Abstract
Calonectria leaf blight (CLB) caused by Calonectria pteridis is one of the main leaf diseases in Brazilian Eucalyptus plantations in warm climates with prolonged periods of rain. The main symptoms are leaf spots followed by intense defoliation in highly susceptible plants. Exploiting the existing inter- and intraspecific variability for defoliation is the best option to control this disease. Nevertheless, nothing is known about the genetic architecture of resistance to CLB in Eucalyptus. We built microsatellite-based genetic maps for E. urophylla × E. camaldulensis (EU11 × EC06) F1 family of 89 plants. Four or five clonal replicates per individual offspring were clonally propagated, totalling 445 plants which were phenotyped for defoliation in the basal third of the branches at 30 days following controlled inoculation with a single-spore isolate. Genetic mapping was performed using a pseudo-testcross, and QTLs detected using composite interval mapping. Five QTLs were detected for resistance to CLB; of them, only one could be validated in two unrelated pedigrees, and its effect was conservatively estimated as controlling between 5 and 10 % of the phenotypic variation when the bias derived from the limited size of the mapping population was taken into account. This work provides a starting point for future studies of the genetics of resistance to CLB, and adds further evidence to the challenge of ascertaining the effects of QTLs detected in a single biparental background across unrelated families.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calonectria leaf blight (CLB) caused by Calonectria pteridis Crous, M.J. Wingf. & Alfenas (=Cylindrocladium pteridis F.A. Wolf) is one of the main leaf diseases in Brazilian eucalypt plantations in warm climates with prolonged periods of rain (Alfenas et al. 2009). The first report of C. pteridis in eucalypts in Brazil occurred in 1995 in 1-year-old plants of E. grandis Hill ex Maid. in southeastern Bahia (Ferreira et al. 1995). Since then, C. pteridis has become one of the most common diseases in commercial plantations in northeastern and northern Brazil, occurring primarily on E. camaldulensis Dehnh., E. cloeziana F. Muell., E. grandis, E. saligna Smith, E. tereticornis Smith, E. urophylla S.T. Blake and the hybrid E. grandis × E. urophylla (“urograndis”) (Alfenas et al. 2009).
For most Eucalyptus species under field conditions, the disease is characterized by spots that are initially small, circular or elongated, and light-gray to light-brown but progress and extend throughout the leaf blade and cause blight and intense defoliation (Alfenas and Ferreira 1979). Intense defoliation caused by the fungus may decrease the tree growth rate due to a reduced photosynthetic area. Defoliation also allows for the increased entrance of light in the subcanopy, leading to increasing weed competition by the understory plants (Alfenas et al. 2009).
The use of resistant genotypes is the most effective and economical method to manage this disease in the field. The broad inter- and intraspecific variability for resistance to CLB, observed in seedling and clonal plantations of eucalypts, has allowed selection of resistant genotypes to reduce losses caused by the disease (Alfenas et al. 2009; Fonseca et al. 2010).
Nothing is known to date on the genetic architecture of resistance to CLB to better support long-term management and control of this increasingly important disease. The identification of QTLs associated with resistance to plant diseases could provide useful hints toward this end while opening avenues for marker-assisted selection. Large sets of microsatellite markers, extensive linkage maps, and QTL studies have been reported for species of Eucalyptus, reviewed by Grattapaglia et al. (2012). QTLs for disease resistance in Eucalyptus have been mapped and validated for Puccinia psidii (Alves et al. 2011; Junghans et al. 2003; Mamani et al. 2010; Rosado et al. 2010) and Teratosphaeria spp. (Freeman et al. 2008). Irrespective of the disease, a common trend across these studies has been the identification of some major-effect loci, explaining a relatively significant amount of the genetic variation in resistance.
This study inaugurates the investigation of the architecture of CLB resistance in Eucalyptus spp. by a QTL mapping approach. We constructed genetic maps using widely used, transferable microsatellite markers for an outbred interspecific F1 family segregating for resistance involving E. urophylla × E. camaldulensis (EU11 × EC06) cross. These maps were used to detect QTLs segregating for resistance to CLB caused by C. pteridis in this particular genetic background. To provide validation data for the mapped QTLs, we studied the co-segregation of the QTL-linked markers with the resistance phenotypes in four additional unrelated families.
