Abstract
Arsenic (As) concentration was analyzed in four sediment-inhabiting animals (Pearsonia of Mollusca Gastropoda, Enchytraeus of Annelida Lumbricidae, Cybister japonicus Sharp of Hexapoda Dytiscidae and Chipangopaludina chinensis of Mollusca Gastropoda) from 19 sampling sites and eight carcasses of red-crowned crane (Grus japonensis) to examine As transfer along the typical food chain in Wuyur catchments, northeastern China. Results indicated that As concentration in the prey of the red-crowned cranes was elevated via the food chain. Geo-accumulation indices at all sites were less than 0, which suggests that this region contained background As concentration. The four aquatic animal families containing As were land snail < water snail < beetle < earthworm. The highest As concentration was found in the liver of the red-crowned cranes (145–441 ppb) followed by the kidneys (116–258 ppb) and muscles (36–94 ppb). The eggshells of red-crowned cranes contained relatively high As concentration, which varied from 35 to 235 ppb, whereas the feathers had the lowest concentration, with an average of 25 ppb. The dietary exposure level to As of the red-crowned crane population in Zhalong Wetland, Northeastern China was lower than the As toxicity threshold concentration. This study reported that eggshells are suitable indicators of As risk levels in red-crowned crane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive quantities of heavy metals, such as arsenic (As), and the mechanism by which these metals are transferred in aquatic biological systems have gained considerable attention as toxic metal accumulation in aquatic organisms increases with each succeeding step in the food chain. Although As is nutritionally essential or beneficial at low concentrations (EPA 1980; Abedin et al. 2002; Williams et al. 2007), its increasing levels cause toxicity to the environment and living organisms, resulting in growth inhibition (Gulz et al. 2005), liver and kidney damages, and death (Shaw et al. 2007). In aquatic environments, As binds to particulate matter and is deposited into sediments (EPA 1980). Nevertheless, some As fractions can be readily absorbed by aquatic plants (e.g., reed) or deposit-feeding benthic organisms and would be elevated into the higher strata of the food chain (Agah et al. 2009). Therefore, As distribution in sediments and As concentration in the bottom level of food chain must be determined to detect net change and assess potential toxic risk in aquatic systems.
Recent researches have shown that damages of metals in animal bodies are proportional to metal content in the environment (Martínez-Villegas et al. 2004; Burger et al. 2008). Environmentally sensitive aquatic benthic-feeding organisms (i.e., earthworm) (Klok et al. 2006; Roodbergen et al. 2008) and water birds are often employed to indicate the pollution levels of a metal-contaminated region (Fasola et al. 1998).
The red-crowned crane (Grus japonensis) is a rare species that has been near extinction since 2000, as indicated in the Red List of Endangered Species generated by the IUCN (BirdLife International 2012). Worldwide, its population is very small at 2750 mature individuals (BirdLife International 2012). Although the resident population in Japan remains stable, the migratory population in mainland Asia continuously declines because of loss and degradation of wetlands for agricultural and industrial development (Harris 2008). Zhalong National Nature Reserve (Zhalong Wetland), located in the downstream Wuyur River catchments, Northeastern China is one of the largest habitant and breeding sites for migratory red-crowned crane (Grus japonensis). Pesticides (e.g., lead arsenate) and As-containing fertilizers had been widely applied in the mid until late 20th century in the large area of arable land surrounding the wetland; these chemicals generated large amounts of heavy metals (such as As), resulting in increased metal accumulation and high probability of cranes to be in contact with these toxic chemicals. Our previous research reported measurable total As concentrations in the sediment (240–10500 ppb) and fish (0.44–28.16 ppb) in this region (Luo et al. 2015). Nevertheless, available As fraction in the sediments, which could be more correlated with As bioaccumulation, remains undefined. Moreover, As accumulation in sediment-inhabiting organisms has not been reported. These organisms (i.e., soil fauna, beetles, and snails) are important prey of red-crowned cranes and preferred environmental risk bio-indicators by most environmental researchers. Therefore, the degree of metal enrichment in the food chain must be determined to conserve red-crowned cranes.
