Abstract
The fall webworm, Hyphantria cunea (Drury) (Lepidoptera: Arctiidae), invaded Japan from North America about 60 years ago. Immediately after its invasion – and for the first three decades – its life cycle was bivoltine, two generations per year throughout its entire distribution in Japan. Thereafter, its life cycle shifted to trivoltine in the southwestern areas of Japan. In the present study we examined the life-history traits proposed to be implicated in this event with the aim of clarifying the mechanism of this life-cycle shift. The critical photoperiod for diapause induction, as defined by the photoperiod at which 50% of individuals enter diapause, was shorter in the trivoltine populations than in their bivoltine counterparts. The temperature sensitivity of the photoperiodic response, as defined by the difference in the critical photoperiod between 20 and 25°C, was greater in the trivoltine populations than in the bivoltine ones. The geographic variation in larval and pupal periods was positively correlated to the latitude of the original localities of the populations. The change in the number of larval instars would be one of the main factors accounting for the regional differences in the developmental period. These results suggest that some life-history traits of H. cunea have changed following its invasion of Japan as an adaptive response to local climates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alien species show various patterns of adaptations in terms of life-history traits following their invasion of new regions (Cox 2004). Substantial modifications in life-history traits and voltinism have been documented in some species of insects closely following their expansion into new habitats (Riedl and Croft 1978; Takeda and Chippendale 1982; Walker et al. 1983). In some invaded and introduced plants, adaptations along a latitudinal gradient have appeared, such at the age of reproduction in wild carrot (Lacey 1988) and the time of flowering in burr medic (Del Pozo et al. 2002). The response of seed germination in cheatgrass to temperature and flowering phenology varies according to local habitat conditions (Rice and Mack 1991; Meyer and Allen 1999). Some introduced vertebrates have evolved gradients in body size in different climatic conditions (Johnston and Selander 1964; Blem 1974; Yom-Tov et al. 1986; Williams and Moore 1989).
Widely distributed insects frequently develop local adaptations along a climatic gradient with respect to their various life-history traits and, consequently, in voltinism (Tauber et al. 1986; Danks 2006). Geographic variation in the photoperiodic control of diapause induction is one of the most remarkable of such adaptations and has been investigated in many species (Beck 1980; Danks 1987). Danilevsky (1965) found a general pattern that insect populations inhabiting higher latitudes have a longer critical photoperiod than those of the same species inhabiting lower latitudes. Other life-history traits, such as developmental rate, diapause intensity and body size, also frequently exhibit geographic clines (Masaki 1961, 1972, 1978; Bradshaw and Lounibos 1977). While there is no doubt that such a clinal variation is formed in each local population through natural selection (Tauber et al. 1986; Danks 1987; Bradshaw et al. 2004), little information is available on either the length of time or the process required for the establishment of such modifications of life-history traits and voltinism. A detailed study of both introduced and invading insects would be useful for obtaining more information on these issues (Tauber et al. 1986).
The distribution of the fall webworm, Hyphantria cunea (Drury) (Lepidoptera: Arctiidae), was limited exclusively to North America before 1940; during the 1940s it invaded central Europe and eastern Asia (Warren and Tadic 1970; Umeya and Itô 1977). The first report of H. cunea in Japan was in Tokyo (35°40′N) in 1945 (Masaki 1975); from there, it expanded its distribution southwards to 32°N and northwards to 41°N (Tate 2000; Gomi et al. 2004). Mitochondrial (mt)DNA analyses suggest that the invasion of Japan was a single event and that the invading individuals originated in a single North American population (Gomi et al. 2004). The winter diapause in the pupal stage is primarily induced by the photoperiod, and early larval instars are stage sensitive to the photoperiod (Masaki 1977a). In the early stage of the invasion, the critical photoperiod, which is defined as a photoperiod that induces diapause in 50% of the individuals, was 14 h and 35 min at 25°C (Masaki et al. 1968) and was relatively stable within the range of 17–25°C (Masaki 1977a).