Materials and methods
Plant material
QTL mapping was carried out in an outbred full-sib family of 89 individuals (“detection population”) derived from an interspecific cross between E. urophylla × E. camaldulensis (EU11 × EC06). QTL validation attempts were performed in samples of individuals, derived from four unrelated full-sib families (“validation populations”) generated by different interspecific crosses, also known to segregate for CLB resistance: (i) 9882 × 6021 [(E. grandis × E. urophylla) × E. grandis] n = 33, (ii) 9882 × 869 [(E. grandis × E. urophylla) × E. urophylla] n = 56, (iii) G26 × G27 (E. grandis) n = 17, and (iv) DG × UGL [(E. dunnii Maiden × E. grandis) × (E. urophylla × E. globulus Labill.)] n = 55.
To evaluate defoliation, five clonal replicates (ramets) of each individual offspring were employed except for the DG × UGL family, for which four replicates per individual were used. Five replicates of a resistant E. grandis clone (CR) and five replicates of a susceptible E. grandis × E. urophylla clone (CS), obtained from the clonal bank of “Clonar Resistência a Doenças Florestais” forest genetics company, were used as controls (checks) of inoculation. Sixty-day-old cuttings were transplanted to 6-L pots containing substrate (MecPlant®, Telêmaco Borba, Paraná, Brazil) enriched with Superphosphate (6.0 kg m−3) and Osmocote® (NPK 19:06:10 at 1.5 kg m−3). The plants were kept in a greenhouse and fertilized biweekly with 100 mL NPK solution (05:10:30 at 6 g L−1) per plant until a stage suitable for inoculation was reached as previously described (Graça et al. 2009).
Inoculation and disease evaluation
A single-spore Calonectria pteridis (LPF#059) isolate collected from among diseased plants in a commercial eucalypt plantation in Monte Dourado, Pará (Brazil) was used for the evaluation of resistance. The inoculum was produced as previously described (Alfenas et al. 2013). Ninety-day-old plants were homogeneously inoculated on both sides of the leaves by mist-spraying 200 mL/plant of inoculum suspension at 1 × 104 conidia/mL. After inoculation, the plants were incubated at 25 °C in a mist chamber for 48 h with an intermittent mist every 1 h for 10 s from 6:00 am to 6:00 pm and every 3 h for 10 s from 6:00 pm to 6:00 am and were subsequently transferred to the greenhouse. The plants were mist irrigated every 2 h with duration of 20 s from 6:00 am to 6:00 pm and every 3 h for 10 s from 6:00 pm to 6:00 am, until evaluation of disease severity. At 30 days after inoculation, the defoliation in the six basal branches of each plant was evaluated (Graça et al. 2009). For this evaluation, the number of leaves on six branches of the basal third of each plant was counted before inoculation and 30 days after inoculation to obtain the percentage (%) defoliation of each plant. A completely randomized design containing four or five replicates per individual offspring clone was employed. Each experimental unit consisted of a single plant.
Microsatellite genotyping
DNA of one replicate of each offspring was extracted as described in Grattapaglia and Sederoff (1994), quantified by NanoDrop (ND-1000 Spectrophotometer), and its concentration adjusted to 2 ng μL−1. Genotyping was performed as described earlier (Faria et al. 2011) in multiplexed systems with four to six co-amplified loci, each labeled with a specific fluorochrome (6-FAM, HEX, or NED) in an ABI 3100XL genetic analyzer (Life Technologies, Foster City, CA, USA). Data were collected using Data Collection and analyzed with GenScan and Genotyper softwares. Allelobin (Idury and Cardon 1997) were used to round up allele size estimates to the unit.
