Abstract
It is difficult to find a genus of Lepidoptera showing the high variability of life history traits observed in Thaumetopoea. There are typical summer feeding close to winter feeding species, and in one special case a recent switch has been detected even within one species, the pine processionary moth, indicating that the natural history traits are constantly evolving at a fast rate. There are species adapted to cold conditions of high mountains and high latitude close to truly Mediterranean and sub-desert region species. All species have gregarious behaviour as larva and are protected against vertebrate predators by urticating setae.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
It is difficult to find a genus of Lepidoptera showing the high variability of life history traits observed in Thaumetopoea. Within the genus there are typical summer-feeding close to winter feeding species, and in one special case a recent switch has been detected even within one species, the pine processionary moth, indicating that the natural history traits are constantly evolving at a fast rate. There are species adapted to cold conditions of high mountains and high latitude close to truly Mediterranean and sub-desert region species (Fig. 2.1). The life cycle is typically annual but some species may spend up to 9 years of prolonged diapause as a pupa in soil, as a possible way to spread the risk of experiencing too harsh conditions for survival. On top of this, all species have a remarkable gregarious behaviour through all the egg (Fig. 2.2) and larval (Fig. 2.3) stages, and are well protected against vertebrate predators by billions of urticating setae they may release when disturbed. Because of all these features, they were called ‘Thaumetopoea’, which means the [insect that] ‘makes wonderful things’ [likely originating from the Greek words “θαύμα” that means “miracle” and “ποιώ” that means “do”] such as shining silk tents on top of trees or long head-to-tail processions at the time of pupation (Figs. 2.4, 2.5, 2.6). Here we summarize the information available for the life history of the most common species, with the general aim of finding what makes a few of them very good indicators of climate change.
(a) Adults of Thaumetopoea and related species with the indication of discriminant morphological traits and detail of scales of forewings: T. pinivora (♂ – ♀ coll. Zoologische Staatssammlung München); T. bonjeani (♂ – ♀ coll. Witt, München); T. libanotica (♂ Liban – Bscharre emerged Beirut, coll. Zoologische Staatssammlung München; ♀ coll. Witt, München); T. ispartaensis (♂ – ♀ Isparta – Senirkent, coll. Padua Univ.); T. cheela (♂ Afghanistan, Sarobi, coll. Zoologische Staatssammlung München; ♀ redrawn from Moore, 1883); T. pityocampa (♂ Veneto, Padova, Colli Euganei; ♀ Alto Adige, Val Venosta, coll. Padua Univ.); T. wilkinsoni (♂ – ♀ Turchia, Sarkikaragas and coll. Witt, Munchen). (b) Adults of Thaumetopoea spp. and related species with the indication of discriminant morphological traits and detail of scales of forewings: T. processionea (♂ Romagna, Forlì; ♀ coll. Witt, Munchen); T. herculeana (♂ – ♀ ex larvae, Portogallo, Vigo, Cabo Home, coll. Padua Univ.); T. solitaria (♂ http://www.nature-of-oz.com; ♀ coll. Witt, Munchen); T. jordana (♂ – ♀ Palestina, Jerusalem; coll. Zoologische Staatssammlung München); T. apologetica (♂ S. Rhodesia, Bulawayo, Glenville; ♀ Ofcolaco Tvl; coll. Schintlmeister); T. dhofarensis (♂ – ♀ Oman, Dhofar, prov. Jebel, Samhan M.ts 900–1,100 m, Tawi Attair; region; coll. Witt, München); Anaphe panda (♂ – ♀ Africa, coll. Oxford University Museum of Natural History); Ochrogaster lunifer (♂1 – Australia, 85 km SE of Broome (Len Willan and CSIRO Entomology); ♂2, ♂♀ 3) Australia, coll. Padua Univ.; ♀ Australia, 5 km E of Broome (Len Willan and CSIRO Entomology)
Larvae of processionary moths, Thaumetopoea (a) T. pinivora; (b) T. pityocampa; (c) T. processionea; (d) T. herculeana (Portugal, Vigo, Cabo Home); (e) T. solitaria; (a–c, e) redrawn from http://www.lepiforum.de; http://www.pyrgus.de; Carlos Gomez de Aizpúra (1986); (d) Credit: L. Berardi
(a) Larval colonies and tents of processionary moths, Thaumetopoea (a) T. pinivora; (b) T. bonjeani; (c) T. pityocampa; (d) T. processionea (Credit: L.M. Nageilesen); (e) T. herculeana. (b) Tents of T. pityocampa on unusual host plants: (f) Himalayan cedar (Cedrus deodara); (g) True fir (Abies sp.); (h, i) Douglas-fir (Pseudotsuga menziesii); (j) Eastern White pine (Pinus strobus); (k) European larch (Larix decidua) (Credit: A. Roques)
2 Natural History of the Pine Processionary Moth, Thaumetopoea pityocampa
2.1 Host Plants and General Distribution
Thaumetopoea pityocampa is oligophagous on Pinus and Cedrus species, both native and introduced. Occasionally it can be found on other conifer species, including those of the genus Pseudotsuga. It typically occurs on isolated trees and stand edges, although inner parts of the stands may also be colonised during outbreaks. The geographic range extends from northern Africa to southern Europe (see Roques et al. 2014, Chap. 3, this volume), from the Atlantic coast to the western part of Turkey.
2.2 Life Cycle
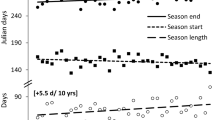
Thaumetopoea pityocampa has a very peculiar one-year development cycle, which is reversed compared to the other species of the genus and to most of other defoliating insects, because the larvae feed across the winter. As a result the adult emergence timing is largely affected by temperature (Démolin 1969a; Huchon and Démolin 1971; Zamoum and Démolin 2005; Pimentel et al. 2010). At colder sites (high elevation or high latitude), adults emerge as early as June whereas emergence can be delayed until September at warmer sites. In individual with annual development, this is regulated by a different length of the summer pupal diapause, which may extend into prolonged diapause under specific circumstances. On the mountains of Corsica Island the development cycle is semivoltine, i.e. with one generation over 2 years (Géri 1983a, 1983b). Figure 2.7 show typical life cycles observed in different parts of the range. In general, females are short lived, probably living for only 1 or 2 days, while males live longer (Zhang and Paiva 1998).
Schematic representation of typical life cycles of the pine processionary moth, Thaumetopoea pityocampa, in different biogeographic zones of France as defined by Huchon and Démolin (1970), Bouhot-Delduc (2005): (a) Italian Alps; (b) expansion area in north-central France = degraded oceanic climate; (c) south oceanic climate; (d) summer population in Portugal; (e) Algeria; (f) southern Turkey (T. wilkinsoni); (g) oceanic climate; (h) endemic areas in the northern coast of the Mediterranean sea and Corsican littoral; (i) Corsica mountains; (j) continental climate; (k) north oceanic climate. Only the dominant annual cycle is figured although possibilities of prolonged diapause exist (Abgrall 2001), except for population (i) where prolonged diapause is obligatory. Dates of 50 % emergence are only indicative because depending on the annual variations in weather conditions.
The dispersal capacity of the female is limited although a few individuals may fly more than 10 km (see Sect. 2.5), whereas the males disperse over much longer distances. Females lay about 150–350 eggs, depending on environmental conditions that may affect also egg size (Zovi et al. 2008; Pimentel et al. 2012). Populations from the very southern edge of the natural range (Eastern Morocco and Saharan Atlas Algeria) have a lower fecundity (105–150 eggs/batch) although they do not differ in the other natural history traits (Zamoum 1998; Zamoum and Démolin 2005; El Alaoui El Fels unpublished data). The eggs are laid in batches on pine needles or twigs shortly after moth emergence and are covered with scales produced by the female. The eggs hatch in July at colder sites and in October at warmer sites. Groups of neonate larvae are full siblings, but larval groups commonly merge at later instars, sometimes forming aggregates of several hundred individuals. Larvae build silk tents right after they hatch from the eggs, and leave them empty when they move to more sun-exposed parts of the tree. The shape and thickness of the silk tent (Fig. 2.4) depends on the number of individuals, host plant, and climate. One colony of larvae can build 2 or 3 of such tents before establishing the final one where larvae stay until the end of their development, which goes through five instars. Initially larvae feed on current-year needles near the oviposition site, but soon after they switch to old needles for the rest of the development. They can go back to current-year needle only when the old ones have been eaten. Feeding mostly occurs at night. Larvae of the third to fifth instar feed when temperature is above 0 °C provided that the colony temperature during the day before has reached 9 °C inside the tent (Battisti et al. 2005). From the third instar larvae develop urticating setae about 0.2 mm in length, situated in groups on the dorsal parts of the abdomen. When larvae are disturbed, setae are actively released, and can cause severe allergic reactions to humans (see Moneo et al. 2014, Chap. 8, this volume). Larvae leave trees in typical head-to-tail processions to search for suitable sites in soil for cocoon spinning as early as December at warmer sites and years, but more typically between February and May. When a suitable site is found, such as open areas and forest edges (Démolin 1971), larvae join in digging through the soil down to a depth of 5–20 cm. Cocoons remain in soil for the next one to a few months until the emergence of moths. A certain proportion of the cocoons enter a prolonged diapause which can be extended over several years (Géri 1983a, 1983b; Démolin 1990).
2.3 Natural Enemies
The natural enemies of T. pityocampa have been firstly reviewed by Biliotti (1958) and since then considered in several studies. A complete list is given in Tables 2.1, 2.2, and 2.3, with additional information on their importance in a Mediterranean country where they have been extensively studied (Zamoum 1998). Figure 2.8 presents some of the parasitoids and predators.
Natural enemies of pine processionary moth. (a–e): egg parasitoids (a, b, c – Baryscapus sp.; d, e – Ooencyrtus sp.); (e, f) – pupal parasitoid, Coelichneumon sp. (e adult; f adult emergence hole on cocoon); (g) adult of a Tachinidae fly ovipositing on larva; (h) larval colony killed by virus; (i) pupae killed by fungi; (j) predation of pupae by hoopoe; (k, l) other signs of predation on pupae
The eggs are mainly parasitized by Baryscapus servadeii and Ooencyrtus pityocampae, while a few other polyphagous species may occur as well. Baryscapus servadeii is restricted to conifer-feeding Thaumetopoea species, on which it develops one generation per year, which is well synchronized with the availability of the host eggs (Battisti 1989). O. pityocampae is polyphagous and has several generations per year on various hosts. The overall parasitism can locally reach up to 45 % (Tsankov et al. 2006).
Larvae are parasitized after the third instar by the tachinid fly Phryxe caudata, which completes two generations per year, with the first emerging in spring from mature larvae and the second in summer-autumn from pupae (Buxton 1990). A number of other species of larval and larval-pupal parasitoids (Diptera and Hymenoptera) have been reported at lower frequencies. Mortality from generalist arthropod predators can be locally high (e.g. the syrphid fly Xanthandrus comtus) and several predators are found inside larval tents (Branco et al. 2008). In contrast, larvae seem to be well protected against vertebrates (Barbaro and Battisti 2011). A number of pathogenic organisms have been described to affect the larvae, the most frequent being cytoplasmic and nuclear viruses (Vago 1959) and entomopathogenic nematodes (Triggiani and Tarasco 2002).
Pupae are parasitized by a number of specialized insects such as the bombylid fly Villa brunnea, the ichneumonid Coelichneumon rudis, and the pteromalid Conomorium pityocampae, which may interfere with the most common predator, the hoopoe Upupa epops (Battisti et al. 2000; Barbaro et al. 2008). They are often contaminated by entomopathogenic fungi, with Beauveria bassiana being the most common one (Battisti et al. 2000).
2.3.1 Population Dynamics
The literature often reports periodic outbreaks of T. pityocampa, although periods may have different lengths. This has been observed on pine in French mountains (from 1959 to 1982, Géri et al. 1985), in the Italian Alps (from 1950 to 2011, Hellrigl 1995; Tamburini et al. 2013), and on pine and cedar in Algeria (Zamoum et al. 2007; Sbabdji and Kadik 2011) and in Morocco (El Alaoui El Fels, unpublished data). The analysis of long-term series (>30 years) of population density, based on the annual survey of tent numbers in endemic French stands which were not treated with any pesticides, tended to indicate the existence of a rough 6-year cycle when a delayed Ricker model is applied (Fig. 2.9; Robinet 2006). Other datasets from Algeria, France, and Spain point to the same direction but still have to be analysed. Pest control and tree growth are important confounding factors and for this reason it is difficult to draw conclusions.
