Abstract
Five heavy metal concentrations, copper (Cu), zinc (Zn), lead (Pb), chromium (Cr), and cadmium (Cd), in the sediments and six typical aquatic animal taxa were analyzed to determine the contamination from heavy metals in the habitat of the red-crowned cranes in Northeastern China. The body burden of these metals in the cranes was analyzed to examine the impact of these hazards on the rare species. Results indicated that all detected concentrations of the five heavy metals in the sediments were higher than the natural background levels. Pb and Cd were the most abundant elements in the sediments, with concentrations ranging from 9.85 to 129.72 mg kg−1 and from 1.23 to 10.63 mg kg−1 (dry weight, dw), respectively. Their absolute fractions were relatively stable phases, i.e., bound to iron-manganese oxides fraction and bound to organic matter fraction at 16.28 and 23.23 mg kg−1 for Pb and 0.33 mg kg−1 and 3.15 mg kg−1 (dw) for Cd. Six common water animal taxa were found to contain detectable heavy metal concentrations. The internal tissues of the red-crowned cranes contained significantly high metal concentrations compared with their external tissues (feather, feces and residual eggshell). Cd concentrations in the feather and liver of red-crowned cranes exceeded a level considered to be potentially toxic in birds, with levels ranging from 0.41 to 3.06 mg kg−1 and 0.37 to 4.42 mg kg−1 (dw), respectively. Similarly, we found increased levels of Pb in the both external and internal tissues, with levels ranging from 0.21 to 3.21 mg kg−1 dw, which indicated likely contamination by the metal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Special concerns have been raised on the excessive quantities of heavy metals and the mechanism by which these metals are transferred into aquatic biological circles because of their toxic effects on the ecosystem. According to Daskalakis and O’Connor (1995), sediments are regarded as a basin for heavy metals discharged into the aquatic environment. Large quantities of heavy metals in aquatic environment are often bound to particulate matter and deposited into sediment (Martínez-Villegas et al. 2004); however, some fractions can be readily taken up by aquatic plants (e.g., reeds) or deposit-feeding benthic organisms and elevated into the higher strata of the food chain (Fisk et al. 2005; Agah et al. 2009). Therefore, the total concentrations of heavy metals and their fractions in each phase in sediments should be determined to detect net change and assess potential toxic risk in an aquatic system.
The red-crowned crane (Grus japonensis) is a precious species that is in the danger of extinction. The species has been included in the International Union for Conservation of Nature and Natural Resources (IUCN) Red List of Endangered Species since 2000 (BirdLife International 2012). Its population worldwide is very small, with an estimated 2,750 mature individuals. Although the resident population in Japan remains stable (Teraoka et al. 2007), the migratory population in mainland Asia continually declines because of the loss and degradation of wetlands for agricultural and industrial development (Harris 2008). Red-crowned cranes are omnivores and typically feed on the aquatic plants (e.g., reed root and stem) and water animals (e.g., fish, shell and aquatic insects). Thus, toxic metals such as Pb and Cd eventually accumulate in the bodies of the red-crowned cranes via the food chain, given that they roost and nest in stable sites for years.
Zhalong National Nature Reserve (Zhalong Wetland), in the downstream Wuyur catchments, Northeastern China (Fig. 1), is one of the largest habitat and breeding sites for migratory red-crowned crane (Grus japonensis). Heavy metals’ contamination in the habitat may contribute to the rapid decline in the red-crowned crane population in this region. Ecological health safety and environmental quality are major concerns and a better understanding of the degree of enrichment of heavy metals in the aquatic system is significant to conserve the endangered species. A complicating factor in determining this is the fact that all rare species in China, including the red-crowned crane, are protected in legislation, and any intentional killing of such species is prohibited. An alternative approach is to investigate the heavy metal concentrations in their habitat (Burger 2002), and examine their outer tissues (e.g., feather and eggshell) (Burger and Gochfeld 1993; Dauwea et al. 2003). In addition, while monitoring the red-crowned crane nests in Zhalong Wetland during 2010–2014, eight red-crowned crane carcasses were salvaged aided by the fire inspectors of Zhalong Wetland. These carcasses could be used to examine dominant metals enriched in the body of the species.