This research aimed to examine As content in red-crowned crane and invertebrates at the bottom of the food chain in Zhalong wetland, China. Results would enrich our understanding of the ecological safety of the health of migratory red-crowned cranes and assist to maintain long-term sustainability of the crane population.
Material and methods
Study area
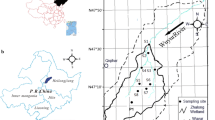
Zhalong Wetland (123°51′E–124°37′E, 46°48′N–47°32′N; the core area is approximately 700 km2 and the buffer zone occupies 1400 km2 lying in outside) is located in the main food production zone of Heilongjian Province in China (Fig. 1). River feeding and precipitation are the major sources of water in this inland reed marsh. The volume of runoff from upper reaches was abruptly decreased from 7.5 × 108 m3 per year in 1980s to less than 1 × 108 m3 per year in the 21st century. Various sludge and wasted water from the surrounding residential area, agricultural land, and industrial workshop containing several types of toxic contaminants, like As, were discharged directly into the wetland without complete disposal treatment. The wastewater discharge volume increased from 0.17 × 108 m3 in 1993 to 0.45 × 108 m3 in 2010. Increasing amount of pollution discharges elevated various toxic element concentrations not only in the sediment but also in the entire biota. In addition, extensive application of agrochemicals in the last century may increase probability of As dietary exposure to crane. Comparing to extensive reports about the essential elements, like Cu and Zn (Cui et al. 2014), and nonessential metals, mainly Pb, Cd and Hg in this crane’s dietary (Luo et al. 2013, 2014), the As content in the crane and on bottom of its food chain were still insufficient.
Location of Wuyur River catchments (a) and in-field sampling design (b, c). The empty area in (b) was the buffer zone of the wetland, and the black area for the core area. The section c is TM image (Band 4, resolution: 30 × 30 m) of the study site pictured in July 2006; the area enclosed by white line in imange c is Wuyur River Catchment, and area enclosed by bold white line is Zhalong Wetland
Sampling scheme
Nineteen sampling sites were designed for collection of reed (an aquatic plant of genus Phragmites australis (Cav.) Trin. ex Steud) and sediment-inhabiting organism. The first set of two sample sites (i.e., S1 and S2) were located in the upper reaches of Wuyur catchments, the second set of 10 sample sites (S3–S10, S15, and S16) were located in buffer zone A of the Zhalong marsh; the third set of 6 sample sites (S11–S14, S17, and S18) were located in the core area of the wetland, and the remaining one sample (i.e., S19) was designed in buffer zone B of the wetland (Fig. 1c). Four sediment-inhabiting organisms, including two soil fauna groups, i.e., a land snail of the genus Pearsonia, Mollusca Gastropoda and an earthworm of the genus Enchytraeus, Annelida Lumbricidae,and two aquatic animal species, i.e., a beetle of the species Cybister japonicas Sharp, Hexapoda Dytiscidae and a water snail of the species Chipangopaludina chinensis, Mollusca Gastropoda, which are typical prey of the red-crowned crane in the wetland (Ma and Li 2002), were collected at designed sampling sites. All prey samples were thoroughly rinsed in-field with distilled water to remove pollutants attached on their body. The organisms were subsequently placed in car refrigerator at −4 °C and transported to the laboratory. Reed roots, rhizomes, and stems and surface sediment were also collected at each site. The samples were immediately packed in dark-colored polyethylene bags, refrigerated, and then transported back to the laboratory.
Oxidation–reduction potential (Eh) and pH of water were measured at each sampling site. Electrical conductivity (EC) of the extract at 1:5 sediment:solution ratio was determined with a conductivity meter (Rhoades 1982). Particle size of sediment was determined by the pipette method (Gee and Bauder 1982). Organic matter content in the sediment was measured according to the method by Jackson (1956, 1958). Physical and chemical properties of water and sediment in study area were given in Table 1.
Red-crowned crane carcasses collection process was collected in the field from 2010 to 2014. A total of eight red-crowned crane carcasses were collected at 8 nesting sites (Table 2), including five males and three females, aided by the fire inspectors of Zhalong Wetland. The direct death cause for these crane samples were starvation because of food shortage in freezing condition, or collision on the power line according to a pathological inspection.