Observed outbreak cycle (thick line) in pine stands of two areas of north-central France (Loiret; Cher) compared to simulations (dashed line) resulting from a delayed Ricker model accounting for temperatures (Modified from Robinet 2006)
Potential factors involved in the population dynamics of T. pityocampa have been seldom studied using long-term data. Tamburini et al. (2013) found that a negative density dependent feedback with a 1-year lag emerged as the most important factor driving the population dynamics in the Southern Alps. Potential mechanisms explaining the observed negative density feedback include deterioration of host quality, increased mortality caused by pathogens, and increase of prolonged diapause as an adaptive mechanism to escape adverse conditions as explained above. Little information is available on the density dependence of natural enemies, which may possibly drive the periodicity in the outbreaks. Some small scale studies have indicated that outbreaks usually result in a deterioration of food quality and a decrease of food quantity that seem to be important factors responsible for the collapse of the population (Avtzis 1986; Battisti 1988; Hódar et al. 2004). Considering the natural enemy species associated with T. pityocampa, a relatively large number of predators and parasitoids are expected to respond numerically to moth density potentially producing negative density feedback on host populations (Zovi et al. 2008). Due to the gregarious behaviour of the larvae, high population density during outbreaks is further expected to trigger the spread of viral diseases with potential negative effect on its populations. In addition, the mechanism of prolonged diapause of the pupae, which may extend up to 8 years under mountain conditions (Battisti unpublished data), is causing an additional noise in the dynamics and in the relationships that the insect may establish with the complex of the natural enemies. All this makes the population dynamics of the pine processionary moth rather unpredictable at the global scale, while locally it may well result that a periodicity may exist.
2.4 Relationships with Climate Change
Several climatic parameters are expected to affect moth population dynamics. Winter temperatures were found to be the main limiting factor for the moth development and range expansion mainly in the northern part of the pest geographical distribution (Huchon and Démolin 1971; Battisti et al. 2005). During the last decades, temperature warming has affected the natural distribution of the pine processionary moth, which has expanded its range both in latitude and in elevation (Hódar and Zamora 2004; Battisti et al. 2005; Buffo et al. 2007; Robinet et al. 2007, 2013). Warmer temperatures may contribute to an improvement of larval performance, and therefore winter survival, in concert with to a decreased probability of occurrence of lethal temperatures (Hódar et al. 2003). The improved performance results from the combined effect of night temperature permissive of feeding (T > 0 °C) and day temperature allowing food digestion when the larvae rest in the tent (T > +9 °C). The high day temperature is obtained through exposure of the larval tents to solar radiation, even when air temperature is far below the threshold of +9 °C (Battisti et al. 2005). The improved larval performance has resulted in a progressive colonization of areas outside of the core range. Furthermore, moth mobility has been found to be favoured by the increase in temperature, as warmer summer nights allow a more frequent achievement of the flight threshold temperature (Battisti et al. 2006). In addition, human-inadvertent translocation of the insect, likely as pupae in the soil of ornamental trees, has overcome the natural dispersal limitation and resulted in the establishment of populations outside of the range, possible because of the improved thermal conditions (Robinet et al. 2011, 2013). A recent long-term study (Tamburini et al. 2013) indicates that both summer temperatures and rainfall significantly affected population growth rate, with different outcomes depending on the local conditions. Although previous studies indicated that low winter temperatures have negative effects on insect performance, these analyses did not show any negative impact on population dynamics. Other potential important climatic factors are rainfall (Pimentel et al. 2011; Hódar et al. 2012) and summer temperatures affecting adult dispersal (Battisti et al. 2006). Extreme events of high temperatures during summer may further contribute to high mortality of early instar larvae (Santos et al. 2011a, 2011b; Robinet et al. 2013) and locally reduce populations. Despite the clear effect of climate on range expansion, its effect on population dynamics and outbreak propensity has not been fully elucidated yet. A recent study (Hódar et al. 2012) has investigated the response of T. pityocampa defoliation to the atmospheric pattern called NAO (North Atlantic Oscillation) finding significant correlations between this global climate index and pest damage. However, it would be important to further evaluate the relative importance of single climatic variables and to consider their effect in combination with endogenous, density-dependent factors.
The scarce knowledge about the drivers of population dynamics is currently preventing to predict large-scale response of the species to climate change. Even if the temperature effects on insect physiology are well known, the influence of the single climatic variables on the population dynamics is still largely unclear (but see Pimentel et al. 2011; Tamburini et al. 2013). This is probably due to the paucity of studies based on long time-series that are able to give a comprehensive view of the moth dynamics and interaction with exogenous factors. The study of Hódar et al. (2012) has been a first step in this direction showing the potential for a good predictive power of climatic models to forecast forest defoliation by T. pityocampa.
2.5 Morphological, Physiological and Behavioural Adaptations of Pine Processionary Moths in the Expanding Areas
Insect range expansion related to climate change (Parmesan 2006; Hill et al. 2011) has often been associated with increased dispersal capacities (Hill et al. 1999, 2011; Thomas et al. 2001), generally explained by the selection during expansion of insects with higher dispersal capacities. Studies carried out during the recent years within the Urticlim project supplied information about the possible changes in the biology of the pine processionary moth in the Paris basin (France) where the species is expanding northwards since the late 1980s. The characteristics of the moth populations were compared along a gradient of decreasing duration of the insect presence, from endemic, core areas (presence observed about a century ago or more), to newly-colonized zones (presence since 15–20 years) and front edge (presence since 5 years at most) (see Fig. 3.7). Not yet invaded zones, located beyond the expansion front, were also used to test experimentally the survival potential of the moth under those conditions. This section briefly presents their main results, which will be extensively published soon.
In 2008, preliminary studies have shown changes in the phenology and quantity of urticating setae (Battisti et al. unpublished data). Bred in the same conditions, populations from the expansion areas developed faster than those from the core area, with a 2-day advance in the adult emergence peak. Meanwhile, larvae from the expansion areas appeared to release about twice as many urticating setae as those from the core area.
In 2009 and 2010, cross-translocation experiments of larval colonies between the different areas of the expansion gradient in the Paris basin were realized in order to assess adult traits, especially those related to dispersal. During autumn, a total of 80 colonies of early-instar larvae (2nd larval stage) were simultaneously collected per site in the core, newly-colonized and front areas (see Fig. 3.7). Each tent lot was then divided into four groups of 20 tents, and one group from each sampled area was translocated and grafted on pines (one larval tent per tree) in the two other areas of the expanding gradient. The two groups remaining per lot were either grafted on the sampling site (control) or in a non-invaded site beyond the front edge. The larvae were allowed to continue their development on the grafted pines until spring, when the mature tents were collected just before the larvae begin to process for pupation. The collected colonies were then stored at the same location, in an outdoor insectary at Orléans, France, where they were put in individual containers filled with soil allowing pupation. Further development was surveyed until the adult emergence. Emerging adults from each combination of sampled/translocated and development areas were used to test for the relative importance of population and environmental effects by comparing morphological (thorax width, wing size), physiological (energy resources) and behavioural (flight capabilities) variables among the different insect groups. Energy resources usable for flight (carbohydrates and fat content) were quantified according to the methods described by Van Handel and Day (1988). Flight capacities were tested in laboratory using flight mills (Fig. 2.10), made up with a horizontal carbon fibre arm plugged on a vertical axis using a ball bearing. Tested insects were plugged on the end of the arm. When flying, they dragged the arm, each revolution of which being detected with an IR transmitter-receiver set and recorded on a computer, allowing to measure the total distance travelled by the insect and other parameters of the flight. Each insect was tested during 24 h. The experiment was repeated during 3 years, from 2008 to 2011.
The dispersal potential of T. pityocampa measured on flight mill appeared significantly more important than the estimations of previous studies based on empirical observations or rough experiments of release-recapture. In 2008–2009, the mean distance covered by males and females were 10.9 km (up to 41 km) and 1.9 km (up to 10.5 km), whereas female flight was considered as very limited, from a few dozen meters up to 2 km (Démolin 1969b). The values observed during the two following years were even much more important with a mean distance averaging 17 and 24 km for males, with flights up to 61 and 56 km in 2009–2010 and 2010–2011, respectively. In the same years, female flight averaged 5.6 and 5.1 km, with maximum values up to 27 and 24 km, respectively. Although measured in a very artificial environment, these data were consistent with the observed expansion speed of the moth (about 5 km a year; Battisti et al. 2005), so they could be representative of the actual flight capabilities of females in the field.
Interestingly, two clines in morphological variables were also observed in 2008–2009. First, the adults originating from the newly-colonized area were smaller, whatever the place they were translocated, than those originating from the core area. Second, those obtained from larvae which had developed in colder areas at the front edge and beyond the front were smaller, whatever their primary origin, than those which had developed in warmer areas (core and recently-colonized areas). These differences were observed in both males and females and for all size variables. Carbohydrates and fat contents were also lower in females originating from the front edge than in those originating from the core area. A similar trend was noticed for female (but not male) flight capabilities, which were less important in females from the front edge or which had developed in colder areas. However, this trend remained not significant statistically. Moreover, these clines were not observed during the two following years which were characterized by unusually severe climatic conditions resulting in very low winter survival and emergence rates.
According to these preliminary studies, moth expansion may be associated to changes in insect features such as phenology, urticating power, size, and partially energy resources and perhaps flight capabilities. The shift in the adult phenology can be integrated in its known variation in the range, where it has been observed that adult flights occur earlier in its northern part (Démolin 1969a). This variation is interpreted as an adaptation to colder climates, with the early flight allowing an earlier larval development, so that the larvae can enter winter in its more cold-resistant fourth larval instar. The clines observed (or suspected) in size and related variables (energy resources, flight capabilities) in relation with population origin and development environment combine their effects in the field, both resulting in smaller insects in the expansion area. The environmental cline may result from a decreasing fitness in the northern areas due to harder climatic conditions. This cline tends to reveal an adaptation of moth populations to the expansion area, and suggests that small and short-dispersing insects may be selected during the expansion process. Similar clines have been observed in species with fragmented range (Hill et al. 2011). It could be due to an Allee effect which reduces the fitness of long-dispersing insects at the range margins. However, contrasting results obtained in the second and third year of the experiment indicate that the expression of the two clines may depend of environmental factors and could for example depend on unusual climatic conditions.
3 Recent Occurrence of a Summer Population of Thaumetopoea pityocampa in Portugal
3.1 Host Plants and General Distribution
The summer population of Thaumetopoea pityocampa was found originally in Portugal in a plantation of maritime pine, Pinus pinaster, which is, to date, the sole host species known for this atypical population. However, since the summer population is expanding to other areas, it can be expected to colonize other conifer species in the future.
3.2 Life Cycle
The life cycle of the atypical summer population has been studied since its discovery (Pimentel et al. 2006; Santos et al. 2007; Pimentel et al. 2010) (Fig. 2.11). Adults start emerging from the soil by the end of April and mate just after emergence. Usually females die after egg laying, but males can persist for 3–4 days. In laboratory experiments both males and females showed a good flight capacity (H. Santos, personal observation). Fecundity was demonstrated to be lower in the summer population, when compared to winter populations, either sympatric or originating from other regions (Santos et al. 2013).
Eggs are mostly laid in the field between the end of April and early July, when adult emergence usually ceases. The oviposition period may slightly vary from year to year (for more detail about the ecology of the summer population see Kerdelhué et al. 2014, Chap. 4, this volume). Hatching starts about one month later. The young larvae are gregarious and start hatching and feeding on P. pinaster, between the end of May and the beginning of July, preferring 1 year old needles. The early instars spin small temporary tents and change location several times. When multiple egg batches are laid on the same tree, colonies can merge in a single tent. Like the typical T. pityocampa populations, from the third instar onwards larvae produce urticating setae and spin definitive, loose tents that will be used until the end of larval development.