The objectives of this research are to report the total concentrations and their speciation distribution by using sequential extraction method of five heavy metals (Cu, Zn, Pb, Cr, and Cd) in the sediments of Zhalong Wetland, and to examine the body burden of five heavy metals in the red-crowned cranes. The present research is the first report on toxic metals’ accumulation in red-crowned cranes in Northeastern China, and the results from this research could help in improving understanding of the ecological health safety of the migratory red-crowned cranes in China and contribute to the conservation of the endangered species.
Materials and methods
Study area
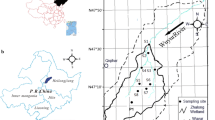
Wuyur River originates from the western foot of Xiaoxin’an Mountain, Northeastern China, where the watershed is an elongated strip that flows through main food production zone of Heilongjian province in China (Fig. 1a, b). The lower reaches of the river disappear after entering the Zhalong Wetland and develop a large area of reed marsh. Zhalong Wetland covers an area of 2,100 km2 (123º51′ to 124º37′E, 46º48′ to 47º32′N). The core area is approximately 700 km2, with the buffer zone occupying 1,400 km2 lying outside of the core area (see details in Fig. 1c). Large areas of pristine reed marsh in the wetland attract more than 500 migratory red-crowned cranes to inhabit and breed from late March to early November (about 8 months) every year.
Geologically, the wetland was formed by alluvial deposits with an average altitude of 140 m with 4,700 km2 of agricultural land around it (Fig. 1c). River feeding as well as precipitation are the major sources of water for this inland reed marsh. Climatically, the wetland has a typical temperate continental monsoon climate with average annual rainfall of 410 mm and potential evaporation 1,500 mm. The volume of runoff from the upper reaches abruptly decreased from 7.5 × 108 m3 per year in the 1980s to less than 1 × 108 m3 per year in the 21st century. Various sludge and wastewater from the surrounding residential area, agricultural land, and industrial workshops containing several types of toxic contaminants including Zn, Cd, and other heavy metals are discharged directly into the wetland without complete disposal treatment. The wastewater discharge volume increased from 0.17 × 108 m3 in 1993 to 0.45 × 108 m3 in 2010 (Luo et al. 2014). Increasing amounts of pollution discharges have elevated the concentrations of various toxic elements not only in sediment but in the entire biota.
Sampling scheme
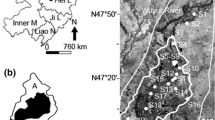
A total of 37 sampling sites were designed for sediment collection. The first set of three sample sites (i.e., S1–S3) were in the upper reaches of Wuyur catchments; the second set of 16 sample sites (S4–S19) were in buffer zone A of Zhalong Wetland; and the third set of 10 sample sites (S20–S29) were in the core area of the wetland. The remaining eight samples were designed in the buffer zone B of the wetland (Fig. 1d). Surface sediment was collected using sediment grab sampler, and immediately packed in dark-colored polyethylene bags, refrigerated, and then transported back to the laboratory.
Six typical aquatic animal taxa, including three invertebrates [water beetle, Cybister japonicus Sharp (Dytiscidae), pond snail, Cipangopaludina chinensis (Viviparidae), and dragonfly, Aeshna mixta (Odonata)] as well as three fish species with body size smaller than 10 cm [common carp, Cyprinus carpio Linnaeus (Cyprinidae), pond loach, Misgurnus mohoity Dybowski (Cobitidae), and Chinese sleeper, Perccottus glehnii Dybowski (Odontobutidae)], that are typical prey of wild red-crowned crane in the wetland, were collected in three regions (buffer zone A, core area and buffer zone B) (see details in Table 1). All prey samples were rinsed thoroughly in field with distilled water to remove pollutants attached to their body, then placed in a car refrigerator at −4 °C, and transported back to the laboratory.
A total of eight carcasses of red-crowned crane were collected to examine five trace elements (Cu, Zn, Pb, Cr and Cd) enriched in the body of the species (see details in Table 2). The direct death cause of these crane samples was starvation due to food shortage in freezing condition as reported by previous research (Luo et al. 2014). The cranes were immediately transferred to the laboratory for dissection. Approximately 1–2 g samples of livers, kidney, and breast muscles were collected using a stainless steel knife. Polyethylene gloves were used throughout the all dissection procedures to prevent contamination. Several flight feathers were also collected from the crane carcasses and washed with distilled water in the laboratory.