Approximately 1–2 g samples of livers, kidney, and breast muscles were collected for acid-digested. Polyethylene gloves were used throughout the all dissection procedures to prevent contamination. Several flight feathers were also collected from the crane carcasses and washed with distilled water in the laboratory. After-hatch residual eggshells were collected from the field in late April and early May of 2012–2014. A total of twelve residual eggshells left by red-crowned cranes were collected at six nesting sites (S3, S4, S10, S14, S15 and S17), aided by the fire inspectors of Zhalong Wetland. Similarly, the eggshells were washed with distilled water in the field and immediately transferred to laboratory.
Microwave digestion and element analysis
The reed rhizome, aquatic animal body, eggshell, and feathers were dried with filter papers and then oven-dried to a constant weight (48 h at 60 °C). The dried samples were ground to homogenous powders in a quartz bowl for acid digestion. Similar processes were performed on the liver, kidney, and muscle samples without washing and drying in the laboratory. All sediment samples were sieved through a 63 μm mesh after indoor air drying for acid digestion.
Each tissue category was acid-digested in a microwave oven (MDS-15, Sineo Microwave Chemistry Technology Col, LTD, Shanghai of China) according to the method by Canli et al. (1998). Triplicate sub-samples of known dry weight (External tissue of the cranes and other organisms: approximately a 0.5 g in dry weight; Internal tissue: 1–2 g in fresh weight) were digested in acid mixture (3 mL HNO3 + 1 mL HCl; both chemicals were analytical reagent and supplied from Shenzhen three chemical co., LTD of China) in a closed Teflon crucible, evaporated slowly to almost dryness (90 °C), and the residue was dissolved in 5 mL 1:1 diluted HCl, and then settled to 25 mL for analysis after the solution has been cooled down to room temperature.
The fraction components of As in the sediments were determined through the sequential extraction procedure by Tessier et al. (1979). The extraction steps used are described as follows:
-
1.
Exchangeable fraction (Exch F): The sediment was extracted at room temperature for 1 h with magnesium chloride solution (1 mol L−1 MgCl2) at pH 7 with continuous agitation.
-
2.
Bound to carbonate fraction (Carbon F): 50 mL 1 mol L−1 M CH3COONa at pH 5 (adjusted using HNO3) was added to the residue from fraction (1). Continuous agitation was maintained for 5 h for complete extraction.
-
3.
Bound to iron-manganese oxides fraction (Fe–Mn F): 50 mL 0.04 mol L−1 NH2OH·HCl in 25 % (v/v) CH3COOH was added to the residue from fraction (2), and continuous agitation was used at 96 ± 3 °C for 6 h.
-
4.
Bound to organic matter (Organic F). H2O2 with 30 % concentration was added to the residue from fraction (3). The sample was placed in water bath until dry and 50 mL 3.2 mol L−1 NH4CH3COOH was added to it for continuous agitation.
-
5.
Residual fraction (Residual F): Residue from fraction (4) was digested with HF-HClO4. This fraction was the most difficult transport form according to the report by Tessier et al. (1979).
The determination of As concentrations in the all sample categories were performed on inductively coupled plasma-mass spectrometry (ICP-MS Agilent 7500ce, Agilent Technologies, Inc. of USA). We estimated the precision and accuracy of the analyses based on a certified reference material: Pseudoscianea crocea (GBW08573) (5080 ± 390 ppb) for aquatic animals. The results agreed with the certified values for all metals, with average recovery rates of 102 %. All of the materials used for sampling and analysis were acid-washed. Moreover, all of the samples were analyzed in triplicate at a relative standard deviation lower than 1.5 %.
The concentrations of As in the red-crowned crane and its prey were given in the form of dry weight (dw) according to their correlations of wet weight form and dry weight form.