In this atypical population, larval development is much faster than in the typical, winter populations, probably because it takes place during the warmer months. By the end of September the larvae have reached the fifth instar and descend from the trees in processions, searching for a suitable place to bury in the soil. Larvae bury between 5 and 15 cm deep where they pupate. The pupae enter a winter diapause, which is just the opposite of the summer diapause of the typical form. It is not clear if the summer population may have prolonged diapause such as in the typical form.
3.3 Natural Enemies
Egg parasitoids attacking the summer population belong to the same species known for the winter populations (Fig. 2.8), although their frequencies are different. Ooencyrtus pityocampae (Hymenoptera, Ooencyrtidae) and Trichogramma embryophagum (Hymenoptera, Trichogrammatidae) are the two main species, followed by Baryscapus servadeii (Hymenoptera, Eulophidae). Eggs are predated by orthopteran species of the family Tettigoniidae.
Larvae are mostly parasitized by Phryxe caudata (Diptera, Tachinidae). Ants, particularly the Argentine ant Linepithema humile (Hymenoptera, Dolicoderinae), are important predators of the summer population, since they can consume up to 100 % of the larvae of an infested tree (Way et al. 1999). Birds from the genus Parus are known to prey on late instar larvae (Pimentel and Nilsson 2009). The fungus Beauveria bassiana (Moniliales, Moniliaceae) is another factor of mortality for the larvae and pupae of the summer population. Parasitoids of the pupal stage so far recorded are P. caudata, Erygorgus femorator (Hymenoptera, Ichneumonidae) Villa brunnea (Diptera, Bombyliidae) and Coelichneumon rudis (Hymenoptera, Ichneumonidae), the three last species being found in very small numbers.
3.4 Population Dynamics
When it was discovered in 1997, the summer population was in an outbreak situation having caused very severe damage to a 10–15 year old pine plantation. The affected area has since expanded northwards and southwards, currently extending about 80 km along the coast line. Within the plantation area, plots with trees over 40–50 years old were not attacked. In urban and peri-urban areas isolated pines, or small tree aggregates, have also been colonized. In the core area, infestation levels remained high, i.e. 25–75 % defoliation, between 1997 and 2005 when they started decreasing, reaching the low levels of damage presently observed, while spots of high density can be found in the expansion area.
3.5 Relationships with Climate Change
Although the expansion range of the summer population has progressed both northwards and southwards in relation to the initial core area, it is still confined to a geographical region with similar climatic conditions. However, under a scenario of global warming, an increase of the maximum temperatures might be limiting for the survival of the larval stage of this population, since it takes place during the summer, consequently precluding its expansion. Yet, an adaptation of this population to hot summers is also plausible (Santos et al. 2011a, 2011b).
4 Natural History of the Eastern Pine Processionary Moth, Thaumetopoea wilkinsoni
4.1 Introduction
The eastern pine processionary moth, Thaumetopoea wilkinsoni, was described as a new species by Tams (1925), who studied specimens found in Cyprus and compared their male genitalia with those of T. pityocampa. Démolin (1969a) found significant morphological overlapping between populations of these two taxa (Fig. 2.1); and, furthermore, both use the same female sex pheromone: (Z)-13 hexadecen-11-ynyl acetate (e.g., Frérot and Démolin 1993). Recent genetic studies (e.g., Salvato et al. 2002) indicated the existence of strong genetic differentiation between the two species, which became separated before the Quaternary ice ages (see Chap. 4). Therefore, we consider it as a separate species although more analyses in the contact zone of western Turkey are needed in order to define the overlapping area and potential introgression.
4.2 Host Plants and General Distribution
Thaumetopoea wilkinsoni develops on native and introduced Pinus spp. in the East Mediterranean, from western Turkey to Israel, and occasionally colonizes Cedrus spp., especially C. atlantica (Halperin 1990a, 1990b). In Turkey, mainly P. nigra, P. brutia brutia, P. halepensis, P. pinea, and P. radiata are colonized (Çanakçıoğlu and Mol 1998); according to İpekdal and Çağlar (2012), T. wilkinsoni preferred P. nigra in a multiple choice experiment. In Israel, T. wilkinsoni occurs mainly on P. canariensis, P. halepensis, and P. brutia brutia; on wet sites it is observed in high densities also on P. brutia eldarica and on P. radiata. In mixed stands the moth seems to prefer the exotic P. canariensis, P. radiata and P. brutia eldarica to the native P. halepensis and P. brutia brutia (Mendel 1988). Two other common exotic species in Israel, P. pinea and P. pinaster, are rarely attacked by the moth. In Cyprus, the moth primarily attacks the native pine, Pinus brutia brutia, the dominant tree species on the island, and, less frequently, P. nigra pallasiana; it also attacks the exotic pine species P. canariensis and P. halepensis, but not P. pinea (Ciesla 2004).
4.3 Life Cycle
Thaumetopoea wilkinsoni has a 1-year development cycle, which is similar to that of T. pityocampa (Fig. 2.7). The first comprehensive study of the moth was conducted by Wilkinson (1926). The adult females fly short distances (<2 km) while males may travel long distances (>50 km) in open areas, and they are strongly attracted to the sex pheromone of the female. Mating may occur on the ground before the female concludes its wing spread. Five instar larval development occurs in autumn and winter, and its duration depends mainly on temperature. At colder sites (high elevation or high latitude) the adults emerge in late July; at warmer sites, in October. In Israel these extremes are manifested by early flight (from late August to mid-September) in the Upper Galilee, and late flight (from late September to mid-October) in the western Negev. Observations in Lebanon (Houri and Doughan 2006) and Israel (Mendel, unpublished) suggested that emergence is triggered by the first significant rainfall. The females survive for 1–2 days and lay about 200 eggs on needles (and rarely on twigs during outbreaks). The eggs hatch in August at colder sites and in November at warmer sites. Özkazanç (2002) recorded a 1-month difference in the beginning of the larval stage, over an 800 m elevation difference (earlier at the higher elevation). The larvae are gregarious, and construct silken tents throughout their development: 2 or 3 fragile temporary webbing during the first two instars, and a permanent massively webbed tent for the last three instars. Studies in Israel suggest that an average tent harbour brood of 2–4 females. The two early instars feed during the day; the older ones pass the day in tents and feed during the night. From the third instar onward the larvae are covered with urticating setae which reach their highest density in the fifth instar. The larvae descend from trees to the ground in a typical procession during spring, until early May. Pupation takes about 4–5 months; possibly shorter at high elevations. Mendel (unpublished data) observed the processions of larvae descending for pupation in mid-July in the Shuff Mountains in Southern Lebanon. Summer pupal diapause can be prolonged up to 9 years (Halperin 1990a, 1990b).
4.4 Natural Enemies
The most common natural enemies are shared with T. pityocampa (Fig. 2.8), starting from the egg parasitoids Ooencyrtus pityocampae and Baryscapus servadeii. In Israel the populations of the former species develop during the summer in the eggs of the caper bug Stenozygum coloratum (S. Samra, personal communication). Three other egg parasitoids of the moth, Pediobius bruchicida, Anastatus bifasciatus, and Trichogramma embryophagum, are usually much less common. Overall, parasitism can be as high as 52 % locally (Avcı 2000; Avcı and Oğurlu 2002; Mirchev et al. 1998, 2004; Pekel 1999; Tsankov et al. 1998), and close to 80 % in extreme cases in Israel, where the parasitism level in urban areas is much lower than in the forests (Mendel unpublished data). The parasitic fly Phryxe caudata attacks the larvae in southern populations (unpublished data). Formica rufa and Calosoma sycophanta are known as larval predators of the eastern pine processionary moth and are used in biological control projects in Turkey (Avcı 2000). The hoopoe Upupa epops and the boar Sus scrofa and several forest bird species, especially Parus major, are known to feed on larvae and/or pupae. On wet sites significant pupal mortality is caused by the entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae.
4.5 Population Dynamics
On an area basis T. wilkinsoni is the dominant forest pest in the Middle East, and the major pine defoliator. Outbreaks of T. wilkinsoni have been observed in the area since the late nineteenth century in Cyprus and the early twentieth century in Turkey (Schimitschek 1944), with the first record of high population density in İstanbul in 1904 (İpekdal 2012). Another recorded outbreak was from İzmir in 1949 (Acatay 1953), and a synchronized outbreak was recorded throughout Turkey between 1996 and 1997 (Gabir 1998). In Israel outbreaks became frequent since the 1950s, with the establishment of large areas afforested with P. halepensis (Halperin 1990a, 1990b).
The number of tents on a single tree can be as high as 100 during outbreaks in southern regions of Turkey (Mol and Küçükosmanoğlu 2002). Low winter temperatures seem to play an important role in the build-up of the moth population at high elevations and high latitudes. In dry areas in Israel outbreaks occur after rainy winters that follow several years of drought. Defoliation of pine forest mainly happens in young stands, and colonization is observed as early as the third year after planting or regeneration after a fire, and reaches its peak about 10 years later. In adult stands the population is low, with only trees on the margin of the plots being lightly infested; heavy thinning coupled with rainy winters encourages population build-up in adult stands. In Israel the moth is also a severe pest of ornamental pine trees in urban areas, where mature trees are prone to attack. This susceptibility in urban habitats is the consequences of continuous tree growth, thanks to ample water supply, via either irrigation or root penetration of sewage systems. City pine trees may be heavily infested year after year, unlike the typical dynamic in the forest, where heavy defoliation results in poor performance of the larvae in the first and second year after a major outbreak.
The urticating setae located on the back of the late instar larvae (see Moneo et al. 2014, Chap. 8, this volume) form the major reason for needing to control the moth population. The moth is a great nuisance both to residents in urban areas, especially children on playgrounds, and to visitors and travellers camping on infested sites (Solt and Mendel 2002). Successful management of the moth is accomplished by aerial application of microbial insecticides based on Bacillus thuringiensis formulations. To prevent T. wilkinsoni infestation on ornamental pine trees, systemic insecticides are applied via stem injection. Since 2006 the use of Monocrotophos (a very effective organophosphate compound) formulations has been banned in Israel. Stem injections to control T. wilkinsoni are applied successfully with commercial products of Emamectin benzoate or Azadirachtin (Mendel, unpublished results).
4.6 Relationships with Climate Change
İpekdal and Çağlar (2012) showed that rising temperatures blur host preferences of T. wilkinsoni, and thus may bring new threats to additional Pinus species in a warmer future. The question whether duration of pupal diapause will be influenced by frequent drought periods is still open (Halperin 1990a, 1990b). The prolonged diapause may also favour the establishment of the moth in loosely managed forests on a warming globe. However, more data are needed to enable development of general inferences to use in future afforestation works and management plans. Simonato et al. (2007) found that the spreading of the moth from the initial range was contiguous, indicating a great ability to colonise areas which become suitable for insect survival. However, in the East Mediterranean the moth already occupies all potential habitats in the infested areas, while it can still expand in the northeast. İpekdal and Beton (2013) showed a possibility of range expansion at the northern edge in Turkey towards Caucasus between 2050 and 2080. In light of the thriving of the moth population in pine plantation in semi-desert areas in Israel, it is suggested that global warming will not cause the retraction of the range at the southern edge of T. wilkinsoni.
5 Natural History of the Northern Pine Processionary Moth, Thaumetopoea pinivora
5.1 Host Plants and General Distribution
Thaumetopoea pinivora is monophagous on Scots pine Pinus sylvestris, but is occasionally found also on black pine, Pinus nigra, and mountain pine, Pinus mugo. Typical habitats include light stands on poor soil, and populations are scattered from south-western Europe (Iberian Peninsula) to northern Europe (around the Baltic Sea).