Fresh feces excreted by red-crowned crane were collected on October 4, 2011 at site S22 [three feces were well-formed (1.8 ± 0.3 g, fresh weight), and one was unformed], and May 2, 2012 at site S26 (two feces were well-formed, 1.5 ± 0.3 g, fresh weight). After-hatch residual eggshells were collected from the field in late April and early May of 2012. The outer parts of the feces were carefully removed and the core parts picked up to avoid the influence of the sediment. Similarly, the eggshells were washed with distilled water in the field and immediately transferred to laboratory.
Microwave digestion and element analysis
All sediment samples were sieved through a 63 μm mesh after indoor air drying for acid digestion following the method by Viklander (1998). After drying body of the aquatic animal, feathers, and eggshell of the red-crowned crane with filter papers, they were oven-dried to constant weight (48 h at 60 °C). The dried samples were ground into homogenous powders in a quartz bowl for acid digestion. Similar processes were performed on the feces samples, without washing and drying in the laboratory.
A total of 0.5 g of each category sample was acid-digested in a microwave according to USEPA (1996) methods. Triplicate sub-samples of known dry weight were digested in acid mixture (3 mL HNO3 + 1 mL HCl; Canli et al. 1998) in a closed Teflon crucible, evaporated slowly to almost dryness (90 °C), and the residue was dissolved in 5 mL 1:1 diluted HCl, and then settled to 25 mL for analysis after the solution has been cooled down to room temperature.
The fraction components of five metals were determined through the sequential extraction procedure by Tessier et al. (1979). The extraction steps used are described as follows:
-
1.
Exchangeable fraction (Exch F): The sediment was extracted at room temperature for 1 h with magnesium chloride solution (1 mol L−1 MgCl2) at pH 7 with continuous agitation.
-
2.
Bound to carbonate fraction (Carbon F): 50 mL 1 mol L−1 M CH3COONa at pH 5 (adjusted using HNO3) was added to the residue from fraction (1). Continuous agitation was maintained for 5 h for complete extraction.
-
3.
Bound to iron-manganese oxides fraction (Fe–Mn F): 50 mL 0.04 mol L−1 NH2OH·HCl in 25 % (v/v) CH3COOH was added to the residue from fraction (2), and continuous agitation was used at 96 ± 3 °C for 6 h.
-
4.
Bound to organic matter (Organic F). H2O2 with 30 % concentration was added to the residue from fraction (3). The sample was placed in water bath until dry and 50 mL 3.2 mol L−1 NH4CH3COOH was added to it for continuous agitation.
-
5.
Residual fraction (Residual F): Residue from fraction (4) was digested with HF-HClO4.
The Cu, Zn, Cr, Cd, and Pb concentrations in the sediment, prey, and external tissues (i.e., feathers, feces and eggshells) were determined using inductively coupled plasma–mass spectrometry (ICP-MS Agilent 7500ce, Agilent Technologies, USA). The precision and accuracy of the applied analytical method was estimated on certified standard reference material: Stream Sediments (GBW07304) [Cu (37 ± 4 mg kg−1), Zn (101 ± 15 mg kg−1), Pb (30 ± 7 mg kg−1), Cr (81 ± 9 mg kg−1), and Cd (0.19 ± 0.03 mg kg−1)] for sediment, and reference materials: Pseudoscianea crocea (GBW08573) (Beijing Shiji Ouke Bio-tech Co., Ltd) for aquatic animal and bird for Cu (1.36 ± 0.13 mg kg−1), Zn (28.8 ± 1.4 mg kg−1), Pb (8.8 ± 1.10 mg kg−1), Cr (0.45 ± 0.04 mg kg−1), and Cd (0.014 ± 0.001 mg kg−1) with the measured values (trace metals in fish muscle and feathers of the red-crowned crane). The results agreed with the certified values for all metals, with average recovery rates of 98 % for Cu, 93 % for Zn, 105 % for Cd, 108 % for Cr, and 104 for Cd in sediment quality control group and 102 % for Cu, 94 % for Zn, 103 % for Pb, 95 % for Cr and 105 % for Cd in aquatic animal and water bird group. All materials used for sampling and analysis were acid-washed. All of the materials used in sampling and analysis were acid-washed and analyzed in triplicate with relative standard deviation lower than 1.5 %.