Assessment of sediment contamination
Geo-accumulation index (I geo) was used to assess As accumulation in sediment introduced by Muller (1969), expressed by the following empirical equation (Sekabira et al. 2010):
where C n is the measured concentration of a heavy metal in sediment and B n represents the geochemical background value of metal n in unpolluted sediments. I geo was classified as following:
For description of As ecotoxicology, individual contamination factor (ICF) for all sampling sites were calculated using fractionation results by dividing the sum of the first four extractions (i.e., exch F, carbon F, Fe–Mn F, and organic F) by the residual fraction for the site (Ikem et al. 2003).
Statistical analysis
SPSS 10.0 for Windows was used for data analysis. Pearson’s correlation coefficients were used to calculate correlations between the wet weight and dry weight and correlation relationship of the As of the sediment versus reed and sediment versus water animals. Analysis of variance (ANOVA) was employed to test whether As concentrations varied significant between the sediments, plant and inhabiting invertebrates in the buffer zone and core area. Possibilities less than 0.05 (P < 0.05) were considered statistically significant.
Results
As concentration in the sediment-inhabiting organisms
As content in the sediments was generally lower than the average natural background values (Table 3). Almost all As concentration in the study area exceeded the tolerable level (1 × 103 to 1.7 × 103 ppb) for agro-economic crops suggested by Kabata-Pendias (2001) but lower than the tolerable level (1 × 104 ppb) for rice plants proposed by Abedin et al. (2002). As contents in the upper reaches of Wuyur River and the buffer zone (3990 ± 2490 ppb average concentration) were significantly higher than those in the core area (1200 ± 1340 ppb average concentration) (F = 8.29, P = 0.01). In general, the most stable form, i.e., residual F, was present by more than 50 % in the absolute fractions in buffer zone A (Table 3; Fig. 2). By contrast, Exch F, which was the most readily absorbed by water and organisms, was only 150 ppb in average. In addition, the organic F and residual F (relatively stable phases), were at 770 and 2350 ppb in average, respectively. Similar results were observed in the core area and buffer zone B.
The ICF at 10 of 19 sampling sites exceeded l and the ICF was positively correlated with As concentration in the reed root, which suggests that most As were readily absorbed by the aquatic animal community (Fig. 3). The highest ICF level for As was calculated in the buffer zone A and core area at 1.78 and 1.79, respectively. As contamination at all sampling sites was approximately equal to the background level (I geo < 0) as assessed using the geo-accumulation index method (Table 3). This finding indicates the presence of background contamination in this region.
Reed rhizome and stem, which are the preferred food of the red-crowned cranes in Zhalong Wetland (Ma and Li 2002), contained measurable levels of As in the following order: stem < rhizome < root (Table 4). The As concentrations in the reed rhizome and root were below the detection limit (ND) to 25.29 ppb and from ND to 67.25 ppb, respectively. The As contents were linearly and positively correlated with sediment concentration (r 2 = 0.62, P < 0.01).
The four sediment–inhabiting organism groups, whose preferred food is reed root and humus in sediments, contained detectable As level in the following order: land snail < water snail < beetle < earthworm (Table 5; Fig. 4). The As concentration in the bodies of water snails was higher than in their shells. The total As concentrations in the slugs of water snails varied from 5.27 to 22.84 ppb, with an average concentration of 11.48 ppb. Although, As was observed in beetle, As concentration significantly varied in different sections (P < 0.001), i.e., As concentration in the remaining section was greater than that in the head section. All the measured As contents in the sediment-inhabiting organisms did not exceed the allowable concentration limit (1000 ppb) recommended by Agah et al. (2009).
As enriched in the red-crown cranes
Endangered water bird (e.g., red-crowned cranes) accumulated measurable As levels in the following order: feather < muscle < eggshell < kidney < liver (Table 6). High As concentration was detected in internal organs, such as liver and kidneys. The relatively high As concentrations detected in the eggshells ranged from 34.98 to 235 ppb. Feathers had the lowest metal contents, which varied from 11.39 to 42.47 ppb. Conversely, the muscles of red-crowned cranes in the Hokkaido, Northern Japan presented higher As content than in the liver (Table 6), with approximately 110 and 90 ppb dw, respectively. In general, As content was also slightly larger or presented similar enrichment than island crane species in Japan.