Linkage analyses and construction of parental maps
Markers segregating in a 1:1 ratio from each parent separately were identified based on a goodness-of-fit chi-square test at α ≤0.05. Markers that did not follow this expectation were excluded from further analysis. Genetic mapping was performed under a pseudo-testcross approach (Grattapaglia and Sederoff 1994) with two datasets, one with markers segregating from the male parent and a second one with markers segregating from the female parent. Fully informative markers with three or four alleles were included in both data sets. To allow for linkage detection between markers in repulsion phase, both datasets were doubled and recoded as described earlier (Grattapaglia and Sederoff 1994). Linkage analyses were performed using MapMaker (Lander et al. 1987). Linked markers were grouped with a threshold LOD score of 3.0 and a maximum recombination fraction (θ) of 0.40. The “first-order” and “compare” commands were then used to identify the most probable marker order within a linkage group. The “ripple” command was used to verify the log likelihood support for the local order. The recombination fractions were transformed to the estimated map distances using the Kosambi map function. Linkage group numbering followed the reference linkage map of Eucalyptus (Brondani et al. 2006) and were drawn using MapChart version 2.1 (Voorrips 2002).
QTL mapping
QTL mapping was carried out for each parental map separately using QTL Cartographer version 2.5 for Windows (Wang et al. 2006) under the backcross model by composite interval mapping. Significance thresholds to declare a QTL were determined via permutation tests (Churchill and Doerge 1994; Doerge and Churchill 1996) using 1000 permutations with three replicates and a significance level of 0.05. The size of the analysis window was maintained at 10 cM with a mapping resolution of 1 cM. Selection of cofactors (potential markers linked to QTLs) to be included as independent variables in a multiple regression model was carried out by a stepwise regression (forward method) using cofactors 0, 5, 8, 12, 15, and 18 under model 6 of QTL Cartographer.
QTL validation
Thirty-three microsatellite markers flanking the mapped QTLs in the detection population EU11 × EC06 were used to cary out a QTL validation attempt in samples of four unrelated families (9882 × 6021, 9882 × 869, G26 × G27, and DG × UGL). Mendelian segregation of each markers in each family was checked using a chi-square (χ 2) test at α ≤0.05. To determine the relative magnitude of the effect of each QTL-linked marker on the defoliation level, a linear regression analysis using an F test was performed, and a coefficient of determination (R2) estimated using GQMOL (Cruz 2008).
Results
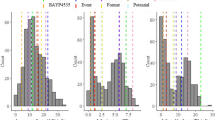
Typical disease symptoms were observed in all individuals following the seventh day after inoculation. The temperature in the mist-irrigation chamber varied from 11.5 to 37 °C (average 23.8 °C). In the greenhouse, the temperature ranged from 10 to 41.5 °C (average 27 °C). All five segregating families displayed a continuous frequency distribution for defoliation (Fig. 1). Family 9882 × 6021 had a larger number of susceptible individuals with defoliation above 50 %, while most individuals of family G26 × G27 were resistant (Fig. 1). The other families, including the QTL mapping family (EU11 × EC06), showed a broad phenotypic distribution. The average defoliation in the five families ranged from 42.2 % for G26 × G27 to 68.9 % for 9882 × 6021 (Table 1). Family EU11 × EC06 had a defoliation mean of 47.5 %, ranging from 3.2 % for the more resistant individuals to 81.4 % for the most susceptible ones. The checks CR and CS displayed a mean defoliation of 18.6 and 63.1 %, respectively (Table 1).
Frequency distribution of defoliation percentage in family a EU11 × EC06 (Eucalyptus urophylla × E. camaldulensis) and in validation families b 9882 × 6021 [(E. grandis × E. urophylla) × E. grandis], c G26 × G27 [E. grandis], d 9882 × 869 [(E. grandis × E. urophylla) × E. urophylla], and e DG × UGL [(E. dunnii × E. grandis) × (E. urophylla × E. globulus)] 30 days after inoculation with Calonectria pteridis under controlled conditions
Microsatellite marker data were grouped and ordered into 11 linkage groups (LG) for each parent of the detection population (Fig. 2). One hundred and thirty microsatellites were amplified, and 104 mapped with 60 fully informative markers, i.e., segregating from both parents. Five QTLs segregating for resistance to defoliation (Rd) were detected. Three of them were mapped in the genetic map of parent EU11, two (Rd1 and Rd2) located on LG1, and a third one (Rd4) on LG6. The other two QTLs were mapped for parent EC06, one on LG2 (Rd3) and one on LG8 (Rd5) (Fig. 2). Estimates of the phenotypic variation (R2) explained by each QTL ranged from 15 to 49 %. The maximum observed LOD score was 8.58 for Rd5, which also showed the greatest R2; while the lowest LOD, still significant, was observed for Rd1. The closest flanking markers to QTLs Rd1, Rd2, Rd3, Rd4, and Rd5 were EMBRA1562, EMBRA70, EG96, EN16, and EMBRA203/EMBRA197, respectively (Table 2).