Statistical analysis
Pearson’s correlation coefficients were used to calculate correlations between the concentrations of five heavy metals in the sediments and aquatic animal tissues. Analysis of variance (ANOVA) was used to test whether the metal concentrations varied significantly between the sediments in the four sampling areas, namely, the upper reaches, buffer A, core area and buffer B. A post hoc comparison (Tukey method) was used as a follow-up test to ANOVA to show the statistical differences between areas. Possibilities of less than 0.05 (p < 0.05) were considered statistically significant.
Results
Heavy metal contamination in sediments
Total concentration of each metal in the sediments of the study sites are given in Table 3. Generally, the total concentrations of five heavy metals in the sediment were higher than natural background values in the buffer zone of this region given by Li and Zheng (1988), in the following order: Zn > Pb > Cr > Cu > Cd. The significantly higher concentrations of Cu and Cd were found in the middle reaches and buffer zone of the wetland, wherein various agricultural activities and other anthropogenic sources are located, e.g., industrial sludge and urban waste, in comparison with metals in the core area wherein human intense impact is avoided (F = 4.88, p = 0.006 for Cu and F = 10.88, p < 0.001 for Cd). Pb and Cd concentrations in the buffers A and B were significantly larger than in the core area (F = 4.70, p = 0.05 for buffer zone A vs. core area and F = 4.81, p = 0.05 for buffer zone B vs. core area for Pb; F = 14.50, p = 0.003 for buffer zone A vs. core area and F = 30.77, p < 0.001 or buffer zone B vs. core area for Cd). Two essential metal concentrations, i.e., Cu and Zn did not exceed the probable effect level values (PELs) that are considered to be potentially toxic in aquatic animals or birds by MacDonald et al. (2000). However, maximum Pb and average Cd concentrations in the buffer zones exceeded the PELs and the tolerable levels for agro-economic crops suggested by Kabata-Pendias (2001). Enrichment factors in the buffer were generally larger than 1.5 for Pb and 15 for Cd, and significantly higher than in the core area.
The most abundant fractions of these metals were in the difficult transport forms, which include Fe–Mn bound, organic bound, and residual bound state, as shown in Table 4. The most readily resolved by water and taken up by aquatic plants and animals fraction (Exch F) were that of Pb and Cd in buffer zone A, with only 1.22 and 0.09 mg kg−1. By contrast, Fe–Mn F and Organic F (relatively stable phases), were at 16.28 and 23.23 mg kg−1 for Pb and 0.33 mg kg−1 and 3.15 mg kg−1 for Cd. Similar results were found in the core area and buffer zone B.
Heavy metals enriched in aquatic animal tissues
Results of heavy metal enrichment in aquatic animal tissues are given in Table 5 and Fig. 2. The five metals were observed in all sampled aquatic animals in the following order: Zn > Cu > Cr > Pb > Cd. Two essential elements, Zn and Cu, were found to prevail in six aquatic animal families, with concentrations ranging from 11.04 to 39.48 mg kg−1 and 0.76 to 4.66 mg kg−1, respectively.
Pb was found in six groups of aquatic animals, with concentrations ranging from 8.37 to 48.84 μg kg−1. Concentrations of the two essential elements, as well as of Pb and Cr did not exceed the limit of allowable concentration recommended by the Joint FAO/WHO food standards program (1990). Cd concentrations in aquatic animals had the largest ratio of observed value/allowable concentration, with the concentration even exceeding the recommended safety limit (50 mg kg−1) at 53.26 mg kg−1 in the Viviparidae group captured in the buffer zone A.
Heavy metals enriched in the red-crown cranes
The concentrations of five trace elements in the external and internal tissues of red-crowned crane are presented in Table 6. Metal concentrations in these tissues followed the order of Zn > Cu > Pb > Cd > Cr. Concentrations of two essential elements were detected in the seven tissues of the red-crowned crane in decreasing order of liver > kidney > gut > muscle > feather > eggshell > feces for Zn, and liver > kidney > muscle > gut > feather > eggshell > feces for Cu. Nonessential trace elements in the tissues of the red-crowned crane varied in the following order: feather > kidney > muscle > liver > gut > eggshell > feces for Pb, and liver > feces > kidney > feather > eggshell > muscle and gut for Cd. Generally, eggshell contained low Cd at 0.55 mg kg−1. Feces appeared to be rich in Cd, with concentrations ranging from 0.37 to 2.08 mg kg−1. Cu concentrations in the feather, eggshell, and internal tissue were found to be significantly different (F = 23.04, p < 0.001 for Cu), whereas difference Zn, Pb, and Cd concentrations among the seven tissues were not to be significant (F = 0.49, p = 0.62 for Zn, F = 1.87, p = 0.17 for Pb; F = 0.63, p = 0.64 for Cd). A comparison of the enrichment level of metals in red-crowned cranes with other birds showed that Cd concentration in the eggshell and internal tissues of the crane was significantly lower than that in rooks, Corvus frugilegus as reported by (Orlowski et al. 2010). Pb concentration in the red-crowned crane was at a similar level to the island red-crowned cranes (Teraoka et al. 2007), but was significantly higher than that in the rook, Corvus frugilegus (Orlowski et al. 2012) and common eider in the Aleutian island of USA, as reported by Burger et al. (2008).