The life cycle of H. cunea was mostly bivoltine throughout its distribution for the first three decades following its invasion of Japan (Masaki 1975). In the bivoltine life cycle, adults of the overwintering generation appear in late spring, while adults of the first generation occur in mid-summer and produce a second generation. Pupae of the second generation enter diapause for overwintering. However, in the mid-1970s partially trivoltine life cycles were reported in two populations occurring about 500 km apart (Arai and Akiyama 1976; Uezumi 1976). Much later, a population in Kobe (34°41′N) was found to be trivoltine (Gomi and Takeda 1990). In this population, both the critical photoperiod for diapause induction and the developmental period were shorter than those found in the population of H. cunea that first invaded Japan (Itô et al. 1968, 1970; Gomi and Takeda 1990, 1991, 1996). Thus, the life-history traits had been modified in the Kobe population in terms of a shift in the life cycle from bivoltine to trivoltine.
Geographic variation of photoperiodic responses
Critical photoperiod for diapause induction
The list of localities where H. cunea was collected for this study is given in Table 1 (Gomi and Takeda 1996; Gomi 1997). The photoperiodic response controlling diapause induction was investigated at 20 and 25°C (Fig. 1), and the critical photoperiod was found to be shorter at 25°C than at 20°C in all but the Sendai population. The correlation between the critical photoperiod and the latitude of origin was significant at each temperature (r = 0.526 for 20°C, df = 10, P = 0.0792; r = 0.696 for 25°C, df = 10, P = 0.0099). At 25°C, the critical photoperiods of the trivoltine populations were similar, and they were shorter than those of the bivoltine populations. A similar result was obtained at 20°C, although the difference in the critical photoperiod between the bivoltine and trivoltine populations was small. The critical photoperiod of the Tsukuba population, originating from the transition zone (Gomi 1996a), was relatively short at 20°C and intermediate at 25°C between the bivoltine and trivoltine populations.
Geographic variation of the critical photoperiod for diapause induction at 25°C in Hyphantria cunea in Japan. The critical photoperiod is defined as the photoperiod at which 50% of individuals enter diapause when the incidence of diapause is investigated in photoperiods at 15-min intervals. Circles bivoltine populations, triangle transition population, squares trivoltine populations
In some native insect and mite species, the critical photoperiod for diapause induction shows a high correlation with the latitude of origin (e.g., r = 0.96 for Chilo suppressalis, r = 0.98 for Wyeomyia smithii and r = 0.99 for Tetranychus urticae) (Kishino 1970; Bradshaw and Lounibos 1977; Vaz Nunes et al. 1990). The codling moth, Cydia pomonella, formed a linear cline (r = 0.87) in the geographic variation of this life-history trait about 200 years after its invasion of North America from Europe (Riedl and Croft 1978; Riedl 1983). In H. cunea, the correlation was relatively low at both 20 or 25°C, and the geographic pattern was step-wise rather than linear. These results suggest that this species has not spent enough time in Japan to form a linear cline in this trait and that the formation of the linear cline can be mediated by a step-wise pattern.
Temperature sensitivity in the photoperiodic response
The temperature sensitivity of the photoperiodic response is one of the more important life-history traits. In the linden bug, Pyrrhocoris apterus, the temperature sensitivity of the photoperiodic response controlling diapause induction was suggested to contribute to stabilization of the life cycle in the transition region between the univoltine and bivoltine areas (Numata et al. 1993; Saulich et al. 1994). A number of insects and mites have been investigated in terms of geographic variations of this trait in order to gain a better understanding of their respective life cycles (e.g. Sauer et al. 1986; Pittendrigh and Takamura 1987; Takafuji et al. 1991; Tanaka 1994).