As concentration in the feather tissues of red-crowned crane in Northeast China was significantly lower (F = 9.36, P < 0.001 for crane versus common eider; and F = 20.16, P < 0.001 for crane versus little egrets) compared with the common eider in USA and little egrets in China. Nevertheless, As level in the eggshells of red-crowned crane was similar to that of little egrets in China. The highest As content value (441 ppb) detected in the liver of red-crowned crane was significantly lower than the toxic level (1 × 104 ppb in fresh weight, approximately 2.5 × 104 to 3.0 × 104 ppb in dry weight) as recommended by Eisler (1988). The difference in As concentration between sex groups, i.e., male crane versus female crane, was significant (F = 6.47, P < 0.001) but was not significant between age groups (i.e., As concentration in the adult crane vs. juvenile crane) (F = 2.75, P = 0.18, Fig. 5).
In addition to toxicity and persistence, bioaccumulation can be an important threat for heavy metal exposure. Similar to As concentration variation, bioaccumulation varied in different organs and tissues in the following order: feather < muscle < kidney < liver. Overall, As was concentrated in the organisms but was not biomagnified in the food chain.
The Linear correlation of external tissues and internal tissues were presented in Fig. 6. As concentration in the eggshell versus the liver presented good and positive linear relations (P < 0.05). However, metal concentration in the eggshell and feather presented extremely weak linear correlation with that in the kidneys and liver (P > 0.05).
Discussion
This study presents two known findings about As accumulation and transfer along the typical food chain in the Zhalong wetland, China. First, four sediment-inhabiting organisms (i.e., land snail, earth worm, beetle, and water snail) contained measurable As levels and elevated As transferred to the consumer at the upper trophic level (i.e., red-crowned crane species). Second, As concentration level in the eggshells of red-crowned crane was high and correlated with that in their internal organs (i.e., liver). However, the dietary exposure to As of these cranes was under the safety level.
According to Fleischer et al. (1974), agricultural practice (mainly application of fertilizers and pesticides) could contribute to As contamination in soils. In the present study, the first set of sediment samples was obtained in the midstream of Wuyur catchments; in this area, runoffs flow through from the large area of arable land and inevitably accumulate some As. These runoffs consequently enter bird habitats, thereby gradually elevating As concentration (S1 < S2 < S3). The buffer zones A and B, which were adjacent to the arable area, are being cultivated; thus, the intensive anthropogenic activity induces the high probability of crane to be in contact with toxic chemicals, such as herbicides and insecticides. By contrast, the core area was protected to some extent by the buffer zone from local human impact. Thus, the maximum total As concentration was detected at S5 and S6, whereas the minimal level was observed in the core area. In addition, the most prevalent form of As in the Zhalong wetland and the most toxic to various water birds in the sediment could be inorganic forms (i.e., arsenate); this finding is similar to results reported by previous research (Eisler 1988; Kabata-Pendias 2001).
As earthworm could enrich high levels of heavy metals because of their feeding habits, they have been proposed as biomonitors for metal contamination (Carpené et al. 2006; Klok et al. 2006; Roodbergen et al. 2008). Similar to the results of previous research, we determined higher As contents in earthworm than that in other sediment-inhabiting organisms, suggesting earthworm is an As-rich soil fauna and could be used as a suitable indicator for the As toxicity risk level posed on this region. However, the presence of As in aquatic animal did not indicate the contamination of particular sediments and habitants as the measured As contents did not exceed the allowable concentration limit (1 × 103 ppb) recommended by Agah et al. (2009).
The kidneys and livers were the most prone to As accumulation in the present research. The highest As content value at 441 ppb was detected in the liver of the red-crowned crane, but this concentration was significantly lower than the toxic level (1 × 104 ppb in fresh weight) as recommended by Eisler (1988). High As content in the liver and kidneys is consistent with the conclusions of Takazawa et al. (2004) and Malik et al. (2010); in their studies, these organs of water fowls are more prone to accumulate toxic metals (such as As) than other internal organs. Researchers also found that some birds, e.g., blue tits (Dauwe et al. 1999) and rook (Orlowski et al. 2010) can sequester significant amounts of some toxic metal (e.g., As in their eggs); therefore, eggshells are suitable indicators of heavy metal contamination, especially in the case of As for such birds. In the present study, eggshells could not be clearly classified if they belong to the carcasses or to any carcass, although a good linear relationship was obtained between eggshell-As versus kidney-As and eggshell-As verus liver eggshell-As. Further research is therefore recommended to confirm these findings.