Discussion
Intensive agricultural practice (mainly the application of fertilizers and pesticides) has proved to be a large contributor to Pb and Cd contaminations (Fleischer et al. 1974). The first set of sediment samples was located in the upper reaches of the Wuyur catchments, and runoffs flowing through from the large area of arable land would inevitably accumulate large amount of toxic substances and consequently enter the birds’ habitats, thereby elevating the concentrations of five heavy metals gradually. Similarly, buffer zones were adjacent to the arable area. Given that the areas are being cultivated, intensive anthropogenic activity induced the high probability of contact of cranes with toxic chemicals such as herbicides and insecticides.
Hazardous chemicals that enter the aquatic ecosystems would inevitably alter the biotic environment and cause unforeseeable consequences in the food chain links of the aquatic system, because some metals (e.g., Pb and Cd) have been observed to be easily taken up by aquatic animals (Zhang et al. 2012). The distinct concentrations of Pb and Cd observed in water animals between sampling locations (see details in Table 5) could be due to the differing amounts of these toxic metals in the sediments because significant linear positive correlation of the five metals (Cu, Zn, Pb, Cr, and Cd) in the sediment versus three water animals (Dytiscidae, Viviparidae, and Odontobutidae taxa) were found as shown in Table 7.
The presence of heavy metals in aquatic animals not only indicates contamination of particular sediments and habitants (Tabari et al. 2010) but also implies a toxic risk to higher strata predator (e.g., red-crowned cranes) in this region because fish and shell are essential foods for large water fowls in Northeastern China. In the present study, Cu and Zn would not impose obvious toxic risks on the red-crowned crane population because the content levels of these metals in both external and internal tissues of the species were below the toxic level. Several researchers have concluded that a certain content level of Cu and Zn is essential for the physiological metabolism of many species (Kabata-Pendias 2001).
The findings in the current study revealed that eggshell contained the lowest Cd concentration when compared with other tissues. This result agrees with that of Mora (2003) and Orlowski et al. (2012) who postulated that Cd was not efficiently transferred from female bird to egg. The feather was found to contain extremely high Pb and Cd concentrations, at 6.12 and 0.65 mg kg−1 in average, which exceeded toxic levels [5.0–11.0 mg kg−1 for Pb (Burger and Gochfeld 1997), and 0.22 mg kg−1 for Cd (Pain et al. 2005)]. However, the use of crane feathers as indicators of Pb and Cd pollution is still being challenged because of the discrepancy due to external contamination (atmospheric deposition) (Dauwea et al. 2003) as types of feather [significant difference may resulted from the molting pattern (Burger et al. 2008)]. By now, no indicator has been provided for feces excreted by birds to be considered to be a potentially toxic level in birds. In the present study, Cd concentrations in the feces significantly exceeded the level in the feather and eggshell at 1.36 mg kg−1 and ranged from 0.37 to 2.08 mg kg−1. Prey fed to cranes generally originated from areas near the nesting colony; therefore, metal levels in the feces probably reflect contaminants acquired from the local environment. Given that any intentional killing of wild cranes or picking their eggs is legally prohibited, feces excreted by wild cranes can be used as a suitable indicator for Cd toxic risk level posed on rare birds, although feces do not always reflect contamination status of Cd in internal organs. Further research is recommended.