Temperature sensitivity, which is defined as the difference in the critical photoperiod for diapause induction between 20 and 25°C (Gomi 1995, 1997), was significantly larger in the trivoltine population than in the bivoltine populations (t test: df = 9, t = 4.85, P < 0.001) and was negatively correlated to the latitude of origin (r = −0.611, df = 10, P = 0.0332) (Fig. 1, 2). These results suggest that rearing temperature influenced the photoperiodic induction of diapause less in the bivoltine and transition populations. Thus, changes in the temperature sensitivity are closely implicated in the shift of the life cycle in H. cunea.
Geographic variation of the temperature sensitivity for the photoperiodic response controlling diapause induction in H. cunea in Japan. Temperature sensitivity is defined as the difference in the critical photoperiod between 20 and 25°C. Circles bivoltine populations, triangle transition population, squares trivoltine populations
Sauer et al. (1986) found that in Pieris brassicae the difference in the critical photoperiod between 15 and 20°C decreased as the latitude of origin increased. In H. cunea, this same geographic trend was observed, with the southern trivoltine populations showing a greater temperature sensitivity in their photoperiodic response than the northern bivoltine populations. A similar result was obtained in the rice stem maggot, Chlorops oryzae, with once again a southern trivoltine population showing greater temperature sensitivity between 20 and 23°C than a bivoltine population (Takeda 1996). These results suggest that the temperature sensitivity of the photoperiodic response is implicated in the life cycle of insects and, consequently, is correlated to the latitudes of inhabited localities.
Geographic variation of developmental traits
Developmental period
Insects are ectothermic and their developmental rates are primarily regulated by ambient temperature. However, in many species of insects, the developmental rate is controlled not only by temperature but also by biological and other physical factors (Danks 1994). Photoperiod is a major factor regulating developmental rates in insects (Masaki 1967, 1972, 1978; Obrycki and Tauber 1981). In some insects, there is very little inter-population difference in the developmental period between different life cycles (Ritland and Scriber 1985; Pullin 1986; Bradford and Roff 1995). Bradford and Roff (1995) suggested that selection pressure is weaker in the developmental period than in the photoperiodic response controlling diapause induction in a cricket, Allonemobius socius. In the rice stem borer, C. suppresalis, however, the developmental period is shorter in the univoltine population than in the bivoltine population occurring at similar latitudes (Kishino 1970). In this moth, the developmental period is shorter in the northern populations than in the southern populations in each voltinism area (Kisino 1974). This type of geographic variation in life-history traits, called a “saw-toothed cline”, is observed in some insects (e.g. Kidokoro and Masaki 1978; Masaki and Walker 1987; Mousseau and Roff 1989; Scot and Dingle 1990; Nylin and Svärd 1991; Ishihara 1998), and has been theoretically analyzed (Roff 2002).
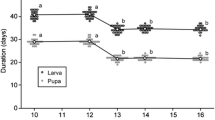
The larval and pupal periods of H. cunea, which were not destined for diapause, positively correlated with their latitudes of origin (Fig. 3), indicating that the developmental period of the bivoltine population occurring in the northern area is longer than that in the trivoltine population occurring in the southern area. However, no conspicuous difference in the developmental period was found among populations occurring around 36°N, which represents the transition zone between the voltinisms. Thus, the developmental period of H. cunea corresponds less clearly to the life cycle than the critical photoperiod for diapause induction.
Regional differences in the number of larval instars
The larvae of H. cunea aggregate and construct nest webs in the field until the fourth instar; thereafter, they disperse and live individually. Two types of H. cunea larvae, the six-instar and seven-instar, have been reported in North America (Morris and Fulton 1970) and Japan (Itô and Miyashita 1968). In Japan, the seven-instar type was predominant during the early stage of the invasion (Itô and Miyashita 1968). Gomi (1996b) and Gomi et al. (2003) investigated the larval developmental period and the incidence of the seven-instar larvae in four populations of H. cunea (Table 2). In the group-rearing experiment, where larvae were reared under crowded conditions throughout the larval stage, the larval period differed significantly among the populations (Tukey-Kramer test: P < 0.05). In the individual-rearing experiments, in which the larvae were reared individually from the fifth instar onwards, the larval period of the six-instar type was not different between the Akita and Saitama populations and between the Kobe and Kumamoto populations (P > 0.05). The larval period of the seven-instar types was significantly longer than that of the six-instar type in the Akita and Kumamoto populations (t test: P < 0.0001), while the incidence of the seven-instar type was significantly higher in the Kumamoto population than in the other populations (Tukey-type multiple comparisons for proportions: P < 0.05). These results suggest that the high incidence of the seven-instar type is one of the underlying factors for the longer larval period.