Genetic map of parents EU11 (Eucalyptus urophylla) (gray) and EC06 (E. camaldulensis) (white) in Kosambi centiMorgans (left to the linkage groups) indicating the QTLs for resistance to CLB caused by Calonectria pteridis. The red bars indicate QTL that were detected in the parent EU11, and the green bars indicate QTL of the parent EC06
Out of the 33 microsatellite markers used to ascertain the effects of the mapped QTLs for CLB resistance in the four unrelated families, only four markers linked to the Rd2 QTL on LG 1 were significantly associated with defoliation level. Markers EMBRA2002 and EMBRA70 were found significant by an F test for parent 6021 (E. grandis), while markers EMBRA222 and EMBRA70 were significant for parent 869 (E. urophylla) (Table 3).
Discussion
This work provides the first insights into the genetic architecture of CLB in Eucalyptus species. Similarly to previous QTL mapping studies for fungal pathogens, only a few QTLs with relatively major effects were detected. Three QTLs (Rd1, Rd2, and Rd4) segregating from the E. urophylla female parent (EU11), explained 22, 15, and 17 % of the phenotypic variation, respectively; and two QTLs (Rd3 and Rd5) segregating from the E. camaldulensis male parent (EC06), explained 16 and 49 % of the phenotypic variation, respectively. Results for CLB resistance also indicate that both parents and therefore both species, contributed to the resistance to CLB in this particular offspring, highlighting the superiority of Eucalyptus hybrids provided by complementary effects derived from different species.
QTL mapping results for CLB are in line with previous studies in other pathosystems such as Eucalyptus × Teratosphaeria cryptica (previously known as Mycosphaerella cryptica), where Freeman et al. (2008) detected two QTLs that explained 52 % of the phenotypic variation, and Eucalyptus × Puccinia psidii, where Alves et al. (2011) detected QTLs explaining between 29.8 and 44.8 % of the phenotypic variation. Estimated effects of QTLs mapped for disease resistance in plants have typically ranged from a few percent to 50 % or more. Such QTLs accounting for a large proportion of the phenotypic variation are commonly referred to as main-effect QTLs or “major QTLs” (StClair 2010). Adopting this criterion, among the five QTLs identified in this work, two of them (Rd1 and Rd5) would be considered major QTLs. The presence of only a few QTLs explaining an apparently substantial part of the phenotypic variation suggests that the genetic control of resistance to CLB in eucalypts may involve some relatively major effect loci.
Nevertheless, the estimated proportions of phenotypic variation (R2) putatively explained by the mapped QTLs in this work should be viewed with caution as they are, most likely, considerably overestimated by the well known “Beavis effect”, given the relatively limited mapping population size (Beavis 1998). In the classic study by Beavis on “power, precision, and accuracy” of QTL mapping, he demonstrated that when sample sizes (n) were small, power to detect true QTL was low, and the bias in the estimated proportion of the genetic variance explained by mapped QTLs can be considerable. For example, if 10 true QTLs are segregating in a biparental mapping population of n = 100 individuals for a trait whose heritability is equal to 0.95, the bias on the estimates of the QTL effects can reach 200 %. Such simulation results were later validated in a formal theoretical framework (Xu 2003) as well as experimentally (Schon et al. 2004).