Although As is toxic at high concentrations, this element is an essential micronutrient for many species (Koller 1980; Kabata-Pendias 2001). According to our previous research (Luo et al. 2015), the daily As intake by red-crowned crane in this region was significantly lower than the toxic level (1.2 × 106 ppb) in the diet recommended by Eisler (1988). Hence, the dietary exposure level of the migratory red-crowned crane population in China to As can be considered under the safety level.
References
Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128:1120–1128
Agah H, Leermakers M, Elskens M, Mohamad Rez Fatemi S, Baeyens W (2009) Accumulation of trace metals in the muscle and liver tissues of five fish species from the Persian Gulf. Environ Monit Assess 157:499–514
BirdLife International (2012) Grus japonensis: IUCN 2012. IUCN red list of threatened species, version 2012. 4. http://www.birdlife.org/datazone/speciesfactsheet (Accessed 5 May 2013)
Burger J, Gochfeld M, Jeitner C, Snigaroff D, Snigaroff D, Stamm T, Volz C (2008) Assessment of metals in down feathers of female common eiders and their eggs from Aleutians: arsenic, cadmium, chromium, lead, manganese, mercury, and selenium. Environ Monit Assess 143:247–256
Canli M, Ay O, Kalay M (1998) Levels of heavy metals (Cd, Pb, Cu, Cr and Ni) in tissue of Cyprinus carpio, Barbus capito and Chondrostoma regium from the Seyhan River, Turkey. Turk J Zool 22:149–157
Carpené E, Andreani G, Monari M, Castellani G, Isani G (2006) Distribution of Cd, Zn, Cu and Fe among selected tissues of the earthworm (Allolobophora caliginosa) and Eurasian woodcock (Scolopax rusticola). Sci Total Environ 363:126–135
Cui J, Zang SY, Zhai DL, Wu B (2014) Potential ecological risk of heavy metals and metalloid in the sediments of Wuyur River basin, Heilongjiang Province, China. Ecotoxicology 23:589–600
Dauwe T, Bervoets L, Blust R, Pinxten R, Eens M (1999) Are eggshells and egg contents of great and blue tits suitable as indicators of heavy metal pollution? Belg J Zool 129:439–447
Eisler R (1988) Arsenic hazards to fish, wildlife, and invertebrates: a synoptic review. Contam Hazard Rev US Fish Wildl Serv Biol Rep 85(1.12)
EPA (1980) Ambient water quality criteria for arsenic. US Environ. Protection Agency Rep. 440/5-80-021
Fasola M, Movalli PA, Gandini C (1998) Heavy Metal, Organochlorine Pesticide, and PCB Residues in Eggs and Feathers of Herons Breeding in Northern Italy. Arch Environ Contam Toxicol 34:87–93
Fleischer M, Sarofim AF, Fassett DW, Hammond P, Shacklette HT, Nisbet ICT, Epstein S (1974) Environmental impact of cadmium. Environ Health Perspect 5:253
Gee GW, Bauder JW (1982) Particle-size analysis. In: Miller RH, Keeney DR (eds) Methods of Soil Analysis: Part 2. Agronomy Monographs: No. 9, 2nd ed. ASA, Madison, pp 383–411
Gulz PA, Gupta SK, Schulin R (2005) Arsenic accumulation of common plants from contaminated soil. Plant Soil 272:337–347
Harris J (2008) Cranes respond to climate change. ICF Bugle 34(1–3):14–15
Ikem A, Egiebor NO, Nyavor K (2003) Trace elements in water, fish and sediment from tuskegee lake, southeastern USA. Water Air Soil Pollut 149:51–75
Jackson ML (1956) Soil chemical analysis. Prentice-Hall, Inc., New York
Jackson ML (1958) Soil chemical analysis. Prentice-Hall, Inc., Englewood Cliffs, NJ
Kabata-Pendias A (2001) Trace elements in soils and plants, 3rd edn. CRC Press, Boca Raton