The incidence of the instar type is affected by sex and environmental factors in H. cunea (Gomi 1996b, 2006; Gomi et al. 2005). Gomi et al. (2005) found that the quality of the host leaves affect the larval period of the Kobe population and that the incidence of the seven-instar type increased when the larvae were reared on hosts in which the larval period was extended. A similar tendency was observed in Helicoverpa armigera (Casimero et al. 2000). The incidence of the seven-instar type has been found to increase at higher temperatures in females (Gomi 2006). In addition, the pupal weight of the seven-instar type is heavier than that of the six-instar type (Gomi 1996b; Gomi et al. 2003, 2005). There is a positive correlation between female body size and fecundity in H. cunea (Morris and Fulton 1970; Gomi 2000), as is the case in many other insects (Honek 1993). Therefore, while females of the seven-instar type have the advantage of a larger body size for fecundity, they suffer the disadvantage of a longer larval period for survival, as in a number of other insects (Fizgerald et al. 1988; Loader and Damman 1991; Slansky 1993; Atkinson 1994).
Univoltine grasshoppers in Europe were observed to have similar patterns of nymphal instar variation as H. cunea, in which the proportions of individuals with the larger number of instars increase with increases in temperature (Willott and Hassall 1998), at low latitudes (Telfer and Hassall 1999) and in females (Cherrill 2005). The temperature-size rule in grasshoppers was theoretically analyzed by Walters and Hassall (2006). In H. cunea, the incidence of the seven-instar type was not significantly different among the Akita, Saitama and Kobe populations. This result appears to be different from that in the grasshoppers, although only a small number of H. cunea populations have been analyzed to date. One reason for this difference may be that H. cunea has not spent enough time in Japan to adapt its developmental traits to the climate. Another explanation may be a difference in voltinism. The developmental traits of univoltine insects would respond more directly to local climate variables than those of multivoltine insects.
Conclusions and perspectives
If insects enter diapause too early in the season, their reproductive potential may be reduced (Taylor 1980, 1981). In the Tsukuba population of H. cunea occurring in the transition zone, the females that entered diapause in the third generation produced a larger egg mass than those that did so in the second generation (Gomi 2000). This result can be applied to the situation in the southwestern areas of Japan, in which the life cycle of H. cunea has shifted from bivoltine to trivoltine, most likely because the weather is warm enough to complete three generations. As such, individuals producing a third generation in the southwestern areas would be favored by natural selection.
In the southwestern areas of Japan, H. cunea had a bivoltine life cycle for the first three decades immediately following its invasion due to their photoperiodic control of diapause (Masaki 1975, 1977b). At present, the populations occurring in the trivoltine areas show shorter critical photoperiods and greater temperature sensitivity than the northern bivoltine populations. These traits of photoperiodic response contribute to the production of a third generation by reducing the incidence of diapause in the second generation. Therefore, individuals showing a short critical photoperiod and a high temperature sensitivity would have been selected for and, consequently, the present trivoltine populations have established themselves in the southwestern areas of Japan. Additional important factors in the life cycle of insects – other than the photoperiodic response controlling diapause induction – are the developmental traits (Zaslavsky 1988). The developmental traits of H. cunea showed local divergence in the present study. The progression of local divergence in developmental traits would modify the timing of photoperiodic induction of diapause and may lead to the formation of a linear geographic cline in the critical photoperiod in the future.