In our study, it is important to note that in spite of having used a mapping population of only 89 plants, a total of 445 plants were actually evaluated phenotypically. This protocol was meant to provide robust estimates of defoliation for each segregating offspring. In other words, we aimed at increasing heritability. Repeatability of phenotypic assessment for CLB defoliation in our experiment, an upper limit to heritability, was estimated at 0.80. Using information from Beavis’ simulations we therefore suggest that the estimates of phenotypic variance explained by the QTLs for CLB are probably upwardly biased by some 300 %, i.e., valid estimates are probably in the range of 5 to 10 % at most. Nevertheless, the robustness of the bioassay and the high trait heritability obtained thereof, provides solid evidence for the existence and genomic location of the QTLs detected in this particular segregating family. This resource-demanding bioassay for this complex pathosystem, involving the need to clonally propagate each segregating individual, puts a significant practical limit to the total number of plants that one can study. This has also been the case for several forest-tree mapping studies to date that had to rely on existing segregating populations given the age that trees had to reach to be adequately phenotyped for wood-quality traits (Neale and Kremer 2011).
A commonly used approach to add evidence in support for a detected QTL is by assessing the ability to detect it in unrelated families. This concept underlies the principle that validated QTLs could be used to carry out marker-assisted selection across families. The few validation experiments reported in trees, however, have had very modest success. A large-effect QTL (Ppr1), which confers resistance to rust, was successfully detected in three genetically unrelated full-sib families (Mamani et al. 2010). Out of five originally mapped QTLs for Mycosphaerella resistance in Eucalyptus, only two of them of larger effect were also detected in a second family and one in a third family (Freeman et al. 2008). QTL validation across families was also investigated for wood quality and growth traits. In Eucalyptus, out of 13 QTLs for wood properties, only three were validated in a second family (Thamarus et al. 2004); and more recently, only 16 % of the 98 QTLs mapped for growth and wood traits were detected when moving to different genetic backgrounds (Freeman et al. 2013). Similar results have been reported for conifers where only a small proportion of QTLs could be detected in unrelated families for different traits (Brown et al. 2003; Devey et al. 2004; Pelgas et al. 2011). Our results are therefore in line with all these previous reports. Among the five QTLs identified in the detection population EU11 × EC06, only one of them, Rd2, was also detected in two unrelated families involving different interspecific hybrids. All these results clearly point to the fact that a significant QTL by background interaction is the norm in such genetically heterogenous tree populations and that a different approach is needed if one intends to actually use this mapping information in breeding for resistance.
The suitability of predicting phenotypes using genome-wide markers across families in a breeding population was recently reported in a genomic selection experiment for wood properties and growth traits in Eucalyptus (Resende et al. 2012). This approach provides much more powerful and less biased QTL discovery when compared to biparental QTL mapping experiments. For one thing, QTL detection involved 738 individuals sampled in 43 different families for one population and 920 individuals of 75 families for the second population. Additionally, a whole-genome prediction approach was used instead of the standard QTL mapping method. By estimating marker effects in a much larger and representative “training” population, while avoiding prior marker selection based on stringent significance tests, genomic prediction captures a wider distribution of marker effects and thus a much larger proportion of the genetic variance for the target trait (Grattapaglia and Resende 2011). In fact genomic prediction was successful in predicting complex phenotypes controlled by several hundred loci across the multiple families within each population. When tested between the two different populations, however, genomic prediction had no appreciable accuracy, indicating that genomic selection models, although successful across families, will still be population-specific due to variable patterns of linkage disequilibrium, inconsistent allelic effects and genotype by environment interaction as one moves to populations outside the one used to train the prediction model.
In conclusion, this work provides a starting point for future studies of the underlying genetic architecture of resistance to CLB, an increasingly important disease in tropical eucalypts. The robust bioassay used, although laborious, provides strong repeatability for phenotype assessment and should therefore be very valuable in upcoming experiments with this disease. At least one major-effect QTL was detected and validated in unrelated pedigrees, whose effect was conservatively estimated as controlling between 5 and 10 % when the expected bias derived from the size of the mapping population was taken into account. The genomic regions corresponding to this QTL might merit further scrutiny if the objective is to discover and characterize disease resistance genes. More importantly, however, the validation results of our study add further evidence to the still largely unrecognized fact that QTLs mapped in single biparental pedigrees will be of limited value when it comes to actual use in marker-assisted selection across families. A whole-genome prediction approach using a large number of families genotyped for several hundred markers provides a much better prospect to develop robust marker-assisted selection methods usable at the population level.