5.2 Life Cycle
The following is a summary of what is currently known mainly based on field observations in Sweden (Aimi et al. 2008; Ronnås et al. 2010), and from what is known from mostly older literature data from Germany and Spain (Koch 1953; Hering 1970; Montoya and Robredo 1972). Thaumetopoea pinivora, at least in northern Europe, has a 2-year development cycle (Fig. 2.12). In Sweden, adults emerge from the cocoons in the soil in July–August (Figs. 2.1 and 2.6). Females are short lived, probably living for only one or a few days. The dispersal capacity of the female is limited, as indirectly shown by molecular markers (Ronnås et al. 2011), whereas the male can disperse over much longer distances. Females develop about 100–200 eggs. The eggs are laid in batches on the pine needles shortly after female emergence and are covered with scales produced by the female. The eggs are laid in the opposite direction compared to those of T. pityocampa, i.e. the female is pointing to the needle base (Fig. 2.2). The eggs hatch in late April the following year. Groups of neonate larvae are full siblings, but larval groups commonly merge at later instars, sometimes forming aggregates of up to 1,000 individuals. Larvae do not build tents, in contrast to T. pityocampa and T. processionea. The larvae go through five instars and feed until late July (Fig. 2.4). The larvae feed on mature needles, even in later instars when current-year needles have developed. The feeding mostly occurs during the night.
First and second instar larvae have a distinct basking behaviour, forming dense clusters at the tip of branches directed towards the sun and thus accumulate heat during the rather cold days in early spring (Ronnås et al. 2010; Battisti et al. 2013). Late-instar colonies, however, hide on the trunk or on a major branch supposedly in order to reduce overheating. From the third instar larvae develop urticating setae about 0.1 mm in length, situated in groups on the dorsal parts of the abdomen. When the larvae are disturbed setae are actively released, and can cause severe allergic reactions in humans (Holm and Larsson 2006). When ready to spin cocoons the larvae leave the trees in typical head-to-tail processions to search for suitable sites in the soil. When a suitable site is found the larvae join in digging through the soil down to a depth of 5–20 cm. The cocoon overwinters and stays in the soil for most of the following summer before emerging in late July. A certain proportion of the cocoons have a prolonged diapause. The Swedish T. pinivora population on Gotland Island has discrete year classes with high larval densities in even years, whereas much lower densities occur in odd years (Larsson et al. 2006) (Fig. 2.12).
5.3 Natural Enemies
Very little specific information is available on natural enemies of T. pinivora. The eggs of Spanish populations share the parasitoids Baryscapus servadeii and Ooencyrtus pityocampae with T. pityocampa in the areas where the two species coexist (Sierra de Guadarrama, Battisti unpublished data) whereas no egg parasitoids have been found in the Swedish population despite thorough searches. Egg predation by birds has been observed in Sweden during winter, accounting for about 20 % of egg loss (Ronnås unpublished data). The larvae of late instars in both Spain and Sweden are parasitized by the tachinid fly Blondelia pinivorae, which complete the development in pupae (Agenjo 1941; Bergstrom and Bystrowski 2011). No population estimates are available, but patch parasitism on Gotland Island can be as high as 20 % (Larsson, unpublished data). In Sweden, mortality from generalist arthropods predators (spiders, ants) can be quite high in young instars (Aimi et al. 2008; Ronnås et al. 2010). The pupae have been found to be infested by fungi but no precise information is available on mortality rates. Occasional cocoon predation by birds has been observed in Sweden, but is most likely of minor importance for the population dynamics. No information on adult predation is available.
5.4 Population Dynamics
Outbreaks of T. pinivora have been documented from central and northern Europe but never over large areas. There are no outbreaks recorded from southern Europe. The first record of high population density was in Germany in 1891 (Altum 1895, 1896). Quantitative estimates of outbreak size are available from Germany (2,500 ha) in 1947–1949 (Gäbler 1951, 1954), Poland: Hel (1,200 ha) in 1952–1960, Leba (1,100 ha) in 1956–1960 (Hajduk 1963); Stegna (940 ha) in 1956–1960, Darzlubie (150 ha) in 1961–1970 (Sliwa 2002), and Russia (Kaliningrad, a few hundred ha) (Maksymov 1978). The outbreak in Sweden on Gotland Island is more recent and started in 2004. The highest density was found on the southernmost tip of the island where about 3,000 ha had high population densities in 2006 (defined as >10 colonies found within a 30 min search). The density slowly declined, but spots with high density still (2014) occur. Despite several years of monitoring of the population we still have no clear picture of what factors are most important in controlling the population. The spatial distribution, and the local character, of the outbreak were tentatively explained by predation from generalist arthropods; plant quality did not seem to be important (Aimi et al. 2008). It should be noted that the outbreak area is characterized by slow-growing pine trees at low density of trees, similar to populations in Kaliningrad and Spain (pers. obs.). It is interesting that there has been virtually no exchange of individuals between the outbreak area and seemingly suitable sites on Gotland Island distant from the outbreak (Ronnås et al. 2011 see also Kerdelhué et al. 2014, Chap. 4, this volume).
5.5 Relationships with Climate Change
Thaumetopoea pinivora belongs to the group of the summer processionary moths, which are generally adapted to high elevation and, in the specific case of T. pinivora, to high latitude. Precise information about the responses of these species to climate change is missing. The natural occurrence of well-established populations at the northern edge of the range in Sweden (Aimi et al. 2008; Ronnås et al. 2011) does not support the hypothesis of a recent range expansion in relation to climate change, as observed in the group of winter processionary moths, and especially T. pityocampa. More work is required in order to understand the effect of climate change on T. pinivora and to the other species of summer processionary moths.
6 Natural History of the Cedar Processionary Moth, Thaumetopoea bonjeani
6.1 Host Plants and General Distribution
Thaumetopoea bonjeani is monophagous on Atlas cedar Cedrus atlantica Manetti in the relic stands of north-western Africa (Algeria and Morocco).
6.2 Life Cycle
Thaumetopoea bonjeani is one of the most serious pests of Cedrus atlantica forests in Algeria (Gachi et al. 1986; Démolin 1988). The larvae feed on the needles of cedar trees and have urticating setae that cause contact dermatitis after the third instar. Adults (Fig. 2.1) appear from the beginning of August to mid-September. The wingspan of the males and females is 25–33 mm and 30–39 mm, respectively. Mating and oviposition occur at night soon after emergence. Females oviposit on the underside of C. atlantica twigs. Egg batches are covered by greyish brown scales, similar to the colour of the bark and therefore the batches are inconspicuous. The number of eggs per egg batch varies between 44 and 340 (mean 150). Eggs hibernate almost for 8 months and hatch in spring (between late March and late April) of the following year. Unlike the pine processionary moth, T. bonjeani does not make structured tent and lives in colonies combined in a form of ball with a very light weave (Fig. 2.4). The mature larvae are the most destructive ones and may cause serious infestation resulting in the complete defoliation of vast areas of cedar forests. The pupation takes place in the first decade of June to the first half of July (Rahim, unpublished data).
6.3 Natural Enemies
Three parasitoid species emerged from T. bonjeani eggs: Ooencyrtus pityocampae (Hym.: Encyrtidae) and Trichogramma sp. (Hym.: Trichogrammatidae) (Démolin 1988). Two tachinid parasitoid are reported from larvae and pupae: Compsilura concinnata and Exorista segregata. The larvae and pupae have been found to be infected by fungi but no precise information is available. Among the predators listed in Djurdjura cedar forest (Algeria) are the carabid beetle Calosoma sycophanta and the staphilinid beetle Ocypus olens (Rahim, unpublished data).
6.4 Population Dynamics
Outbreak populations of T. bonjeani were observed in Algeria first in 1982 and two major outbreaks were recorded, the first between 1982 and 1990 in Belzma over about 500 ha (Démolin 1988), and the second between 2009 and 2011 in Djurdjura over about 150 ha (Rahim, unpublished data). In Morocco an outbreak was recorded in Kétama (Central Rif) over 120 ha in 1989 (El Alaoui El Fels, unpublished data). More work is certainly required to understand the population dynamics of the species. Defoliation by T. bonjeani most certainly contributes to the decline of the relic cedar stands in the Algerian mountains.
6.5 Relationships with Climate Change
Although outbreaks have been observed only recently and at the southern edge of the range, there is not a clear link between climate change and the performance of T. bonjeani. More work is required to address the effect of climate change on T. bonjeani and to the other species of summer processionary moths.
7 Natural History of the Eastern Cedar Processionary Moth, Thaumetopoea ispartaensis
7.1 Host Plants and General Distribution
Thaumetopoea ispartaensis is monophagous on Taurus cedar Cedrus libani (Doğanlar and Avcı 2001) in relic stands of Taurus Mountains (southern Turkey).
7.2 Life Cycle
Thaumetopoea ispartaensis (described as Traumatocampa ispartaensis by Doğanlar and Avcı in 2001) is one of the most serious pests of Cedrus libani forests in Isparta region and on Taurus Mountains in southern Turkey. All the information reported here comes from the original description (Doğanlar and Avcı 2001). The elevational range is between 1,250 and 1,650 m. The larvae feed on the needles of cedar trees and have urticating setae that cause contact dermatitis after the third instar. The wingspan of the males and females is 26–29 mm and 34–37 mm, respectively. Adults (Fig. 2.1) appear from mid-August to the end of September, earlier at lower elevation. Mating starts a few hours after adult emergence and oviposition occurs during the same night. Females oviposit on the underside of C. libani twigs. Egg batches are covered by greyish brown scales, similar to the colour of the bark and therefore the batches are inconspicuous. The number of eggs per egg batch varies between 39 and 245 (mean 121). Eggs hibernate for almost 7 months and hatch in spring (between late March and late April) of the following year. The larvae live together in greyish silky tents until the fifth final instar, which is reached in 2.5 months, generally between end of April and mid-July. The silk tents are quite loose in comparison to those of the pine processionary moth. The mature larvae are the most destructive ones and may cause serious infestation resulting in the complete defoliation of vast surfaces of cedar forests. Pupation takes place in the first half of July, at a depth of 5–15 cm of soil, in sunlit cedar forest floors, especially along roadsides. Pupae develop in grey-brown cocoons (Avcı 2003). There is no observation about the occurrence of prolonged diapause of the pupae.
7.3 Natural Enemies
Avcı (2003) reported three parasitoid species reared from T. ispartaensis eggs: Ooencyrtus pityocampae and Ooencyrtus sp. (near masii) (Hym.: Encyrtidae), both solitary species, Trichogramma brassicae (Hym.: Trichogrammatidae), a gregarious species. O. pityocampae was found to be the most abundant species, followed by O. sp. near masii and T. brassicae. Parasitism in T. ispartaensis eggs varied between 7.4 % and 11.3 % in 1999 and 2000, respectively.
Avcı and Kara (2002) reported six tachinid parasitoid species for T. ispartaensis larvae and pupae: Blondelia nigripes, Carcelia iliaca, Compsilura concinnata, Exorista segregata, Pales processioneae, Phryxe caudata. Blondelia nigripes was reported to be the most common one, parasitizing up to 4.6 % of the pupae.
A few vertebrate and invertebrate predators have been observed, the most important being Formica rufa (Avcı and Carus 2005). More observations are required to produce a complete list of both parasitoids and predators.
7.4 Population Dynamics
Thaumetopoea ispartaensis population in Isparta has been studied since 1998 and an outbreak was recorded between 1999 and 2003 (Avcı and Carus 2005), but it was impossible to see even a single colony in some years such as 2012. We do not know if this is a pattern of regular fluctuation or just irregular outbreaks, as possibly indicated by a dendro-ecological analysis (Avcı and Carus 2005). More work is certainly required to understand population dynamics of the species.
7.5 Relationships with Climate Change
As T. pinivora, T. ispartaensis belongs to the group of the summer processionary moths adapted to high elevation. So far, no study has been carried out about its specific response to climate change.
8 Natural History of the Lebanon Cedar Processionary Moth, Thaumetopoea libanotica
8.1 Host Plants and General Distribution
Thaumetopoea libanotica is monophagous on Lebanon cedar Cedrus libani in relic stands of Lebanon Mountains at high elevation (around 1,900 m) (Kiriakoff and Talhouk 1975).
8.2 Life Cycle
Thaumetopoea libanotica is generally found at low density and the information is scarce. In the original description of the species, Kiriakoff and Talhouk (1975) reported that a single colony was found on a cedar tree in May and the larvae were in the second instar. They were taken to the laboratory and reared until pupation, which happened at the end of June with the larvae in the fifth instar. Adults (Fig. 2.1) emerged in August.