The climate of the Earth has warmed over the past 100 years, and there is ample evidence that climate changes have already affected a wide spectrum of organisms (Walther et al. 2002). In the pitcher-plant mosquito, Wyeomyia smithii, the critical photoperiod for diapause induction has become shorter in the northern area of the USA as a result of adaptation to recent global warming (Bradshaw and Holzapfel 2001). Preliminary evidence suggests that the life cycle of H. cunea is being influenced by the effect of global warming (unpublished data). Global warming may therefore be a strong modifying factor for the seasonal adaptation of H. cunea.
References
Arai Y, Akiyama Y (1976) Life cycle of Hyphantria cunea Drury (Lepidoptera: Arctiidae) in a mulberry field of Kumagaya district with special reference to the third generation (in Japanese with English summary). Jpn J Appl Entomol Zool 20:125–128
Atkinson D (1994) Temperature and organism size – a biological law for ectotherms? Adv Ecol Res 25:1–58
Beck SD (1980) Insect photoperiodism, 2nd edn. Academic Press, New York
Blem CR (1974) Geographic variation of thermal conductance in the house sparrow (Passer domesticus). Comp Physiol Biochem 47A:101–108
Bradford MJ, Roff AD (1995) Genetic and phenotypic sources of life history variation along a cline in voltinism in the cricket Allonemobius socius. Oecologia 103:319–326
Bradshaw WE, Holzapfel CM (2001) Genetic shift in photoperiodic response correlated with global warming. Proc Natl Acad Sci USA 98:14509–14511
Bradshaw WE, Lounibos LP (1977) Evolution of dormancy and its photoperiodic control in pitcher-plant mosquitoes. Evolution 31:546–567
Bradshaw WE, Zani PA, Holzapfel CM (2004) Adaptation to temperate climates. Evolution 58:1748–1762
Casimero V, Tsukuda R, Nakasuji F, Fujisaki K (2000) Effect of larval diets on the survival and development of larvae in the cotton bollworm, Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Appl Entomol Zool 35:69–74
Cherrill A (2005) Body size and phenology of the grasshopper species Chorthippus brunneus with variable numbers of female instars (Orthoptera: Acrididae). Entomol Gen 28:219–231
Cox GW (2004) Alien species and evolution: the evolutionary ecology of exotic plants, animals, microbes and interacting native species. Island Press, Washington D.C.
Danilevsky AS (1965) Photoperiodism and seasonal development of insects. Oliver and Boyd, London
Danks HV (1987) Insect dormancy: an ecological perspective. Biological Survey of Canada, Ottawa
Danks HV (1994) Diversity and integration of life-cycle controls in insects. In: Danks HV (ed) Insect life-cycle polymorphism: theory, evolution and ecological consequences for seasonality and diapause control. Kluwer, Dordrecht, pp 5–40
Danks HV (2006) Key themes in the study of seasonal adaptations in insects. II. Life-cycle patterns. Appl Entomol Zool 41:1–13
Del Pozo A, Ovalle C, Aronson J, Avedaño J (2002) Ecotypic differentiation in Medicago polymorpha L. along an environmental gradient in central Chile. I. Phenology, biomass production and reproductive patterns. Plant Ecol 159:119–130
Fizgerald TD, Casey T, Joos B (1988) Daily foraging schedule of field colonies of the eastern tent caterpillar Malacosoma americanum. Oecologia 76:574–578
Gomi T (1995) Effect of temperature on diapause response to photoperiod in Hyphantria cunea with special reference to local divergence. Appl Entomol Zool 30:490–492
Gomi T (1996a) Mixed life cycles in the transitional zone between voltinisms in the fall webworm, Hyphantria cunea. Experientia 52:273–276