Klok C, Van der Hout A, Bodt J (2006) Population growth and development of the earthworm Lumbricus rubellus in a polluted field soil: possible consequences of the godwit (Limosa limosa). Environ Toxicol Chem 25:13–219
Koller LD (1980) Immunotoxicology of heavy metals. Int J Immunopharmacol 2:269–279
Li J, Zheng CJ (2008) Handbook of environmental background concentration of heavy metals in China. China Environmental Science Press, Beijing (in Chinese)
Luo J, Yin X, Ye Y, Wang Y, Zang S, Zhou X (2013) Pb and Cd bioaccumulations in the habitat and preys of red-crowned cranes (Grus japonensis) in Zhalong wetland, northeastern China. Bio Trace Elem Res 156:134–143
Luo J, Ye Y, Wang Y (2014) Dietary exposure of the red-crowned crane (Grus japonensis) to total and methyl mercury in Zhalong wetland, northeastern China. Bio Trace Elem Res 159:210–218
Luo J, Ye Y, Yin X (2015) Bioaccumulation and dietary exposure of the red-crowned cranes (Grus japonensis) to arsenic in Zhalong wetland, northeastern China. Aquatic Ecosys Health Manage 18:121–129
Ma Y, Li X (2002) Research on the red-crowned crane. Shanghai Scientific and Technological Education Press, Shanghai (in Chinese)
Malik N, Biswas AK, Qureshi TA, Borana K, Virha R (2010) Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environ Monit Assess 160:267–276
Martínez-Villegas N, Flores-Vélez LM, Domínguez O (2004) Sorption of lead in soil as a function of pH: a study case in México. Chemosphere 57:1537–1542
Muller G (1969) Index of geo-accumulation in sediments of the Rhine River. Geo J 2:108–118
Orlowski G, Kasprzykowski Z, Dobicki W, Pokorny P, Polechoński R (2010) Geographical and habitat differences in concentrations of copper, zinc and arsenic in eggshells of the Rook Corvus frugilegus in Poland. J Ornithol 151:279–286
Rhoades JD (1982) Soluble salts. In: Miller RH, Keeney DR (eds) Methods of Soil Analysis: Part 2. Agronomy Monographs: No. 9, 2nd ed. ASA, Madison, pp 167–179
Roodbergen M, Klok C, Van der Hout A (2008) Transfer of heavy metals in the food chain earthworm Black-tailed godwit (Limosa limosa): comparison of a polluted and a reference site in the Netherlands. Sci Total Environ 406:407–412
Sekabira K, Oryem Origa H, Basamba TA, Mutumba G, Kakudidi E (2010) Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int J Environ Sci Tech 7:435–446
Shaw JR, Jackson B, Garor K, Stanton S, Hamilton JW, Stanton B (2007) The influence of exposure history on arsenic accumulation and toxicity in the Killifish, Fundulus Heteroclitus. Environ Toxicol and Chem 26:2704–2709
Takazawa Y, Kitamura K, Yoshikane Y, Morita M (2004) Discovery of fenthion poisoning in two Japanese cranes (Grus japonensis) found dead in Hokkaida, Japan. Bull Environ Contam Toxicol 73:947–954
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Tech 41:6854–6859
Zhang Y, Ruan L, Fasola M, Boncompagni E, Dong Y, Dai N, Gandini C, Orvin E, Ruiz X (2006) Little Egrets (Egretta Garzetta) and trace metal contamination in wetlands of China. Environ Monit Assess 118:355–368
Acknowledgments
This research was funded by the scientific research projects of Heilongjiang Provincial Department of Education (Grant no.12541886) and Qiqihar municipal science project (Grant no. RKX-201301).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Luo, J., Ye, Y. & Gao, Z. Arsenic content in red-crowned crane (Grus japonensis) and invertebrates at the bottom of food chain in Zhalong wetland, northeastern China. Ecol Res 30, 803–812 (2015). https://doi.org/10.1007/s11284-015-1278-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-015-1278-y