocturnal hypoxemia with oxidative stress in the airway. In contrast, no difference was documented between the three study groups regarding NO x concentrations in the EBC.
It should be acknowledged that no therapeutic intervention for OSA was part of this investigation and hence it cannot be claimed that the demonstrated association between H2O2 levels and severity of OSA is causal. Nevertheless, higher H2O2 EBC concentrations have been found in adults with OSA relative to control subjects [24]. Moreover, results of the current investigation are in agreement with findings of the study by Biltagi et al., in which 8-isoprostane EBC concentration, a surrogate marker of oxidative stress in the airway, was higher in children with OSA than in healthy subjects and increased in parallel to OSA severity [11]. Isoprostanes are products of the lipid peroxidation of arachidonic acid by oxygen-free radicals (reactive oxygen metabolites) [25].
Superoxide radical (O · −2 ) and H2O2 are some of the most important reactive oxygen metabolites that can lead to generation of the highly reactive hydroxyl radical and the subsequent oxidation of biologic substrates [26]. In healthy subjects, the mitochondrial respiratory chain is a major source of O · −2 and oxidative stress. Regularly, the production of free radicals is counterbalanced by the antioxidant systems, including amongst others, superoxide dismutase which converts O · −2 to H2O2, and catalase that transforms H2O2 into water. Apart from the respiratory chain, O · −2 is produced by neutrophils, monocytes and macrophages against invading micro-organisms (respiratory burst) and by vascular cells via the action of NADPH oxidase [27].
Studies in adults with OSA reveal that neutrophils from the systemic circulation are primed for enhanced respiratory burst and O · −2 release [28]. Since increased numbers of neutrophils have been found in induced sputum of children and adults with OSA, it can be speculated that abrupt changes in alveolar partial oxygen pressure accompanying episodes of obstructive apnea or hypopnea induce oxidative stress [28–31]. Recruitment of neutrophils could be mediated by leukotrienes which are synthetized under the influence of oxidative stress [32, 33]. It should be noted, however, that raised H2O2 concentrations have been reported in EBC from children with common cold, asthma, allergic rhinitis and cystic fibrosis [34], and for this reason subjects with such conditions have been specifically excluded from the present study.
NO x concentration in EBC of children with OSA did not differ from that in healthy subjects. Nitrites and nitrates are stable end-products of the nitric oxide (NO) metabolism and their levels reflect activity of inducible nitric oxide synthetase expressed by neutrophils, eosinophils, and other inflammatory cells in the respiratory tract [35]. A single pediatric study and one investigation in adults have shown significantly higher morning exhaled NO concentration in overweight subjects with OSA than in overweight and normal-weight control participants [24, 36]. Nevertheless, three other investigations in adults have not identified differences in terms of exhaled NO between obese patients with OSA and obese control subjects [37–39]. The limited number of participants may be another factor explaining lack of significant differences among the three study groups regarding NO x concentration in EBC.
In summary, the current pediatric investigation provides preliminary evidence for an association of OSA with oxidative stress in the airway. Pediatric OSA is not only the result of an imbalance between mechanical forces, but it is also related to systemic and airway inflammation and possibly oxidative stress.
References
Gozal D, Capdevila OS, Kheirandish-Gozal L (2008) Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med 177:1142–1149
Amin R, Somers VK, McConnell K, Willging P, Myer C, Sherman M, McPhail G, Morgenthal A, Fenchel M, Bean J, Kimball T, Daniels S (2008) Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension 51:84–91
Goodwin JL, Kaemingk KL, Fregosi RF, Rosen GM, Morgan WJ, Sherrill DL, Quan SF (2003) Clinical outcomes associated with sleep-disordered breathing in Caucasian and Hispanic children—the Tucson Children's Assessment of Sleep Apnea study (TuCASA). Sleep 26:587–591
Katz ES, D'Ambrosio CM (2008) Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 5:253–262
Suzuki YJ, Jain V, Park AM, Day RM (2006) Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med 40:1683–1692
Kaditis A, Alexopoulos E, Ntamagka G, Chaidas K, Karathanasi A, Gougoura S, Papathanasiou AA, Liakos P, Zintzaras E, Gourgoulianis K (2010) Serum nitrite and nitrate levels in children with obstructive sleep-disordered breathing. Sleep Med 11:258–62
Lavie L (2009) Oxidative stress—a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis 51:303–312
Zanconato S, Carraro S, Corradi M, Alinovi R, Pasquale MF, Piacentini G, Zacchello F, Baraldi E (2004) Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. J Allergy Clin Immunol 113:257–263
Dut R, Dizdar EA, Birben E, Sackesen C, Soyer OU, Besler T, Kalayci O (2008) Oxidative stress and its determinants in the airways of children with asthma. Allergy 63:1605–1609
Goldbart AD, Krishna J, Li RC, Serpero LD, Gozal D (2006) Inflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndrome. Chest 130:143–148