Gomi T (1996b) A mechanism for the decrease in developmental period of a trivoltine population of Hyphantria cunea (Lepidoptera: Arctiidae). Appl Entomol Zool 31:217–223
Gomi T (1997) Geographic variation in critical photoperiod for diapause induction and its temperature dependence in Hyphantria cunea Drury (Lepidoptera: Arctiidae). Oecologia 111:160–165
Gomi T (2000) Effects of timing of diapause induction on winter survival and reproductive success in Hyphantria cunea in a transition area of voltinism. Entomol Sci 3:433–438
Gomi T (2006) Sexual difference in the effect of temperature on the larval development in Hyphantria cunea (Drury) (Lepidoptera: Arctiidae). Appl Entomol Zool 41:303–307
Gomi T, Takeda M (1990) The transition to a trivoltine life cycle and mechanisms that enforce the voltinism change in Hyphantria cunea Drury (Lepidoptera: Arctiidae) in Kobe. Appl Entomol Zool 25:483–489
Gomi T, Takeda M (1991) Geographic variation in photoperiodic responses in an introduced insect, Hyphantria cunea Drury, in Japan. Appl Entomol Zool 26:357–363
Gomi T, Takeda M (1996) Changes in life-history traits in the fall webworm within half a century after introduction to Japan. Funct Ecol 10:384–389
Gomi T, Inudo M, Yamada D (2003) Local divergence in developmental traits within a trivoltien area of Hyphantria cunea Drury (Lepidoptera: Arctiidae). Entomol Sci 6:71–75
Gomi T, Muraji M, Takeda M (2004) Mitochondrial DNA analysis of the introduced fall webworm, showing its shift in life cycle in Japan. Entomol Sci 7:183–188
Gomi T, Hirochika M, Nagasaka M, Hagihara H, Fukuda T (2005) Effects of diet on life-history traits in a trivoltine Kobe population of Hyphantria cunea (Drury) (Lepidoptera: Arctiidae). Appl Entomol Zool 40:475–482
Honek A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492
Ishihara M (1998) Geographic variation in insect development period: effect of host plant phenology on the life cycle of the bruchid seed feeder Kytorhinus sharpianus. Entomol Exp Appl 87:311–319
Itô Y, Miyashita K (1968) Biology of Hyphantria cunea Drury (Lepidoptera: Arctiidae) in Japan. V. Preliminary life tables and mortality data in urban areas. Res Popul Ecol 10:177–209
Itô Y, Miyashita K, Yamada H (1968) Biology of Hyphantria cunea Drury (Lepidoptera: Arctiidae) in Japan. VI. Effect of temperature on development of immature stages. Appl Entomol Zool 3:163–175
Itô Y, Shibazaki A, Iwahashi O (1970) Biology of Hyphantria cunea Drury (Lepidoptera: Arctiidae) in Japan. XI. Results of road survey. Appl Entomol Zool 5:133–144
Johnston RF, Selander RK (1964) House sparrows: rapid evolution of races in North America. Science 144:548–550
Kidokoro T, Masaki S (1978) Photoperiodic response in relation to variable voltinism in the ground cricket, Pteronemobius fascipes Walker (Orthoptera: Gryllidae). Jpn J Ecol 28:291–298
Kishino K (1970) Ecological studies on the local characteristics of seasonal development in the rice stem bore, Chilo suppressalis Walker. II. Local characteristics of diapause and development (in Japanese with English summary). Jpn J Appl Entomol Zool 5:1–11
Kisino K (1974) Ecological studies on the local characteristics of the seasonal development in the rice stem borer Chilo suppressalis Walker (in Japanese with English summary). Tohoku Agric Sta Bull 47:13–114
Lacey EP (1988) Latitudinal variation in reproductive timing of a short-lived monocarp, Daucus carota (Apiaceae). Ecology 69:220–232
Loader D, Damman H (1991) Nitrogen content of food plants and vulnerability of Pieris rapae to natural enemies. Ecology 72:1586–1590
Masaki S (1961) Geographic variation of diapause in insects. Bul Fac Agric Hirosaki Univ 7:66–98