The results in this paper showed that the livers and kidneys of red-crowned cranes were more prone to the accumulation of the toxic metals, such as Pb and Cd, than other internal organs as found in other studies. A previous study has shown that Cd concentrations exceeding 3 mg kg−1 dw in liver or kidney were presumptive evidence of environmental hazardous exposure (Scheuhammer 1987), and larger than 40 mg kg−1 Cd in liver or 100 mg kg−1 in kidney were indicative of toxicosis (Degernes 2008). Liver Pb >1.7 mg kg−1 dw was generally considered diagnostic for Pb toxicosis (Degernes 2008). Compared with these data, samples in this paper contained average concentrations of Cd, at 2.21 mg kg−1, and were below those associated with toxic effect threshold; however, the highest concentration of Cd at 4.42 mg kg−1, and liver Pb (1.83 mg kg−1) and kidney Pb (1.85 mg kg−1) exceeded the Cd and Pb exposure levels (3 mg kg−1 for Cd and 1.5 mg kg−1 for Pb). According to Mochizuki et al. (2008), the highest concentration of Cd (≤179 mg kg−1 dw) was found in kidney of several marine and water birds. Thus, such a high level of Pb and Cd in body of the red-crowned crane can be considered, at the very least, to indicate potential Pb and Cd toxic risk to the birds.
The results in the present research showed that internal tissues of the red-crowned crane contained increased concentrations of Pb and Cd when compared with other birds. According to Wenzel et al. (1996), the level of toxic metals in free-living birds is closely related to their concentration in the diet. Hence, it is believed that the relatively high levels of Pb and Cd in the body of the crane could be attributed to the uptake in their daily diet. Therefore, the inputs of heavy metals into the Wuyur River catchments should be reduced to ensure that the healthy state of this critical crane habitat is maintained for the long-term sustainability of the crane population. However, certifying whether the detectable level of the heavy metals was the main contributor to the decline of the bird population can be difficult, although the red-crowned crane population and nesting numbers scale have continued to decline significantly in recent decades as expected as shown in Fig. 3. Several other factors, such as frequent fishing and fire accidents have also been proved to be principal causes for the decline of this endangered species (Ma and Li 2002).
References
Agah H, Leermakers M, Elskens M, Mohamad RFS, Baeyens W (2009) Accumulation of trace metals in the muscle and liver tissues of five fish species from the Persian Gulf. Environ Monit Assess 157:499–514
BirdLife International (2012) Grus japonensis: IUCN red list of threatened species, version 2012. 4. http://www.birdlife.org/datazone/speciesfactsheet (Accessed 5 May 2013)
Burger J (2002) Food chain differences affect heavy metals in bird eggs in barnegat bay, New Jersey. Environ Res Sec A 90:33–39
Burger J, Gochfeld M (1993) Lead and cadmium accumulation in eggs and edgling seabirds in the New York Bight. Environ Toxicol Chem 12:261–267
Burger J, Gochfeld M (1997) Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res 75:160–172
Burger J, Gochfeld M, Jeitner C, Snigaroff D, Snigaroff D, Stamm T, Volz C (2008) Assessment of metals in down feathers of female common eiders and their eggs from Aleutians: arsenic, cadmium, chromium, lead, manganese, mercury, and selenium. Environ Monit Assess 143(1–3):247–256
Canli M, Ay O, Kalay M (1998) Levels of heavy metals (Cd, Pb, Cu, Cr and Ni) in tissue of Cyprinus carpio, Barbus capito and Chondrostoma regium from the Seyhan River, Turkey. Turk J Zool 22:149–157
Collings SE, Johnson MS, Leah RT (1996) Metal contamination of angler caught fish from Mersey Estuary. Mar Environ Res 41:281–297
Daskalakis KD, O’Connor TP (1995) Distribution of chemical concentrations in US coastal and estuarine sediment. Mar Environ Res 40:381–398
Dauwea T, Bervoetsb L, Pinxtena R, Blustb R, Eens M (2003) Variation of heavy metals within and among feathers of birds of prey: effects of molt and external contamination. Environ Pollut 124:429–436
Degernes LA (2008) Waterfowl toxicology: a review. Vet Clin North Am Exot Anim Pract 11:283–300
