Abstract
Objective
To assess the clinical significance of nasal nitric oxide (nNO) and fractional exhaled nitric oxide (FeNO) concentrations in children with sleep-disordered breathing (SDB).
Methods
Enrolled in this study were 30 children with SDB and 15 healthy children. The nNO and FeNO concentrations were measured noninvasively using a NIOX MINO system (Aerocrine AB, Solna, Sweden). SPSS statistics 20.0 software (IBM SPSS statistics 20.0, Armonk, NY, USA) was used to analyze the data.
Results
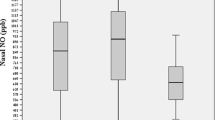
The median (25th and 75th percentiles) nNO concentration of SDB children measured in parts per billion (ppb) was 111.0 (44.0; 349.0) ppb; FeNO concentration of SDB children was 12.0 (9.8; 14.0) ppb. The nNO concentration of healthy children was 52.0 (22.0; 139.0) ppb; FeNO concentration of healthy children was 12.0 (10.0; 16.0) ppb. Compared to healthy children, nNO concentration was significantly higher in children with SDB (Z = −2.215, P = 0.027). Correlation analysis showed that SDB children’s nNO concentration directly correlated with apnea–hypopnea index (AHI; r = 0.429, P = 0.018), and inversely correlated with nadir oxygen saturation (SaO2; r = −0.482, P = 0.007). No other polysomnographic parameters significantly correlated with nNO concentration.
Conclusion
Our data suggest that nNO concentration might be useful for diagnosis and evaluation of disease severity in SDB children. Furthermore, these results suggest that nNO concentration has a greater prognostic value than FeNO concentration.
Zusammenfassung
Ziel
Ziel war die Bestimmung der klinischen Bedeutung der nasalen Stickstoffmonoxid- („nasal nitric oxide“, nNO) und der fraktionierten exhalierten Stickstoffmonoxidkonzentration („fractional exhaled nitric oxide“, FeNO) bei Kindern mit schlafbezogenen Atemstörungen (SBAS).
Methoden
An der Studie nahmen 30 Kinder mit SBAS und 15 gesunde Kinder teil. Die nNO- und die FeNO-Konzentration wurden nichtinvasiv unter Einsatz des Systems NIOX MINO® (Fa. Aerocrine AB, Solna, Schweden) gemessen. Für die Auswertung der Daten wurden die Software SPSS Statistics 20.0 (IBM SPSS Statistics 20.0, Armonk/NY, USA) verwendet.
Ergebnisse
Die nNO-Konzentration der Kinder mit SBAS lag bei 111,0 (44,0; 349,0) ppb („parts per billion“, 10-9) im Median (25. und 75. Perzentile). Die FeNO-Konzentration der Kinder mit SBAS betrug 12,0 (9,8; 14,0) ppb. Dagegen lag die nNO-Konzentration der gesunden Kinder bei 52,0 (22,0; 139,0) ppb. Die FeNO-Konzentration der gesunden Kinder betrug 12,0 (10,0; 16,0) ppb. Im Vergleich zu gesunden Kindern war die nNO-Konzentration bei Kindern mit SBAS signifikant höher (z =−2,215; p =0,027). Die Korrelationsanalyse zeigte, dass die nNO-Konzentration der Kinder mit SBAS direkt mit dem Apnoe-Hypopnoe-Index (AHI) korreliert war (r =0,429; p =0,018), eine umgekehrte Korrelation bestand mit dem Nadir der arteriellen Sauerstoffsättigung (SaO2; r =−0,482; p =0,007). Jedoch war die nNO-Konzentration nicht signifikant mit anderen polysomnographischen Parametern korreliert.