Masaki S (1967) Geographic variation and climatic adaptation in a field cricket (Orthoptera: Gryllidae). Evolution 21:725–741
Masaki S (1972) Climatic adaptation and photoperiodic response in the band-legged ground cricket. Evolution 26:587–600
Masaki S (1975) Biology of Hyphantria cunea Drury (Lepidoptera: Arctiidae) in Japan: a review. Rev Plant Protect Res 8:14–28
Masaki S (1977a) Life cycle programming. In: Hidaka T (ed) Adaptation and speciation in the fall webworm. Kodansha, Tokyo, pp 31–60
Masaki S (1977b) Past and future. In: Hidaka T (ed) Adaptation and speciation in the fall webworm. Kodansha, Tokyo, pp 129–148
Masaki S (1978) Climatic adaptation and species status in the lawn ground cricket. II. Body size. Oecologia 35:343–356
Masaki S, Walker TJ (1987) Cricket life cycles. Evol Biol 21:349–423
Masaki S, Umeya K, Sekiguchi Y, Kawasaki R (1968) Biology of Hyphantria cunea Drury (Lepidoptera: Arctiidae) in Japan. III. Photoperiodic induction of diapause in relation to the seasonal life cycle. Appl Entomol Zool 3:55–66
Meyer SE, Allen PS (1999) Ecological genetics of seed germination regulation in Bromus tectorum L. I. Phenotypic variance among and within populations. Oecologia 120:27–34
Morris RF, Fulton WC (1970) Models for the development and survival of Hyphantria cunea in relation to temperature and humidity. Mem Entomol Soc Can 70:1–60
Mousseau TA, Roff DA (1989) Adaptation to seasonality in a cricket: Patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution 43:1483–1496
Numata H, Saulich AH, Volkovich TA (1993) Photoperiodic responses of the linden bug, Pyrrhocoris apterus, under conditions of constant temperature and under thermoperiodic conditions. Zool Sci 10:521–527
Nylin S, Svärd L (1991) Latitudinal patterns in the size of European butterflies. Holarctic Ecol 14:192–202
Obrycki JJ, Tauber MJ (1981) Phenology of three coccinelid species: thermal requirements for development. Ann Entomol Soc Am 74:31–36
Pittendrigh CS, Takamura T (1987) Temperature dependence and evolutionary adjustment of critical night length in insect photoperiodism. Proc Natl Acad Sci USA 84:7169–7173
Pullin AS (1986) Effect of photoperiod on the life-cycle of the peacock butterfly Inachis io. Entomol Exp Appl 41:237–242
Rice KJ, Mack RN (1991) Ecological genetics of Bromus tectorum. I. A hierarchical analysis of phenotypic variation. Oecologia 77:77–83
Riedl H (1983) Analysis of codling moth phenology in relation to latitude, climate and food availability. In: Brown VK, Hodek I (eds) Diapause and life cycle strategies in insects. Dr W Junk, The Hague, pp 233–252
Riedl H, Croft BA (1978) The effects of photoperiod and effective temperatures on the seasonal phenology of the codling moth (Lepidoptera: Tortricidae). Can Entomol 110:455–470
Ritland DB, Scriber JM (1985) Larval development rates of three putative subspecies of tiger swallowtail butterflies, Papilio glaucus, and their hybrids in relation to temperature. Oecologia 65:185–193
Roff DA (2002) Life history evolution. Sinauer Assoc, Sunderland
Sauer KP, Spieth HR, Grüner C (1986) Adaptive significance of genetic variability photoperiodism in Mecoptera and Lepidoptera. In: Taylor F, Karvan R (eds) The evolution of insect life cycles. Springer, Berlin Heidelberg New York, pp 153–172
Saulich AH, Volkovich TA, Numata H (1994) Control of seasonal development by photoperiod and temperature in the linden bug, Pyrrhocoris apterus in Belforod, Russia. Zool Sci 11:883–887
Scot SN, Dingle H (1990) Developmental programmes and adaptive syndromes in insect life-cycles. In: Gilbert F (ed) Insect life cycles. Springer, London, pp 69–85