Fisk A, deWit C, Wayland M, Kuzyk ZZ, Burgess N, Lethcer R, Branue B, Norstrom R, Blum SP, Sandau C, Lie E, Larsen HJS, Skaare JU (2005) An assessment of the toxicological significance of anthropogenic contaminants in Canadian arctic wildlife. Sci Total Environ 351–352:57–93
Fleischer M, Sarofin AF, Fassett DW, Hammond P, Shacklette HT, Nisbet IC, Epstein S (1974) Environmental impact of cadmium: a review by the panel on hazardous trace substances. Environ Health Perspect 5:253–323
Harris J (2008) Cranes respond to climate change. ICF Bugle 34(1–3):14–15
Joint FAO/WHO food standards program (1990) Guideline levels for cadmium and lead in food. Codex committee of food additives and contamination, 22nd session, Haugue, March 1924
Kabata-Pendias A (2001) Trace elements in soils and plants, 3rd edn. CRC Press, New York
Li J, Zheng CH (1988) Handbook of environmental trace element in China. China Environment Press, Beijing (in Chinese)
Luo J, Ye Y, Yin X (2014) Bioaccumulation and dietary exposure of the red-crowned cranes (Grus japonensis) to arsenic in Zhalong Wetland, Northeastern China. Aquat Ecosys Health Manag (In press)
Ma Y, Li X (2002) Research on the red-crowned crane (Grus japonensis). Shanghai Scientific and Technological Education Press, Shanghai (in Chinese)
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxic 39:20–31
Martínez-Villegas N, Flores-Vélez LM, Domínguez O (2004) Sorption of lead in soil as a function of pH: a study case in México. Chemosphere 57:1537–1542
Mochizuki M, Mori M, Hondo R, Ueda F (2008) Anew index for evaluation of cadmium pollution in birds and mammals. Environ Monit Assess 137:35–49
Mora M (2003) Heavy metals and metalloids in egg contents and eggshells of passerine birds from Arizona. Environ Pollu 125:393–400
Orlowski G, Kasprzykowski Z, Dobicki W, Pokorny P, Polechoński R (2010) Geographical and habitat differences in concentrations of copper, zinc and arsenic in eggshells of the Rook Corvus frugilegus in Poland. J Ormithol 151:279–286
Orlowski G, Kamiński P, Kasprzykowski Z, Zawada Z, Koim-Puchowska B, Szady-Grad M, Klawe JJ (2012) Essential and nonessential elements in nesting rooks Corvus frugilegus from eastern Poland with a special emphasis on their high cadmium contamination. Arch Environ Contam Toxicol 63:601–611
Pain DJ, Meharg AA, Ferrer M, Taggart M, Penteriani P (2005) Lead concentrations in bones and feathers of the globally threatened Spanish imperial eagle. Biol Conserv 121:603–610
Scheuhammer AM (1987) The chronic toxicity of aluminum, cadmium, mercury, and lead in bird: a review. Environ Pollut 46:263–295
Tabari S, Soheil S, Saravi S, Bandany GA, Dehghan A, Shokrzadeh M (2010) Heavy metals (Zn, Pb, Cd and Cr) in fish, water and sediments sampled form Southern Caspian Sea. Iran. Toxicol Ind Health 26:649–656
Teraoka H, Kumagai Y, Iwai H, Haraguchi K, Ohba T, Nakai K, Satoh H, Sakamoto M, Momose K, Masatomi H, Hiraga T (2007) Heavy metal contamination status of Japanese cranes (Grus japonensis) in east Hokkaido, Japan-extensive mercury pollution. Environ Toxicol Chem 26:307–312
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
USEPA (1996) Method.3050B-Acid digestion of sediments, sludges, and soils. U.S. Environmental Protection Agency, USA
Viklander M (1998) Particle Size Distribution and Metal Content in Street Sediments. J Environ Eng 124:761–766
Wenzel C, Adelung D, Theede H (1996) Distribution and age-related changes of trace elements in kittiwake Rissa tridactyla nesting form a isolated colony in the German Bight. North Sea Sci Total Environ 103:13–26
Zhang Z, Song X, Wang Q (2012) Cd and Pb contents in soil, plants, and grasshoppers along a pollution gradient in Huludao city, Northeast China. Bio Trace Elem Res 145:403–410
Acknowledgments
This research was funded by the School of Supporting Youth’s Academic Backbone Project in Heilongjiang Province of China (Grant no.1253G063) and Qiqihar municipal science project (Grant no.RKX-201301).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, J., Ye, Y., Gao, Z. et al. Heavy metal contaminations and influence on the red-crowned crane (Grus japonensis) in Wuyur catchments, Northeastern China. Environ Earth Sci 73, 5657–5667 (2015). https://doi.org/10.1007/s12665-014-3820-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3820-6