References
Alfenas AC, Ferreira FA (1979) A mancha de folha do eucalipto no Brasil causada por três espécies de Cylindrocladium—Uma revisão da descrição da doença. Revista Árvore 3:47–56
Alfenas AC, Zauza EAV, Mafia RG, Assis TF (2009) Clonagem e doenças do eucalipto. 2 ed. Editora UFV, Viçosa
Alfenas RF, Pereira OL, Freitas RG, Freitas CS, Dita MAD, Alfenas AC (2013) Mass spore production of Calonectria pteridis on Eucalyptus spp. under different environmental conditions. Tropical Plant Pathology 38:406–413
Alves AA, Rosado CCG, Faria DA, Guimarães LMS, Lau D, Brommonschenkel SH, Grattapaglia D, Alfenas AC (2011) Genetic mapping provides evidence for the role of additive and non-additive QTLs in the response of inter-specific hybrids of Eucalyptus to Puccinia psidii rust infection. Euphytica 183(1):27–38
Beavis WD (1998) QTL analyses: power, precision, and accuracy. In: Paterson AH (ed) Molecular dissection of complex traits. CRC, Boca Raton, Florida, pp 145–162
Brondani RP, Williams ER, Brondani C, Grattapaglia D (2006) A microsatellite-based consensus linkage map for species of Eucalyptus and a novel set of 230 microsatellite markers for the genus. BMC Plant Biol 6:20
Brown GR, Bassoni DL, Gill GP, Fontana JR, Wheeler NC, Megraw RA, Davis MF, Sewell MM, Tuskan GA, Neale DB (2003) Identification of quantitative trait loci influencing wood property traits in loblolly pine (Pinus taeda L.). III. QTL verification and candidate gene mapping. Genetics 164:1537–1546
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138(3):963–971
Cruz CD (2008) GQMOL: Programa para análise de genética quantitativa molecular. Versão 2008.6.1. Disponível em: <http://www.ufv.br/dbg/gqmol/gqmol.htm>, Desenvolvido pelo setor de Genética da Universidade Federal de Viçosa
Devey ME, Groom KA, Nolan MF, Bell JC, Dudzinski MJ, Old KM, Matheson AC, Moran GF (2004) Detection and verification of quantitative trait loci for resistance to Dothistroma needle blight in Pinus radiata. Theor Appl Genet 108(6):1056–1063
Doerge RW, Churchill GA (1996) Permutation tests for multiple loci affecting a quantitative character. Genetics 142(1):285–294
Faria DA, Mamani EMC, Pappas GJ, Grattapaglia D (2011) Genotyping systems for Eucalyptus based on tetra-, penta-, and hexanucleotide repeat EST microsatellites and their use for individual fingerprinting and assignment tests. Tree Genetics & Genomes 7:63–77
Ferreira FA, Alfenas AC, Moreira AM, Demuner NL (1995) Mancha-de-pteridis doença foliar de eucalipto em áreas tropicais brasileiras. Fitopatol Bras 20:107–110
Fonseca SM, Resende MDV, Alfenas AC, Guimarães LMS, Assis TF, Grattapaglia D (2010) Manual Prático de Melhoramento Genético do Eucalipto. Editora UFV, Viçosa
Freeman JS, Potts BM, Vaillancourt RE (2008) Few Mendelian genes underlie the quantitative response of a forest tree, Eucalyptus globulus, to a natural fungal epidemic. Genetics 178(1):563–571
Freeman JS, Potts BM, Downes GM, Pilbeam D, Thavamanikumar S, Vaillancourt RE (2013) Stability of quantitative traits loci for growth and wood properties across multiple pedigrees and environments in Eucalyptus globulus. New Phytol 198:1121–1134
Graça RN, Alfenas AC, Maffia LA, Titon M, Alfenas RF, Lau D, Rocabado JMA (2009) Factors influencing infection of eucalyptus by Cylindrocladium pteridis. Plant Pathol 58(5):971–981
Grattapaglia D, Resende MDV (2011) Genomic selection in forest tree breeding. Tree Genet Genomes 7:241–255
Grattapaglia D, Sederoff R (1994) Genetic linkage maps of Eucalyptus grandis and Eucalyptus urophylla using a pseudo-testcross: mapping strategy and RAPD markers. Genetics 137:1121–1137