Slansky F Jr (1993) Nutritional ecology: the fundamental quest for nutrients. In: Stamp NE, Casey TM (eds) Caterpillars. Chapman and Hall, New York, pp 29–91
Takafuji A, So P-M, Tsuno N (1991) Inter- and intra-population variations in diapause attribute of the two-spotted spider mite, Tetranycus urticae Koch, in Japan. Res Popul Ecol 33:331–334
Takeda M (1996) Photoperiodic induction, maintenance and termination of winter diapause in two geographic ecotypes of the rice stem maggot, Chlorops oryzae Matsumura (Diptera: Chloropidae). Appl Entomol Zool 31:379–388
Takeda M, Chippendale GM (1982) Phenological adaptations of a colonizing insect: the southwestern corn borer, Diatraea grandiosella. Oecologia 53:386–393
Tanaka S (1994) Diapause as a pivotal factor for latitudinal and seasonal adaptation in Locusta migratoria in Japan. In: Danks HV (ed) Insect life-cycle polymorphism: theory, evolution and ecological consequences for seasonality and diapause control. Kluwer, Dordrecht, pp 173–190
Tate K (2000) Occurrence of Hyphantria cunea in Hakodate (in Japanese). Shin-rin Hogo 280:43
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, New York
Taylor F (1980) Optimal switching to diapause in relation to the onset of winter. Theor Popul Biol 18:125–133
Taylor F (1981) Ecology and evolution of physiological time in insects. Am Nat 117:1–23
Telfer MG, Hassall M (1999) Ecotypic differentiation in the grasshopper Chorthippus brunneus: life history varies in relation to climate. Oecologia 121:245–254
Uezumi Y (1976) The first outbreak of Hyphantria cunea Drury in Nara Prefecture (in Japanese). Shokubutsu-boeki (Plant Prot) 31:355–356
Umeya K, Itô Y (1977) Invasion and establishment of a new insect pest in Japan. In: Hidaka T (ed) Adaptation and speciation in the fall webworm. Kodansha, Tokyo, pp 1–12
Vaz Nunes M, Koveos DS, Veerman A (1990) Geographic variation in photoperiodic induction of diapause in the spider mite (Tetranychus urticae): a causal relation between critical nightlength and circadian period? J Biol Rhythms 5:47–57
Walker TJ, Reinert JA, Schuster DJ (1983) Geographical variation in flights of the mole cricket, Scapteriscus spp. (Orthoptera: Gryllotalpidae). Ann Entomol Soc Am 76:507–517
Walters RJ, Hassall M (2006) The temperature-size rule in ectotherms: may a general explanation exist after all? Am Nat 167:510–523
Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Warren LO, Tadic M (1970) The fall webworm, Hyphantria cunea (Drury). Ark Agric Exp Sta Bull 759:1–106
Williams CK, Moore RJ (1989) Phenotypic adaptation and natural selection in the wild rabbit, Oryctolagus cuniculus, in Australia. J Anim Ecol 58:495–507
Willott SJ, Hassall M (1998) Life-history responses of British grasshoppers (Orthoptera: Acrididae) to temperature change. Funct Ecol 12:232–241
Yom-Tov Y, Green WO, Coleman JD (1986) Morphological trends in the brushtail possum, Trichosurus vulpecula, in New Zealand. J Zool London 208:583–593
Zaslavsky VA (1988) Insect development. Springer, Berlin Heidelberg New York
Acknowledgments
This study was supported by aid from the Japan Society for the Promotion of Science, the Japan Science and Technology Corporation and the Nippon Life Foundation.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gomi, T. Seasonal adaptations of the fall webworm Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) following its invasion of Japan. Ecol Res 22, 855–861 (2007). https://doi.org/10.1007/s11284-006-0327-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-006-0327-y