Schlussfolgerung
Den vorliegenden Daten zufolge ist die nNO-Konzentration möglicherweise nützlich für die Diagnosestellung und die Beurteilung des Schweregrads bei Kindern mit SBAS. Darüber hinaus ist nach den vorliegenden Ergebnissen die nNO-Konzentration dabei von höherer prognostischer Aussagekraft als die FeNO-Konzentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Airway inflammation resulting from activation of mast cells and antigen-specific type 2 T‑helper cells (Th2 cells) results in production of cytokines, including interleukins (IL)-4, IL-5, and IL-13. In turn, release of these factors causes upregulation of epithelial inducible nitric oxide synthase (iNOS) expression and increased NO generation [1]. Measurements of fractional exhaled (Fe)NO and nasal (n)NO provide a simple, rapid, objective, reliable, and noninvasive method for monitoring airway inflammation. These may provide information on the primary location and extent of airway inflammation, to help guide the management of obstructive sleep apnea (OSA) syndrome [2, 3]. OSA is clinically significant sleep-disordered breathing (SDB; [4]). SDB is the most common sleep-related disorder and is characterized by snoring (to different extents) when asleep, sleeping with an open mouth, an element of choking, heavy respiratory effort and sweating at night, restless sleep, frequent nocturnal awakening, daytime sleepiness, restless motor activity, inattention, poor school performance, and aggressive behavior. Prior studies evaluating nNO and FeNO concentrations in OSA patients have not been consistent [2–6]. While the abovementioned international clinical studies focused on adults, there has been limited research on children. The current study was designed to compare nNO and FeNO concentrations between SDB and healthy children, in order to assess the clinical significance of NO release in SDB children.
Materials and methods
Patient recruitment
The children treated in our department between January 2014 and January 2015 were initially selected for this study. We excluded patients < 60 months of age because they were too young to cooperate during the examination. All children were recorded during nocturnal sleep for a minimum of 7 h via video-polysomnography (Compumedics E‑series, Abbotsford, Victoria, Australia), which recorded basic polygraphic parameters allowing clinical polysomnography (PSG) evaluation. Sleep stage and arousal indices were scored manually in 30-second epochs and respiratory events were identified, in all cases according to the American Academy of Sleep Medicine (AASM) standard criteria [7]. The sizes of adenoid and tonsils were assessed via fiberoptic nasal endoscopy (Olympus, Shinjuku, Tokyo, Japan) and physical examination, respectively. Adenoid size was recorded based on fiberoptic nasal endoscopy with assessment of percentage choanal obstruction: 1+: 0 to 25 %; 2+: 26 to 50 %; 3+: 51 to 75 %; and 4+; 76 to 100 % [8]. Tonsil size was the percentage decrease in pharyngeal luminal diameter: 1+: 0 to 25 %; 2+: 26 to 50 %; 3+: 51 to 75 %; and 4+: 76 to 100 % [8]. Based on symptoms, physical examination, fiberoptic nasal endoscopy, and PSG findings, we selected 30 SDB children (24 males and 6 females, 61–163 months of age; Tab. 1). SDB was diagnosed based on an apnea–hypopnea index (AHI) ≥ 1 per hour (/h) in the absence of nasal inflammatory diseases [4].
As a control group, 15 healthy children matched for age and gender ratio (11 boys and 4 girls, 62–163 months of age) were recruited among the children of hospital staff during the same period. These healthy children had no clinical symptoms of disease. They did not have sleep-related disorders and they did not snore at night, sleep with an open mouth, or exhibit apnea. All controls were also subjected to physical examination, fiberoptic nasal endoscopy and PSG analysis. Their PSG findings indicated an AHI < 1/h of total sleep time (TST) and an obstructive apnea index (OAI) < 1/h (Tab. 1).
Exclusion criteria included chronic lung disease, central hypoventilation syndrome, immune deficiency disease, diabetes, tuberculosis, asthma, systemic metabolic storage diseases, morbid obesity, a history of upper and/or lower airway surgery, and respiratory infection within the 4 weeks preceding the study. There were no children who smoked actively and/or passively.
The study was approved by the Medical Ethics Committee of Guang Dong Women and Children’s Hospital. All parents or guardians provided informed consent.