Biltagi MA, Maguid MA, Ghafar MA, Farid E (2008) Correlation of 8-isoprostane, interleukin-6 and cardiac functions with clinical score in childhood obstructive sleep apnoea. Acta Paediatr 97:1397–1405
Kaditis AG, Ioannou MG, Chaidas K, Alexopoulos EI, Apostolidou M, Apostolidis T, Koukoulis G, Gourgoulianis K (2008) Cysteinyl leukotriene receptors are expressed by tonsillar T cells of children with obstructive sleep apnea. Chest 134:324–331
Goldbart AD, Goldman JL, Veling MC, Gozal D (2005) Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med 172:364–370
Rosias PP, Dompeling E, Dentener MA, Pennings HJ, Hendriks HJ, Van Iersel MP, Jobsis Q (2004) Childhood asthma: exhaled markers of airway inflammation, asthma control score, and lung function tests. Pediatr Pulmonol 38:107–114
Robroeks CM, Rosias PP, van Vliet D, Jobsis Q, Yntema JB, Brackel HJ, Damoiseaux JG, den Hartog GM, Wodzig WK, Dompeling E (2008) Biomarkers in exhaled breath condensate indicate presence and severity of cystic fibrosis in children. Pediatr Allergy Immunol 19:652–659
Kostikas K, Koutsokera A, Papiris S, Gourgoulianis KI, Loukides S (2008) Exhaled breath condensate in patients with asthma: implications for application in clinical practice. Clin Exp Allergy 38:557–565
Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, Becher G, van Beurden WJ, Corradi M, Dekhuijzen R, Dweik RA, Dwyer T, Effros R, Erzurum S, Gaston B, Gessner C, Greening A, Ho LP, Hohlfeld J, Jobsis Q, Laskowski D, Loukides S, Marlin D, Montuschi P, Olin AC, Redington AE, Reinhold P, van Rensen EL, Rubinstein I, Silkoff P, Toren K, Vass G, Vogelberg C, Wirtz H (2005) Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J 26:523–548
Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL (2002) Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL (2002) 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11:1–190
Brodsky L (1989) Modern assessment of tonsils and adenoids. Pediatr Clin North Am 36:1551–1569
Iber C, Ancoli-Israel S, Chesson A, Quan SF, American Academy of Sleep Medicine (2007) The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st edn. American Academy of Sleep Medicine, Westchester, Illinois
Gallati H, Pracht I (1985) Horseradish peroxidase: kinetic studies and optimization of peroxidase activity determination using the substrates H2O2 and 3,3',5,5'-tetramethylbenzidine. J Clin Chem Clin Biochem 23:453–460
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Petrosyan M, Perraki E, Simoes D, Koutsourelakis I, Vagiakis E, Roussos C, Gratziou C (2008) Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath 12:207–215
Folco G, Murphy RC (2006) Eicosanoid transcellular biosynthesis: from cell–cell interactions to in vivo tissue responses. Pharmacol Rev 58:375–388
Conner EM, Grisham MB (1996) Inflammation, free radicals, and antioxidants. Nutrition 12:274–277
Lavie L (2003) Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7:35–51
Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F (2000) Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 162:566–570
Sanidas D, Garnham A, Mian R (2000) Activation of human leukocytes by acute hypoxia. Exp Physiol 85:263–266
Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM (2005) Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172:915–920
Li AM, Hung E, Tsang T, Yin J, So HK, Wong E, Fok TF, Ng PC (2007) Induced sputum inflammatory measures correlate with disease severity in children with obstructive sleep apnoea. Thorax 62:75–79
Jakobsson PJ, Steinhilber D, Odlander B, Radmark O, Claesson HE, Samuelsson B (1992) On the expression and regulation of 5-lipoxygenase in human lymphocytes. Proc Natl Acad Sci USA 89:3521–3525
Steiner DR, Gonzalez NC, Wood JG (2001) Leukotriene B(4) promotes reactive oxidant generation and leukocyte adherence during acute hypoxia. J Appl Physiol 91:1160–1167
Liu J, Thomas PS (2005) Exhaled breath condensate as a method of sampling airway nitric oxide and other markers of inflammation. Med Sci Monit 11:MT53–MT62
Gaston B, Drazen JM, Loscalzo J, Stamler JS (1994) The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 149:538–551
Verhulst SL, Aerts L, Jacobs S, Schrauwen N, Haentjens D, Claes R, Vaerenberg H, Van Gaal LF, De Backer WA, Desager KN (2008) Sleep-disordered breathing, obesity, and airway inflammation in children and adolescents. Chest 134:1169–1175
Carpagnano GE, Spanevello A, Sabato R, Depalo A, Turchiarelli V, Foschino Barbaro MP (2008) Exhaled pH, exhaled nitric oxide, and induced sputum cellularity in obese patients with obstructive sleep apnea syndrome. Transl Res 151:45–50
Olopade CO, Christon JA, Zakkar M, Hua C, Swedler WI, Scheff PA, Rubinstein I (1997) Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest 111:1500–1504
Agusti AG, Barbe F, Togores B (1999) Exhaled nitric oxide in patients with sleep apnea. Sleep 22:231–235
Acknowledgement
This work was funded by the University of Thessaly Research Committee.
Conflict of interest
None of the authors has any conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malakasioti, G., Alexopoulos, E., Befani, C. et al. Oxidative stress and inflammatory markers in the exhaled breath condensate of children with OSA. Sleep Breath 16, 703–708 (2012). https://doi.org/10.1007/s11325-011-0560-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-011-0560-7