8.3 Natural Enemies
No information is available.
8.4 Population Dynamics
No information is available.
8.5 Relationships with Climate Change
No information is available.
9 Natural History of the Oak Processionary Moth, Thaumetopoea processionea
Nicolas Meurisse, Axel Schopf, Traian Manole, Irina Ionescu-Mălăncuş, and Andrea Battisti
9.1 Host Plants and General Distribution
Thaumetopoea processionea feeds on Quercus species across Europe and the Near East. In Europe, its preferred species are Q. cerris, Q. pubescens Q. petraea, Q. pyrenaica, and Q. robur (Dissescu and Ceianu 1968; Pascual 1988; Stigter et al. 1997). In the Near East, it is also found on Q. boissieri and on the evergreen Q. callyprinos (Démolin and Nemer 1999; Halperin and Sauter 1999). Typical habitats for this thermophilic species in the northern part of its range are urban and avenue trees, forest edges and open forests (Stigter et al. 1997; Offenberg 2000). Colonisations of closed canopy oak stands may occasionally occur, leading to tree dieback after repeated defoliation over several years, or making trees more vulnerable to secondary pests or pathogens (Dissescu and Ceianu 1968; Maksymov 1978; Pascual 1988; Baker et al. 2009; Lobinger 2010).
A population restricted to the mountainous area surrounding the Dead Sea Transform was first identified as the subspecies T. processionea pseudosolitaria (Halperin and Sauter 1999). A recent morphological study examined the external and internal characters of a large variety of specimens from all over Europe and the Middle East; it was concluded that all populations from Europe and the Middle East belong to T. processionea (Groenen 2010).
9.2 Life Cycle
Thaumetopoea processionea has a 1-year development cycle. The following is a summary of its life cycle observed in most European countries. Moths (Fig. 2.1) emerge from the cocoons on the trunks (old tents) from mid-July to mid-September (Dissescu and Ceianu 1968; Pascual 1988; Wagenhoff and Delb 2011; Williams et al. 2013). Emergences from cocoons are observed from the late afternoon (c.a. 4 p.m.) to the mid of the night (c.a. 2 a.m.) (Dissescu and Ceianu 1968; Pascual 1988; Lobinger 2012). These are usually followed, in the next hours, by an important flight activity (Dissescu and Ceianu 1968).
Observations performed at the edge of the distribution range allowed to deduct that the dispersal ability of females is 5–20 km per year (Stigter et al. 1997; Groenen and Meurisse 2012). Males may disperse over distances of 50–100 km, and are occasionally captured in light traps in areas where the species is not established (Denmark: Lovgren and Dalsved 2005; Franzen and Johannesson 2005; southern coast of Sweden: Skule and Vilhelmsen 1997; island of Jersey and south and southeastern coasts of England: Wedd 2002; Waring et al. 2003; Clancy 2008; Townsend 2009). The eggs (Fig. 2.2) are laid shortly after female emergence on the terminal branches of oak trees, preferably on the tallest part of the tree and on diameters usually comprised between 3.5 and 10 mm (Dissescu and Ceianu 1968; Bin and Tiberi 1983). Egg batches contain about 50–200 eggs, depending on the nutritional conditions of the larval stages (Dissescu and Ceianu 1968). All eggs are covered with scales produced by the female. Shortly after the egg mass is deposited, embryogenesis begins. In autumn, already fully-developed first instar larvae are found within the shells of the eggs (pharate larvae). At midwinter time, these pharate larvae become highly freeze-tolerant and can withstand temperatures as low as −30 °C (Meurisse et al. 2012).
The eggs hatch in April or early May of the following year, mainly depending on the local temperature regime in the preceding weeks (Pascual 1988; Custers 2003; Wagenhoff and Delb 2011; Meurisse, et al. 2012; Wagenhoff et al. 2012). Hatching is usually well synchronized with the time of the specific oak bud flushing (Stigter et al. 1997; Wagenhoff and Veit 2011). Neonates are able to withstand starvation periods of up to 3 weeks, reflecting the species’ close adaptation to variable inter-annual, between-tree and within-tree budburst phenology (Wagenhoff and Veit 2011; Meurisse et al. 2012; Wagenhoff et al. 2012). Groups of neonate larvae consist of full siblings, but larval groups commonly merge at later instars, sometimes forming aggregates of up to 1,000 individuals.
The larvae usually go through six instars and feed until mid-June or early July, depending on the local conditions (Pascual 1988; Wagenhoff and Delb 2011). In Rumania the whole larval development takes 96–100 days in the field and 76–79 days in the laboratory at a temperature of 20–22 °C (Dissescu and Ceianu 1968). Feeding and movements of young larvae may occur during daytime, presumably taking advantage of higher day temperatures (Wagenhoff et al. 2012). Older larvae are active at night, and congregate during the day to rest in a silk tent at branches or on the trunk (Fig. 2.4). The typical big communal tents on the lower part of the trunks are constructed by the fifth or sixth instars (Maksymov 1978). The tents are bag-shaped and consist of an assemblage of silk, hairs, faeces and old larval skins. The larvae pupate in the tent (Fig. 2.6). Tents can be partly in the ground under warm and dry weather conditions such as in Romania (Dissescu and Ceianu 1968) and, more recently, in the Netherlands and Germany (Hellingman, personal communication).
From the third instar onwards, larvae develop urticating setae on specific organs situated on the dorsal parts of the abdomen. Setae are harpoon-shaped and about 50–400 μm in length (Lamy and Novak 1987; Lamy 1990; Battisti et al. 2011; Petrucco Toffolo et al. 2014). They are actively released in the air when the larvae are disturbed, then causing intense irritation, ocular and respiratory problems, as well as severe allergic reactions in nearby animals and humans (Lamy 1990; Maier et al. 2003; Spiegel et al. 2004).
9.3 Natural Enemies
Little specific information is available on natural enemies of T. processionea. Eggs are parasitized by chalcidoid parasitoids (Hymenoptera) such as the eupelmid Anastatus bifasciatus and the encyrtid Ooencyrtus masii, but at relatively low rates of about 15 % (Biliotti 1952; Dissescu and Ceianu 1968; Maksymov 1978; Bin and Tiberi 1983; Mirchev et al. 2003). Late instar larvae and pupae are mostly impacted by the tachinid flies Carcelia iliaca and Pales processionea (Fig. 2.8). High rates of parasitism relating to these two species, up to 76 %, have been observed in Belgium, in the Netherlands and in France (Grison 1952; Tschorsnig 1993; Stigter et al. 1997; Meurisse, unpublished data). In Bavaria and Baden-Württemberg, the macrotype tachinid Phorocera grandis parasitized up to 70 % of mature larvae (Lobinger 2010; Tschorsnig and Wagenhoff 2012). Other tachinids (Blondelia nigripes, Compsilura concinnata, Pales pavida, Phryxe semicaudata, Zenillia libatrix) and the braconid species Meteorus versicolor, as well as ichneumonids (Pimpla processionea, P. rufipes, Coccygomimus turionellae, Theronia atalantae) and pteromalids (Dibrachys cavus, Psychophagus omnivorus, Pteromalus puparum) (Hymenoptera), have been observed as larval or pupal parasitoids (Maksymov 1978; Bogenschütz et al. 1988; Tiberi and Bin 1988; Tiberi et al. 1991; Tschorsnig 1993; Stigter et al. 1997; Zeegers 1997; Kara and Tschorsnig 2003; Cerretti and Freidberg 2009; Zwakhals 2005). Little is known, however, regarding their numerical impacts on T. processionea populations. All instars are actively preyed by the larvae and the adults of Calosoma inquisitor and, more significantly, C. sycophanta. High predation levels have been observed in France, Germany, and Romania (Dissescu and Ceianu 1968; Koch, pers. Obs.; Meurisse, pers. Obs.). Adults of the silphid Xylodrepa quadripunctata, the reduviid bugs Rhinocoris iracundus and R. annulatus as well as Troilus luridus (Pentatomidae) were observed as larval predators (Maksymov 1978). Furthermore, larval mortality by generalist arthropods predators (spiders, ants), or by birds (cuckoo, hoopoe, tits) has been reported (Gäbler 1954; Wagenhoff et al. 2012), but no precise information is available. Late instar larvae and pupae have also been found to be infected by microsporidia infecting midguts and fat bodies (Hoch et al. 2008). From surveys performed in Belgium, France, the Netherlands and Germany, Endoreticulatus spp. was found to be as the most widespread species (Hoch and Meurisse, unpublished data). The more aggressive Vairimorpha and Nosema spp. were less common (Table 2.4).
9.4 Population Dynamics
All over its range, T. processionea is known for the unpredictable nature of its outbreaks (Agenjo 1941; Gómez-Bustillo 1978; Maksymov 1978; Furth and Halperin 1979; Pascual 1988; Krehan 1993; Tomiczek and Krehan 1996; Jans and Franssen 2008). Epidemics, however, are highly synchronized locally, over distances of ca. 30 km. Synchronization rapidly decreases at larger distances, but still remains significant at large spatial scales of several hundred kilometres (Meurisse, unpublished). A striking outbreak, for instance was simultaneously observed in France, Belgium, the Netherlands and Germany in 1995–1996, and followed by an important decline in the same countries in 1997 (Groenen and Meurisse 2012). From 2000 to 2009, a heavy outbreak of T. processionea was observed in oak forests in Bavaria (Germany) causing oak tree mortality of 10–15 % mainly due to secondary infestations by buprestids and Armillaria sp. (Lobinger 2011).
Underlying factors for outbreaks and collapses are not known in detail, and there is still controversy if T. processionea outbreaks are either cyclic or eruptive (Wagenhoff and Veit 2011). Analysis of recent outbreaks in northern France indicates a possible 9–10 year periodicity (Meurisse, unpublished), while epidemics in Germany are reported to be rather chronic (Lehmann 2009).
9.5 Relationships with Climate Change
Well-established populations of T. processionea are known from the nineteenth century in most parts of Europe, with no evidence of any long-term latitudinal shift related to climate change (Groenen and Meurisse 2012). However, it is noticeable that, in recent years, outbreaks developed with an increasing frequency and intensity in many parts of Europe (Lempke 1989; Stigter and Romeijn 1992; Stigter et al. 1997; Maier et al. 2003; Jans and Franssen 2008; Wagenhoff and Delb 2011; Groenen and Meurisse 2012). Nowadays, the latitudinal distribution of T. processionea is still more restricted than that of its host-tree Q. robur, so that the permanent establishment of the species in Northern Europe could be restricted by climatic factors. Specific mechanisms, however, are not well known. Hypotheses include severe late winter conditions affecting the eggs, or temperature conditions in early spring, as they possibly affect neonate survival through high mismatching between hatching and budburst (Wagenhoff and Veit 2011; Meurisse et al. 2012; Wagenhoff et al. 2012). In Hungary, it was shown that a dry and hot May-July period favours T. processionea densities, particularly if it lasts 2–3 consecutive years (Klapwijk et al. 2013).
10 Natural History of the Pistachio Processionary Moth, Thaumetopoea solitaria
10.1 Host Plants and General Distribution
Thaumetopoea solitaria is oligophagous on several pistachio species, both wild and cultivated, in south-eastern Europe and in the Near East. It occurs mainly on Pistacia palaestina, P. terebinthus (both named “terebinth” in English), P. atlantica (Mt. Atlas mastic tree or Persian turpentine tree) and P. vera (pistachio nut). There are single records of the moth on P. lentiscus and other related Anacardiaceae such as Schinus and Rhus species (Halperin 1983). Single references on members of other tree families (such as Cupressus sempervirens and Fraxinus) (De Freina and Witt 1987) are doubtful. Freyer (1838) suggested that the larvae displayed solitary behaviour, which led the moth to be labelled with the name ‘solitaria’, which is inappropriate as indicated by Rauber (1858–1866) and Agenjo (1941).