Nitric oxide measurement
The nNO and FeNO concentrations were measured noninvasively using a NIOX MINO system (Aerocrine AB, Solna, Sweden) and were expressed as parts per billion (ppb; 1 ppb = 1/10−9). All measurements were conducted between 2:00 and 9:00 pm in a room maintained at 20–30 °C with relative humidity 20–60 %. The environment was clean and measurements were obtained away from windows (sources of dust and pollen) and volatile gases. Interruptions from mobile phones and other strong electromagnetic signals within 2 m were avoided. Subjects were instructed not to drink or eat anything during the 2 h prior to NO testing, and not to drink liquids containing caffeine for 24 h before the test. Subjects were also instructed not to eat foods rich in nitrogen, such as sausage, offal, lettuce, and spinach for 24 h before test. The children abstained from intensive physical activities on the day of the measurements.
Exhaled nitric oxide
Exhaled NO was measured using the online standardized single-breath technique. In the sitting position, children were asked to grasp the handle and mouthparts tightly and inhale heavily after a heavy exhalation. Thereafter, the children were instructed to slowly exhale at a constant flow of 50 ml/s for 6 s. The instrument then automatically determined FeNO concentrations.
Nasal nitric oxide
After resting for approximately 15 min, children were seated with their mouths closed. An olivary probe was placed on the right nostril and children continued to breathe normally. The instrument continuously pumped nasal gas into the sampling tube at a flow rate of 2 ml/s for 2 min. The instrument then analyzed the nNO concentrations automatically in the total sample.
Polysomnography measurement
All children were recorded during nocturnal sleep for a minimum of 7 h via video-PSG that recorded basic polygraphic parameters allowing clinical PSG evaluation. These parameters were the electrooculogram (EOG), submental and bilateral tibialis electromyograms (EMG), the electrocardiogram (EKG), oronasal air flow (measured using thermistors), thoracic and abdominal pneumograms (recorded using strain gauges), oxygen saturation (SaO2; pulseoxymetric data), and video recording under infrared light. Respiratory parameters were evaluated using standard techniques. Electroencephalogram (EEG) arousal indices were calculated using the AASM criteria [7]. Detailed video-PSG reports were obtained after all recordings had been independently evaluated by sleep experts.
Statistics
Statistical analyses were performed using SPSS software (IBM SPSS statistics 20.0, Armonk, NY, USA). Dataset distributions were assessed by the one-sample Kolmogorov–Smirnov test. Normally distributed data are expressed as mean ± standard deviation (SD) and non-normally distributed data by the median (25th and 75th percentiles). Continuous variables were compared by the independent samples student’s t‑test or the Mann–Whitney test, depending on distribution. Correlations analyses were obtained using Spearman’s rank correlation tests. Categorical variables were compared by the Kruskal–Wallis test. A p‑value < 0.05 was considered statistically significant.
Results
Nitric oxide concentrations
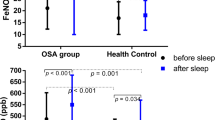
Children with SDB exhibited a higher nNO concentration than healthy children, while FeNO concentrations did not differ significantly (Tab. 2; Figs. 1 and 2). The two groups were well matched in terms of months of age (Z = −0.676, P = 0.499), gender ratio (Z = 0.016, P = 0.899), and body mass index (BMI; Z = −0.437, P = 0.662).
Relationship between nitric oxide concentration and polysomnographic parameters
Correlation analysis showed that nNO concentration directly correlated with AHI (r = 0.429, P = 0.018) and inversely correlated with nadir SaO2 (r = −0.482, P = 0.007; see Figs. 3 and 4). No significant correlation of nNO concentration with gender, months of age, height, weight, BMI, FeNO level, adenoid and tonsil size, total arousal index (ARtotI), respiratory arousal index (RAI), spontaneous arousal index (SAI), NREM sleep (1 stage, 2 stage, 3 stage), REM sleep, or percentage of TST with SpO2 < 90 % was found (P > 0.05).
FeNO concentrations of SDB children were similar to healthy children; therefore, no correlation analysis of FeNO concentrations was performed.