Grattapaglia D, Vaillancourt RE, Shepherd M, Thumma BR, Foley W, Kulheim C, Potts BM, Myburg AA (2012) Progress in Myrtaceae genetics and genomics: Eucalyptus as the pivotal genus. Tree Genetics & Genomes 8:463–508
Idury RM, Cardon LR (1997) A simple method for automated allele binning in microsatellite markers. Genome Res 7(11):1104–1109
Junghans DT, Alfenas AC, Brommonschenkel SH, Oda S, Mello EJ, Grattapaglia D (2003) Resistance to rust (Puccinia psidii Winter) in eucalyptus: mode of inheritance and mapping of a major gene with RAPD markers. Theor Appl Genet 108(1):175–180
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newberg LA (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1(2):174–181
Mamani EMC, Bueno NW, Faria DA, Guimarães LMS, Lau D, Alfenas AC, Grattapaglia D (2010) Positioning of the major locus for Puccinia psidii rust resistance (Ppr1) on the Eucalyptus reference map and its validation across unrelated pedigrees. Tree Genetics & Genomes 6(6):953–962
Neale DB, Kremer A (2011) Forest tree genomics: growing resources and applications. Nat Rev Genet 12(2):111–122
Pelgas B, Bousquet J, Meirmans PG, Ritland K, Isabel N (2011) QTL mapping in white spruce: gene maps and genomic regions underlying adaptive traits across pedigrees, years and environments. BMC Genomics 12:145
Resende MDV, Resende MFR, Sansaloni CP, Petroli CD, Missiaggia AA, Aguiar AM, Abad JM, Takahashi EK, Rosado AM, Faria DA, Pappas GJ, Kilian A, Grattapaglia D (2012) Genomic selection for growth and wood quality in Eucalyptus: capturing the missing heritability and accelerating breeding for complex traits in forest trees. New Phytol 194:116–128
Rosado TB, Tomaz RS, Ribeiro Junior MF, Rosado AM, Guimarães LMS, de Araújo EF, Alfenas AC, Cruz CD (2010) Detection of QTL associated with rust resistance using IBD-based methodologies in exogamic Eucalyptus spp. populations. Crop Breeding and Applied Biotechnology 10:321–328
Schon CC, Utz HF, Groh S, Truberg B, Openshaw S, Melchinger AE, Schön CC (2004) Quantitative trait locus mapping based on resampling in a vast maize testcross experiment and its relevance to quantitative genetics for complex traits. Genetics 167:485–498
StClair DA (2010) Quantitative disease resistance and quantitative resistance loci in breeding. Annu Rev Phytopathol 48:247–268
Thamarus KA, Groom K, Bradley A, Raymond CA, Schileck LR, Williams ER, Moran GF (2004) Identification of quantitative trait loci for wood and fiber properties in two full-sib progenies of Eucalyptus globulus. Theor Appl Genet 190:856–864
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93(1):77–78
Wang S, Basten CJ, Zeng ZB (2006) Windows QTL Cartographer 2.5. North Carolina State University, Raleigh, NC
Xu SZ (2003) Theoretical basis of the Beavis effect. Genetics 165:2259–2268
Acknowledgments
The authors thank Jari for the financial support and for encouraging the researchers to study this important disease. We also thank FAPEMIG and CNPq for the financial support and for the postdoctoral scholarships to LMSG and TGZ. We are also thankful to “Clonar Resistência a Doenças Florestais” for providing rooted cuttings of the susceptible and resistant plants, used as controls in the inoculation assays.
Data submission standards
http://dendrome.ucdavis.edu/treegenes/literature/literature_detail.php?id=26496
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Burdon
Rights and permissions
About this article
Cite this article
Zarpelon, T.G., da Silva Guimarães, L.M., Faria, D.A. et al. Genetic mapping and validation of QTLs associated with resistance to Calonectria leaf blight caused by Calonectria pteridis in Eucalyptus . Tree Genetics & Genomes 11, 803 (2015). https://doi.org/10.1007/s11295-014-0803-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-014-0803-4