10.2 Life Cycle
Information about the biology of the pistachio processionary moth was supplied mainly by Davatchi (1958) in Iran, Serafimovski (1975) in Macedonia, Halperin (1983) and the present Authors in Israel, Mourikis et al. (1998) in Greece, Mart et al. (1995) and Karadağ et al. (2007) in Turkey, and Mirchev et al. (2012) in Bulgaria. Detailed adult descriptions were supplied by Agenjo (1941) and by Doğanlar and Doğanlar (2005). Agenjo (1941) noticed clear morphological differences between adults from the Mediterranean area and those named T. solitaria var. iranica collected in Iran.
The main flight period occurs between the second half of August and early November, but sampling of egg clusters in the Golan heights suggested that flight may occur even in December. However, monitoring emergence from collected pupae in Israel and Turkey suggested that the major flight period was in mid-September. The sex ratio is about 1:1. Both sexes display grey forewing and white hind wing, most conspicuously in the males. The wingspan is 20–28 mm for males and 25–35 mm for females. The males start to emerge in the late afternoon, the females a little later, and both complete emergence by around 10 p.m. The female sex pheromone differs from those of other studied processionary moths, in having 18 carbons and 2 double bonds in the 13 and 15 positions of the chain (Z13, Z15-18Al; Frérot and Démolin 1993). Mating was observed during the first half of the night. The females live for 1–2 days and the males may survive for a day longer.
The eggs are initially yellow and turn grey eventually. They are glued to branches of 3–18 mm in diameter, with the preferred diameter being 4–7 mm (Mirchev et al. 2006). The clusters contain 80–260 eggs in one layer comprising 5–12 rows. Reports on female fecundity vary between areas. The clusters are covered with dark grey scales that closely mimic the colour of the outer bark of the branch. The females tend to concentrate the eggs clusters on particular trees and on certain branches within each tree, so that the clusters clearly display a clumped distribution. The eggs pass through an obligatory diapause, and hatching is usually synchronized with bud swelling and leaf bursting; for example, February–March in Israel and March–April in Turkey.
The neonate larva is 1.6–1.8 mm in length, and the mature fifth instar larva 25–35 mm. Larvae are covered with conspicuous long grey hairs and display a gregarious behaviour during feeding and resting periods. During the first two instars, larvae feed on young leaves and rest along thin branches close to the feeding sites. Older, more advanced instar larvae consume more mature foliage, on which they feed only during darkness, usually in the evenings, and they tend to rest deep inside the canopy. Whereas the early instars are usually active under spring low temperatures the more mature larvae favour periods of high temperature. When fed on semi- or fully grown leaves the young instars gnaw the margin of the leaf, whereas from the third instar onward the larvae consume the entire leaf tissue. The later instars tend to feed on the high canopy, therefore larva-infested spots can be detected by the occurrence of top growth (flags) where most or all of the leaves were consumed. Silk strands with larval faeces and larval exuviae attached to them are usually found close to the resting spots. In Israel, the feeding period lasts 50–60 days for those hatched in February (Halperin 1983). Karadağ et al. (2007) showed that relative humidity had a significant effect on the duration of the development period: for example at 26 °C, at 50 or 75 % RH the feeding period was 34 or 25 days, respectively. The threshold temperature for larval development is about 6.5 °C, and 564–570 degree-days are required for larval development from hatching to pupation (Karadağ et al. 2007).
Pupation occurs in litter and upper soil, usually not deeper than 10 cm below the surface in Israel. In Iran, however, pupation was reported to occur at 15–25 cm deep (Davatchi 1958). The pupae are enclosed in delicate silk webbing. The diapause lasts 141–180 days; some pupae may exhibit diapause prolongation by 1–2 additional years (Halperin 1983). Laboratory observations suggest that adults that emerged after prolonged diapause tend to appear earlier in the season than those that passed only one season in diapause.
10.3 Natural Enemies
Karadağ et al. (2007) showed that high relative humidity adversely affected the survival of advanced-instar larvae. Little information is available on the natural enemies of the eggs and larvae. Egg mortality was addressed mainly by Mirchev et al. (2006): in Bulgaria successful hatching ranged from 75 to 81 %, in Israel it was over 95 %. Mirchev et al. (2006) reported on activity of egg parasitoids (9.5 %) and egg predation (about 3 %) but supplied no information regarding the species involved. No sign of egg parasitism was observed in Israel. Mirchev et al. (2012) reported that 5.6 % of newly hatched neonates died because of Beauveria bassiana fungal infection, and Halperin (1990a, 1990b) found that about 9 % of pupating larvae in the laboratory were killed by this fungus. Kugler (1979) reported on five species of parasitic flies (Diptera; Tachinidae), all of which are considered highly polyphagous; Drino imberbis was by far the most dominant species in Israel (Halperin 1983).
10.4 Population Dynamics
Intense defoliation of the infested wild trees is rarely widespread in any area. In Israel the species is a major concern because of the urticating setae of the larvae (see Chap. 8), which are a serious nuisance to foresters, visitors and, especially, to hiking and camping travellers: usually the signs of infestation are inconspicuous, therefore contact with the larval setae while passing though infested vegetation – usually during the summer – is often inevitable. In Turkey and Iran outbreaks occur in pistachio plantations and may cause serious economic damage, especially in mature plantations, where heavy defoliation of young foliage occurs and many of the fruitlets are damaged by the larvae (Anonymous 1995). Although major outbreaks in either forest areas or pistachio plantations are not frequent, when they do occur they may continue for several years. The larvae of T. solitaria are highly susceptible to Bacillus thuringiensis formulations, but their susceptibility decreases progressively with further moults (Er et al. 2007; Gindin et al. 2008).
10.5 Relationships with Climate Change
No information is available on the impact of global warming on the geographic range of T. solitaria.
11 Natural History of Thaumetopoea jordana
11.1 Host Plants and General Distribution
Thaumetopoea jordana is monophagous on Rhus tripartita, a species of Anacardiaceae of the Saharo-Arabian region (Furth and Halperin 1979), and was found on this host for the first time in the lower part of the Jordan River valley and the Dead Sea (Jordan and Israel) (Trougth 1954). In captivity, the larvae complete their development when fed with another Anacardiaceae, Schinus terebinthifolius (Halperin 1990a, 1990b), but pupae did not survive. It has been found later also in the Asir Mountains of Saudi Arabia, where it was thought to live on the same host plant (Wiltshire 1982).
11.2 Life Cycle
All the information comes from the papers of Trougth (1954), Furth and Halperin (1979), and Halperin (1990a, 1990b). The moth main flight period and oviposition occurs in October-December. However, emergence may happen also through the summer (May–September) (e.g. Aharoni 1912; Amsel 1935). The eggs are glued to the bark, in clusters 18–32 mm in length and 5–14 mm in width. Egg clusters laid on thin twigs are cylindrical whereas those laid on thicker branches are flat. The clusters are covered with shiny gold-brown scales. The incubation period lasts about 6 weeks and the mean number of eggs per cluster is 197 (min 105, max 258). Larvae raised at constant temperatures of 20, 25 and 30 °C complete their development in 70, 48 and 40 days, respectively, while those raised outdoors, in November-March, on trees of Schinus terebinthifolius, require about 150 days for full development. The larvae of T. jordana do not build a tent and moulting takes place in groups on branches, or in the soil beneath the host plant. When disturbed, the larvae fall from the tree and try to get back to it. The larvae generally bask in the sun during daytime and feed during night, searching for the leaves still on the twigs of R. tripartita. The larvae have a silver and black appearance because of the long silver hairs that cover the black integument and the mirrors carrying the urticating setae. Pupation occurs in March–April in the soil and a prolonged diapause of 1–2 years may occur.
11.3 Natural Enemies
High mortality was observed in rearing but the factors were not identified. Only the parasitoids Ooencyrtus sp. (close to masii) (Hymenoptera, Encyrtidae) and Palesisa aureola (Diptera, Tachinidae) emerged from eggs and larvae, respectively (Furth and Halperin 1979). The tachinid was reclassified according to Cerretti and Freidberg (2009), and T. jordana seems to be its only host known so far (Cerretti, personal communication).
11.4 Population Dynamics
The species is rare and associated with relic areas of Rhus tripartita, which generally occur in clumps of few shrubs.
11.5 Relationships with Climate Change
There are no data about the potential impact of climate change on T. jordana, although it has become less abundant in the recent years and repeated surveys in Wadi Qilt conducted in the springs of 2010–2012 failed to find egg clusters or larval colonies.
12 Natural History of Thaumetopoea herculeana
12.1 Host Plants and General Distribution
Thaumetopoea herculeana is associated with open areas covered with low natural vegetation of truly Mediterranean type of the Iberian Peninsula and on the southern rim of the Mediterranean basin until the Near East. The larvae feed on Geraniaceae (Erodium moschatum and E. arborescens), and Cistaceae (Helianthemum vulgare, H. croceum and Cystus salviaefolius), which are not crop plants.
12.2 Life Cycle
There is large uncertainty about the life cycle of this species. According to Gómez-Bustillo (1979), the moth’s flight period extends from June in the northern and central Spain to September/October in Andalusia. Agenjo (1941) reported moth catches in May, August, September, and October. Larvae are reported to be present from October to April (Gomez de Aizpura 1986). During our small survey in coastal NW Spain, larvae of different instars (second to fifth) were found in April and gave origin to adults emerged in July under laboratory conditions, while in the field moths have been observed between July and September (unpublished data). It is thus possible that the life cycle depends on local weather patterns and host plant phenology. Moths lay eggs on low plants, grouping them in cylindrical batches. Larvae show a gregarious behaviour as they stay in groups of about 20–30 individuals piled up on the ground, forming a small cluster without silk (Fig. 2.4). A silk protection is reported just in the northern and central part of Spain, but not in Andalusia (Gómez-Bustillo 1978). In the sunny days they move in processions with a triangular shape, separating to feed on the low vegetation around, and regrouping again after it. They usually feed during the night and in the morning.
Larvae have a bluish grey appearance, and are covered by hairs of the same colour during the first two instars; in the next larval instars yellow-green tufts interspersed with grey appear on the back until the last moulting, probably to mimic the surrounding vegetation (for example Ulex europaeus). Larvae pupate in the ground at a low depth between March and April in the south of Spain. Larvae were not reported to be irritant (Agenjo 1941) although they carry urticating setae as in the other species of the genus (unpublished data).
12.3 Natural Enemies
The larvae are parasitized by unknown tachinids and preyed by the carabid beetle Macrocarabus lusitanicus (Pino, unpublished data from north-western Spain).
12.4 Population Dynamics
No information is available.
12.5 Relationships with Climate Change
No information is available.
13 Pheromones Within Processionary Moths, Thaumetopoea spp.
13.1 Introduction
The field of chemical ecology and the identification of chemical signals open the opportunity to manipulate the insect behaviour in order to decrease the impact of the pest on crop or forest. Sex pheromones in Lepidoptera are female-produced chemicals that attract the conspecific males, induce courtship behaviour and mediate reproduction (Fig. 2.13). A significant number of the sex pheromones in Lepidopteran pests were identified and are compiled on the website “The Pherobase” (http://www.pherobase.com). In some social lepidopteran species chemical communication was also evidenced to contribute to maintain the cohesion of larvae colony through the deposit of a trail pheromone (Fitzgerald 1995, 2008; Ruf et al. 2000). The development of new control methods like larval disruption could be of interest.
13.2 Sex Pheromones in Thaumetopoea spp.
13.2.1 Winter Processionary Moths
Sex pheromone study in processionary moths started in 1980 with the identification of an unique and original pheromone structure in Thaumetopoea pityocampa, (Z)-13-hexadecen-11-ynyl acetate (Yne 11, Z13-16: Ac) (Guerrero et al. 1981) (Fig. 2.14a). It was the first time that such a chemical with an enyne bond was identified as a sex pheromone. This compound was then synthetized using different routes (Camps et al. 1983; Gardette et al. 1983; Shani et al. 1983). It displayed a high attraction for conspecific males when formulated as a bait in traps, leading to the development of new tools for monitoring populations of pine processionary moth (see Martin et al. 2014, Chap. 9, this volume). The synthesis did not provide a pure component and a limited proportion of the E11 isomer is present in the synthetic chemical. Different behavioural and trapping experiments showed that this isomer did not act as a synergist neither as a negative signal especially when in little amount (Quero et al. 1995).