Discussion
NO is a molecule involved in oxidative stress and airway inflammation; reactive NO metabolites may cause tissue damage through oxidation and nitration [9]. The concentration of exhaled NO (eNO) is correlated with airway inflammation [10]. Orally exhaled gases contain NO derived from lower airways, while the NO in nasal exhalant (nNO) is produced mainly in the upper respiratory tract, predominantly by the sinuses and, to a lesser extent, the nasal mucosa [10]. Inflammation has been described to involve the upper airways, including the nose, the uvula, the soft palate, and the pharyngolaryngeal tract [2]. Airway inflammation in OSA is supposed to be the consequence of inflammatory cytokine release due to hypoxia and reperfusion cycles during episodes of apnea, or to snoring-related mechanical trauma [2]. Local upper airway inflammation promotes oropharyngeal inspiratory muscle dysfunction and a progressive local neurogenic lesion, thus amplifying upper airway narrowing and collapsibility, which can exacerbate SDB [11]. In the current study, children with SDB exhibited a higher nNO concentration than healthy children, and while SDB children’s nNO concentration directly correlated with AHI, it correlated inversely with nadir SaO2. Thus, nNO might be useful for SDB diagnosis and severity evaluation.
Olopade et al. [12] found that nNO concentrations in OSA patients were significantly higher than in healthy subjects. Culla et al. [2] showed that OSA patients had significantly increased oral NO (oNO) concentrations (measured soft mouthpiece) as compared to healthy subjects. Torretta et al. [3] selected adenoid hypertrophy in children with obstructive but without allergic symptoms, whose nNO concentrations were higher than healthy children and had a wide range. Our results were similar to those of above studies. We selected SDB children who had concomitant tonsil and/or adenoid hypertrophy. Nasal, tonsillar, and pharyngeal tissues are potential inflammation sites in patients with OSA [13]. Mechanical trauma and hypoxemia cause local pharyngeal inflammation [12]. Repetitive airway closing and opening during apneic episodes and nonspecific mucosal epithelial cell hyperactivity of hypertrophic adenoids and tonsils causes regulation of inflammatory cytokine expression and increased NO generation. In addition, intermittent hypoxia and reperfusion during repetitive episodes of nocturnal apnea, as well as in the process of ischemia/reperfusion injury, seemed to be responsible for the insurgence of airway inflammation and therefore for the generation of reactive oxygen species [14]. Low oxygen tension is an important trigger for activation of polymorphonuclear neutrophils, which adhere to the endothelium and release free oxygen radicals [14]. Therefore, there was local and systemic inflammation in our SDB children and their nNO concentrations were increased. Prior studies, such as those by Culla et al. [2] and Chua et al. [15], also found that FeNO concentrations in OSA adult patients with AHI > 5/h were significantly higher than in healthy subjects. However, in our study, the results showed that FeNO concentrations in SDB children were similar to those of healthy children, i.e., without an increasing tendency, which was not consistent with the above findings. In our study, SDB children’s median nadir SaO2 concentrations were 88.0 % (25th and 75th percentiles 84, 91.3), and their percentage of TST with SpO2 < 90 % was 0.2 (25th and 75th percentiles 0, 0.5). It was obvious that intermittent hypoxia and low oxygen tension in these children was milder; hence their whole body, particularly the lower airways, might be not exhibit the significant inflammation noted in adults.
In addition, Chua et al. [15] and Culla et al. [2] showed that FeNO positively correlated with AHI, and negatively correlated with nadir SpO2. At present, there are few studies on factors which influence nNO. In the current study, the FeNO concentration in SDB children was similar to that of healthy children, so no correlation analysis was performed for FeNO concentration; however, our SDB children’s nNO concentration was directly related to AHI and inversely related to nadir SaO2, and was not significantly correlated with any other PSG parameters. These findings are similar to those of Culla et al. (correlation between oNO and PSG parameters; [2]). As we know, AHI and nadir SpO2 were used in the diagnosis and severity evaluation of SDB [4]. From what has been discussed above, we may safely draw a conclusion that FeNO and nNO concentration has potential value for diagnosing SDB and evaluating SDB’s severity, and that nNO concentration is more prognostic than FeNO concentration in SDB children.