Because the lepidopteran species producing a single component in a sex pheromone are rare (http://www.pherobase.com), the search for possible minor components was actively carried out using all the improvements in pheromone research technologies (Quero et al. 1997). The conclusion was that this pheromone is really characterized by a single component.
The sister species of Thaumetopoea pityocampa, T. wilkinsoni from Asia Minor, was shown to share the same sex pheromone, thus also including a single component (Frérot and Démolin 1993). Field experiments confirmed that T. wilkinsoni and T. pityocampa were attracted by the same compound. These two processionary moths shared also the same behavioural traits concerning female calling behaviour and mating period, which were observed during the first part of the night, some hours after imago moulting. Actually, individuals of T. wilkinsoni collected in Israel were shown to be capable of interbreeding with T. pityocampa when artificially kept together (Démolin, unpublished data).
An enyne pheromone pattern was also found in Thaumetopoea jordana. In this species the sex pheromone was identified as a complex blend of Yne 11, Z13-16: Ac 10 %, (Z)-13-hexadecen-11-yn-1-ol (Yne 11, Z13-16: OH) 50 %, (Z)-13-hexadecen-11-yn-1-al (Yne 11, Z13-16: Al) 40 % (Frérot and Démolin 1993) (Fig. 2.14a, b, c). Unlike T. pityocampa and T. wilkinsoni, this species does not develop on conifers but on Anacardiaceae, and, phylogenetically, it likely belongs to a different clade within the genus Thaumetopoea (see Kerdelhué et al. 2014, Chap. 4, this volume, part 2).
13.2.2 Summer Processionary Moths
All the summer processionary moths were subject to pheromone identification, except T. libanotica still under investigation and for which results are not yet been published.
In the oak tree processionary moth, T. processionea, the sex pheromone was investigated from a gland extract obtained from a French population. The analyses by GC-MS lead to the identification of a mixture of (Z,Z)-11,13-hexadecadienyl acetate 95 % (Z11, Z13-16: Ac) and (Z,Z)-11,13-hexadecadienol 5 % (Z11, Z13-16: OH) (Frérot and Démolin 1993) (Fig. 2.15a, b). Field experiments confirmed the attractiveness of this mixture but in Spain the females were significantly more attractive than the synthetic blend. Reinvestigation of the pheromone composition in a Spanish population evidenced an additional minor component, corresponding to an isomer of the previously identified diene acetate, (E,Z)-11,13-hexadecadienyl acetate (E11, Z13-16: Ac) (Fig. 2.15c) (Quero et al. 2003). The E,E isomer was shown to inhibit male attraction, pointing out that the purity of the main component is a crucial point to be kept in mind for trapping success (Breuer et al. 2003). Indeed, dienic compounds are hardly obtained free of geometric isomer by synthesis. They do not remain stable when formulated in caps and left under outdoor conditions. However 0.1 % of the E,E isomer can be tolerated. The E,Z isomer present in the extract was not proven to increase the level of attraction and should be as the other isomers present in the synthetic chemical. Therefore, the described formulation is as follows: Z11, Z13-16: Ac 88 %, E11, Z13-16: Ac 7 % and Z11, Z13-16: OH 5 %.
Later, Gries et al. (2004) also reinvestigated the sex pheromone composition in a German population of oak processionary moth. The presence of Z11, Z13-16: Ac was confirmed but a second additional compound was identified, (Z,Z)-11,13, 15-hexadecatrienyl acetate (Fig. 2.15d). The blend of 50/50 of both components was further reported as attractant in field trappings. These contrasted results depending on the population origin point out the need for further behavioural and/or field experiments on sex pheromones produced by the different populations all over the range of oak processionary moth.
Two other summer processionary moths, but conifer-feeding, Thaumetopoea pinivora and T. bonjeani, were described as closely related species based on systematics and biology. Male genitalia of both species bear the same pattern such as female abdominal scales do. Larvae of both species have a gregarious behaviour but without building a conspicuous tent. Sex pheromone identifications corroborated the similarities between both species which appeared to share the same blend, (Z,Z)-11,13-hexadecadienal 80 % (Z11, Z13-16: Al, Fig. 2.16a) and (Z,Z)-11,13-hexadecadienol 20 % (Z11, Z13-16: OH) (Fig. 2.16 a, b) (Frérot et al. 1990). The sex pheromone of a related conifer-feeding moth, Thaumetopoea libanotica, was investigated by Nemer and Frérot (unpublished data) by GC-MS analyses of pheromone glands and field trappings. The work confirmed that this species is very closely related to T. bonjeani.
Sex pheromones were also tentatively identified in another summer processionary moth but developing on Pistacia spp. under desert conditions, Thaumetopoea solitaria. Analysis of a few female gland samples evidenced a single compound characterized by 18 carbons and 2 double bonds at the putative positions 13 and 15 on the carbon chain. These results obtained around 1990 should be reinvestigated once the reproductive behaviour, i.e. timing of calling behaviour or mating, will be better known.
13.2.3 Concluding Remarks
Pheromone compounds identified in Thaumetopoea spp. shared the same pattern: a long chain with 16 atoms of carbons and bonds located at positions 11 and 13. The systematics of the group appeared homogeneous on the base of the pheromone chemistry, characterised by the enyne bond which is original within Lepidoptera. There is a correlation between the biological group to which the processionary moth belongs, winter versus summer, and sex pheromone chemistry.
Most of the identified pheromones are efficient for male trapping except in some populations of T. processionea that should be re-investigated. The sex pheromone of Thaumetopoea herculeana a processionary moth developing on Cistaceae and Geraniacae (Gomez Aizpurùa 1986), remains to be studied.
13.3 Trail Pheromones in Pine Processionary Larvae
Thaumetopoea pityocampa accomplishes as a larva different types of displacements called “processions”. The first type of procession is related to food consumption and concerns larvae of all instars developing together into a silky tent but feeding outside on pine needles. Larvae leave the tent together, following each other to forage on needles and once the feeding achieved crawl back to the protective tent by following the same path.
A second type of procession is exhibited by the last-instar, mature larvae searching for pupation into the soil. This behaviour was minutely described since a long time by Fabre (1899). The gregarious behaviour was associated by a head-to-tail displacement with tight links between all individuals forming the procession subject to tactile stimuli produced by movement of cuticular setae located at the end of the forehead larva abdomen. The stimulus acting on the head of the following larva were evidenced as an important cue for the procession (Démolin 1962, 1969a, 1969b). More broadly, tactile contacts were considered by Démolin (1969a, 1969b, 1971) as the basis of a “social memory” steering the initiation of the procession behaviour, and then reinforcing it along the way.
13.3.1 Trail Pheromones During Foraging Processions of Early-Instar Larvae
Early-instar larvae of T. pityocampa were known for a long time to maintain a social link via a silky pathway, a kind of “Ariadne” strand that can contribute to guide the larvae back to the tent during the feeding procession. However, the evidence of a trail pheromone was obtained only rather recently (Fitzgerald 2003). The second-instar larvae were experimentally showed to leave a marking trail during a foraging procession, and it was proved that this trail is acting on the behaviour of conspecific larvae, maintaining the procession cohesion. As the role of the silk produced by the larvae was investigated in this process, it appeared that no left silk seems necessary to mark trails nor to maintain processions. However, silk may play a subordinate role to trail pheromone, its function having being hypothesized to enhance steadfastness (Fitzgerald 2003). Despite their essential role in allowing larvae to move efficiently to and from the tent, chemical cues appear of less importance than tactile ones. Fitzgerald (2003) indeed noticed that the larvae unable to spin silk or to mark the substrate with the trail pheromone are still able to form processions suggesting that tactile cues alone may be adequate to allow larvae to form and maintain processions.
The trail pheromone is likely to be deposited by the ventral part of the larva body, with the ventral tip of the abdomen as the primary source. Fitzgerald (2003) also demonstrated that an acetone extract of both larvae and silk induces an attractive response on second-instar larvae. However, choice tests revealed that the effectiveness of the extracts largely varies over time, early-instar larvae preferring fresh trials. Moreover, recent, yet unpublished experiments additionally suggested that the pheromone trail is not specific to the larval colony. The trail left by a colony induces similar positive reactions on larvae randomly selected from other colonies (Yart et al., unpublished data).
13.3.2 Trail Pheromones During Pupation Processions of Mature Larvae
According to Fitzgerald (2003), the role/importance of chemical cues might have declined when prepupal larvae are leaving the tent in search of pupation sites. Neither silk nor the deposit of a trail signal seems to be required. The same observation was previously reported by Démolin (1971), who also quoted that lost or isolated larvae were seeking for conspecific. During this step, thigmotactic cues could take the priority, with a crucial role of stimuli associated with setae found on the tip of the abdomen of the preceding larva in the procession (Démolin 1969a, 1969b, 1971; Fitzgerald 2003). In contrast, Fabre (1899) reported that mature larvae are following silky strands during pupation processions. Our own, recent results (Yart et al., unpublished data) were also contrasting with those of Fitzgerald and Démolin cited above. A large production of silk was observed at all instar larvae, even the last one. Prepupal larvae were also found to be able to deposit and to discern a chemical trail since they preferentially use in a Y maze (glass Y-shape tube) the branch where conspecific larvae passed through (64 % against 36 %; n = 546, binomial test, p < 0.001). Unfortunately, the GC-MS analyses of larvae extracts did not lead to identification of any chemicals responsible for this behaviour.
13.3.3 Conclusions
Candidate compounds as trail markers were identified in other gregarious lepidopteran species. Fitzgerald (1995) and Fitzgerald and Webster (1993) showed 5β-cholestane-3-one to be a component of the trail marker for the foraging larvae of the eastern tent caterpillar, Malacosoma americanum (Lepidoptera: Lasiocampidae). Ruf et al. (2000) observed that the larvae of another lasiocampid moth, Eriogaster lanestris, readily followed trails prepared with the same compound. However, the identity of possible trail markers remains still unknown in pine processionary moth despite efforts (Fitzgerald 2003). Such an identification is a challenge for the future, and could be promising for the development of larval disruption techniques.
14 General Conclusions
The life history traits of the processionary moths of the genus Thaumetopoea can be generally divided into three groups (see also Kerdelhué et al. 2014, Chap. 4, this volume). The first two, i.e. the summer and winter pine processionary moths, are associated with coniferous host plants while the third is associated with broadleaf trees and shrubs. The switch to coniferous host can be seen as one of the amazing traits of the genus, also because it has been shown to be successful in terms of speciation and performance. It has coincided with the switch from the summer feeding to the winter feeding, and consequently with the change of the pupal diapause from winter to summer. These dramatic changes in the life history seem to be justified by the possibility to feed for a longer period of time, and mainly when competition with other herbivores is almost absent. The best example of such an adaptation is the group of the winter pine processionary moths (T. pityocampa and T. wilkinsoni), which are by far the most widely distributed and abundant species in the genus. Because of this special adaptation to winter feeding, based on a precise physiological reaction to temperature, they also result to respond directly to climate change by expanding the range into previously unsuitable regions. Although similar responses have been claimed for species of the other groups, both conifer and broadleaf feeding, there is no clear indication that climate change is driving the range expansion or any other type of recent shift, such as pestilence level. This is, however, an open question and must be addressed in the future. In addition, there are many other aspects of the life histories which are still poorly known and would contribute to a better comprehension of the ecology of the species. The availability of data about the species of Central Africa, Arabic peninsula, and south-western Asia is also essential for the definition of the evolutionary history of the genus and to predict the effects of the global change.
References
Abgrall, J. F. (2001). Le réseau surveillance processionnaire du pin en France 1969–1989. Conception – historique – résultats. Groupement de Nogent sur Vernisson: Cemagref, Division Ecosystèmes Forestiers et Paysages, 104 p.
Acatay, A. (1953). Investigation of pine processionary moth (Thaumetopoea pityocampa Schiff. = Thaumetopoea wilkinsoni Tams) and its control on the islands. İstanbul University Faculty of Forestry Journal, 3, 29–47 [in Turkish].
Agenjo, J. (1941). Monografía de la Familia Thaumetopoeidae (Lep.). EOS, Revista Española de Entomología, 17, 69–130.