Berry and Gleeson [16] suggested that higher CO2 concentration, hypoxia, simple ventilation driving, and mechanical trauma were considered as arousal pathogenesis. Chua et al. [15] showed that FeNO was positively correlated with arousal index (AI). Their subjects were adults with a longer history and more severe symptom hypoxia, which led to exacerbated airway and systemic hypoxia, which could change sleep structure and respiratory rhythm. In our study, SDB children’s symptoms and hypoxia were milder than adults’; hence, their sleep structure may not exhibit the significant changes noted in adults. Therefore, SDB children’s nNO concentration was not significantly correlated with total AI (ARtotI), respiratory arousal index (RAI), spontaneous arousal index (SAI), NREM sleep (1 stage, 2 stage, 3 stage), REM sleep, or percentage of TST with SpO2 < 90 %. Tonsillar and adenoidal size might just reflect the severity of SDB from one aspect of the disease, but not reflect the inflammatory conditions, and these did not significantly correlate with nNO concentration.
This study had several shortcomings: SDB children’s FeNO and nNO concentrations were not monitored after treatment. Therefore, it was not possible to analyze the change in FeNO and nNO concentrations before and after treatment. It is difficult to thoroughly reveal the clinical significance of FeNO and nNO concentrations for diagnosis SDB and guiding therapeutic decisions.
References
Mahr TA, Malka J, Spahn JD (2013) Inflammoeometry in pediatric asthma: a review of fractional exhaled nitric oxide in clinical practice. Allergy Asthma Proc 34(3):210–219
Culla B, Guida G, Brussino L et al (2010) Increased oral nitric oxide in obstructive sleep apnoea. Respir Med 104:316–320
Torretta S, Bossi A, Capaccio P et al (2010) Nasal nitric oxide in children with adenoidal hypertrophy: A preliminary study. Int J Pediatr Otorhinolaryngol 74:689–693
Riva Tauman MD, Louise M et al (2004) Sleep pressure score: a new index of sleep disruption in snoring children. Sleep 27(2):274–278
Petrosyan M, Perraki E, Simoes D et al (2008) Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath 12:207–215
Foresi A, Leone C, Olivieri D, Cremona G (2007) Alveolar-derived exhaled nitric oxide is reduced in obstructive sleep apnea syndrome. Chest 132:860–867
Berry RB, Brooks R, Gamaldo CE et al (2012) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.0. American Academy of Sleep Medicine, Darien
Franco RA, Rosenfeld RM, Rao M (2000) First place-resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surgery 123:9–16
Bove PF, Van der Vliet A (2006) Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic Biol Med 41(4):515–527
Scadding G, Scadding GK (2009) Update on the use of nitric oxide as a noninvasive measure of airways inflammation. Rhinology 47(2):115–120
Friberg D, Ansved T, Borg K, Carlsson-Nordlander B, Larsson H, Svanborg E (1998) Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med 157(2):586–593
Olopade CO, Christon JA, Zakkar M et al (1997) Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest 111:1500–1504
Goldbart AD, Goldman JL, Li RC, Brittian KR, Tauman R, Gozal D (2004) Differential expression of cysteinyl leukotriene receptors 1 and 2 in tonsils of children with obstructive sleep apnea syndrome or recurrent infection. Chest 126:13–18
Depalo A, Carpagnano GE, Spanevello A et al (2008) Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med 263(1):70–78
Chua AP, Aboussouan LS, Minai OA et al (2013) Long-term continuous positive airway pressure therapy normalizes high exhaled nitric oxide levels in obstructive sleep apnea. J Clin Sleep Med 15;9(6):529–535
Berry RB, Gleeson K (1997) Respiratory arousal from sleep: mechanisms and significance. Sleep 20:654–675
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Huang, Y. Zou, F. Mai, X. Zhang, Y. Liu, and X. Lin state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Additional information
Redaktion
P.K. Plinkert, Heidelberg
B. Wollenberg, Lübeck
Yaping Huang and Yu Zou contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Huang, Y., Zou, Y., Mai, F. et al. Sleep-disordered breathing children. HNO 64, 169–174 (2016). https://doi.org/10.1007/s00106-016-0120-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00106-016-0120-3