Aharoni, J. (1912). Die Tierwelt des Jordan-Tal und Totes Meer-Gebiets. In M. Blackenhorn (Ed.), Naturwissenschaftliche Studien am Toten Meer und im Jordantal (pp. 395–441). Berlin: Verlag Friedlaender.
Aimi, A., Larsson, S., Ronnås, C., Frazão, J., & Battisti, A. (2008). Growth and survival of larvae of Thaumetopoea pinivora inside and outside a local outbreak area. Agricultural and Forest Entomology, 10, 225–232.
Altum, D. (1895). Zur Lebensweise und Bekampfung des Keifernprozessionspinners, Cnethocampa pinivora Tr. Zeitschrift fur Forst und Jagdwesen, 27, 373–395.
Altum, D. (1896). Neuere Beobachtungen uber den Kiefernprozessionspinner, Cnethocampa pinivora Tr. Zeitschrift fur Forst und Jagdwesen, 28, 649–652.
Amsel, H. G. (1935). Weitere Mitteilungen ueber palaestinensische Lepidopteren. Veroeffentlichungen des Deutschen Kolonialund Uebersee-Museums Bremen, 1, 223–277.
Anonymous. (1995). The technical guide of agricultural control (pp. 147–148). T.C. Ankara: The Ministry of Agriculture of Turkey.
Avcı, M. (2000). Türkiye’nin farklı bölgelerinde Thaumetopoea pityocampa (Den. & Schiff.) (Lep.: Thaumetopoeidae) nın yumurta koçanlarının yapısı, parazitlenme ve yumurta bırakma davranışları üzerine araştırmalar. Türkiye Entomoloji Dergisi, 24, 167–178.
Avcı, M. (2003). Parasitism of egg-batches of the cedar processionary moth Traumatocampa ispartaensis in Turkey. Phytoparasitica, 31, 118–123.
Avcı, M., & Carus, S. (2005). The Impact of Cedar Processionary Moth [Traumatocampa ispartaensis (Doğanlar, Avcı) (Lepidoptera:Notodontidae)] Outbreaks on Radial Growth of Lebanon Cedar (Cedrus libani A. Rich.) Trees, Turkey. Journal of Pest Science, 78, 91–98.
Avcı, M., & Kara, K. (2002). Tachinidae parasitoids of Traumatocampa ispartaensis from Turkey. Phytoparasitica, 30, 361–364.
Avcı, M., & Oğurlu, İ. (2002). Göller Bölgesi çam ormanlarında çam kese böceği [Thaumetopoea pityocampa (Den. & Schiff.)] önemi, biyolojisi ve doğal düşmanları. In M. Kanat (Ed.), Proceedings of the symposium for pine processionary moth problem in Turkey and solutions, 24–25 April 2002, Kahramanmaras, Turkey (pp. 28–36) [in Turkish].
Avtzis, N. (1986). Development of Thaumetopoea pityocampa Schiff. (Lepidoptera: Thaumetopoeidae) in relation to food consumption. Forest Ecology and Management, 15, 65–68.
Baker, R., Caffier, D., Choiseul, J. W., De Clercq, P., Dormannsné-Simon, E., Gerowitt, B., Karadjova, O. E., Lövei, G., Lansink, A. O., Makowski, D., Manceau, C., Manici, L., Perdikis, D., Puglia, A. P., Schans, J., Schrader, G., Steffek, R., Strömberg, A., Tiilikkala, K., van Lenteren, J. C., & Vloutoglou, I. (2009). Evaluation of a pest risk analysis on Thaumetopoea processionea L., the Oak Processionary moth, prepared by the UK and extension of its scope to the EU territory, Scientific Opinion of the Panel on Plant Health, European Food Safety Authority. The EFSA Journal, 491, 1–63.
Barbaro, L., & Battisti, A. (2011). Birds as predators of the pine processionary moth (Lepidoptera: Notodontidae). Biological Control, 56, 107–114.
Barbaro, L., Couzi, L., Bretagnolle, V., Nezan, J., & Vetillard, F. (2008). Multi-scale habitat selection and foraging ecology of the Eurasian hoopoe (Upupa epops) in pine plantations. Biodiversity and Conservation, 17, 1073–1087.
Battisti, A. (1988). Host-plant relationships and population dynamics of the pine processionary caterpillar Thaumetopoea pityocampa (Denis & Schiffermüller). Journal of Applied Entomology, 105, 393–402.
Battisti, A. (1989). Field studies on the behavior of two egg parasitoids of the pine processionary moth Thaumetopoea pityocampa. Entomophaga, 34, 29–38.
Battisti, A., Bernardi, M., & Ghiraldo, C. (2000). Predation by the hoopoe on pupae of Thaumetopoea pityocampa and the likely influence on other natural enemies. BioControl, 45, 311–323.
Battisti, A., Stastny, M., Netherer, S., Robinet, C., Schopf, A., Roques, A., & Larsson, S. (2005). Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecological Applications, 15, 2084–2096.
Battisti, A., Stastny, M., Buffo, E., & Larsson, S. (2006). A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Global Change Biology, 12, 662–671.
Battisti, A., Holm, G., Fagrell, B., & Larsson, S. (2011). Urticating hairs in arthropods: Their nature and medical significance. Annual Review of Entomology, 56, 203–220.
Battisti, A., Marini, L., Pitacco, A., & Larsson, S. (2013). Solar radiation directly affects larval performance of a forest insect. Ecological Entomology, 38, 553–559.
Bergstrom, C., & Bystrowski, C. (2011). The identity of Blondelia pinivorae (Ratzeburg) (Diptera: Tachinidae), a parasitoid of processionary moths (Lepidoptera: Thaumetopoeidae). Stuttgarter Beiträge zur Naturkunde A, Neue Serie, 4, 321–334.
Biliotti, E. (1952). Difficulties in deciding the period at which to apply control measures against Thaumetopoea processionea and T. pityocampa. Revue de Pathologie Vegetale, 31, 115–120.
Biliotti, E. (1958). Les parasites et prédateurs de Thaumetopoea pityocampa Schiff. (Lepidoptera). Entomophaga, 3, 23–24.
Bin, F., & Tiberi, R. (1983). Notizie preliminari sui parassitoidi oofagi di Thaumetopoea processionea L. in Italia centrale (Hym., Chalcidoidea; Lep., Thaumetopoeidae). Redia, 66, 449–459.
Bogenschütz, H., Schwartz, G., & Limberger, S. (1988). Auftreten und Bekämpfung des Eichenprozessionsspinners, Thaumetopoea processionea L., in Südwestdeutschland 1986 bis 1988. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft, 245, 427–428.
Bouhot-Delduc, L. (2005). Dynamique des populations de la processionnaire du pin et extension de son aire de colonisation de 1981 à 2004 en France. In Les Cahier du DSF 1–2005, La santé des forêts (France) en 2003 et 2004, Ministère de l’Agriculture, de l’Alimentation, de la Pêche et des Affaires Rurales, Paris, 6 p.
Branco, M., Santos, M., Calvão, T., Telfer, G., & Paiva, M.-R. (2008). Arthropod diversity sheltered in Thaumetopoea pityocampa (Lepidoptera: Notodontidae) larval tents. Insect Conservation and Diversity, 1, 215–221.
Breuer, M., Kontzog, H. G., Guerrero, A., Camps, F., & De Loof, A. (2003). Field trials with the synthetic sex pheromone of the Oak Processionary moth Thaumetopoea processionea. Journal of Chemical Ecology, 29, 2461–2468.
Buffo, E., Battisti, A., Stastny, M., & Larsson, S. (2007). Temperature as a predictor of survival of the pine processionary moth in the Italian Alps. Agricultural and Forest Entomology, 9, 65–72.
Buxton, R. D. (1990). The influence of host tree species on timing of pupation of Thaumetopoea pityocampa Schiff. (Lep., Thaumetopoeidae) and its exposure to parasitism by Phryxe caudata Rond. (Dipt., Larvaevoridae). Journal of Applied Entomology, 109, 302–310.
Camps, F., Coll, J., Guerrero, A., & Riba, M. (1983). Simple and stereoselective synthesis of sex pheromone of processionary moth Thaumetopoea pityocampa (Denis and Schiff.). Journal of Chemical Ecology, 9, 869–875.
Çanakçıoğlu, H., & Mol, T. (1998). Orman Entomolojisi Zararlı ve Yararlı Böcekler. İstanbul Üniversitesi Orman Ekolojisi, 541 p [in Turkish].
Cerretti, P., & Freidberg, A. (2009). Updated checklist of the Tachinidae of Israel. The Tachinid Times, 22, 9–19. http://www.nadsdiptera.org/Tach/TTimes/TT22.pdf
Ciesla, W. M. (2004). Forests and forest protection in Cyprus. Forestry Chronicle, 80, 107–113.
Clancy, S. P. (2008). The immigration of Lepidoptera to the British Isles in 2006. Entomologists Record and Journal of Variation, 120, 209–276.
Custers, C. J. L. (2003). Climate change and trophic synchronisation. A case study of the Oak Processionary caterpillar. Master’s thesis, Wageningen UR University, The Netherlands.
Davatchi, A. (1958). Etude biologique de la faune entomologique des pistacia sauvages et cultivés. Revue de Pathologie Vegetale et d’Entomologie Agricole de France, 37, 49–52.
De Freina, J., & Witt, T. J. (1987). Die Bombyces und Sphinges der Westpalaearktis (Insecta, Lepidoptera). Band 1. Forschung & Wissenschaft Verlag GmbH, München, 708 p.
Démolin, G. (1962). Comportement des chenilles de Thaumetopoea pityocampa au cours des processions de nymphose. Comptes Rendus de l’Académie des Sciences, 254, 733–744.
Démolin, G. (1969a). Bioecología de la procesionaria del pino, Thaumetopoea pityocampa Schiff. Incidencias de los factores climáticos. Boletin del Servicio de Plagas Forestales, 23, 9–24.
Démolin, G. (1969b). Comportement des adultes de Thaumetopoea pityocampa Schiff. Dispersion spatiale, importance écologique. Annales des Sciences Forestières, 26, 89–102.
Démolin, G. (1971). Incidences de quelques facteurs agissant sur le comportement social des chenilles de Thaumetopoea pityocampa Schiff. (Lepidoptera) pendant la période des processions de nymphose. Répercussion sur l’efficacité des parasites. Annales de Zoologie, Ecologie Animale (H.S.), 33–56.
Démolin, G. (1988). Intensification de la protection phytosanitaire des forêts. Algérie 1986–1987. La processionnaire du cèdre: Thaumetopoea bonjeani. FAO, Rapport scientifique et rapport iconographique, Roma, Italy, 21 p.
Démolin, G. (1990). Réflexions générales sur la diapause et les diapauses renforcées chez la processionnaire du pin, Thaumetopoea pityocampa Schiff. In Les Colloques de l’ INRA 52- Cycles saisonniers chez les invertébrés, Dourdan, France.
Démolin, G., & Nemer, N. (1999). Insectes défoliateurs de Quercus callyprinos Webb. et de Quercus infectoria Olv. au Liban. IOBC Bulletin, 22, 65–99.
Dissescu, G., & Ceianu, I. (1968). Cercetari asupra bioecologiei omizii procesionare a stejarului (Thaumetopoea processionea L.) (pp. 1–120). București: Centrul de Documentare tehnică pentru Economia Forestieră [in Romanian, Russian summary].
Doğanlar, M., & Avcı, M. (2001). A new species of Traumatocampa Wallengren (Lepidoptera: Thaumetopoeidae) feeding on cedar from Isparta (Türkiye). Tuerkiye entomoloji dergisi, 25, 19–22.
Doğanlar, M., & Doğanlar, O. (2005). Türkiye Thaumetopeidae Türleri, Tannimlari, Dağilis Alanlari, Doğal Düşmanlari ve Mücadele Yöntemleri. Mustafa Kemal Üniversitesi. Ziraat Fakültesi, Bitki Koruna Bölümü. Antakya-Hatay, Turkey, 56 p.
Er, M., Karada, S., & Mart, C. (2007). Effectiveness of Bacillus thuringiensis var. kurstaki on Thaumetopoea solitaria Frey. (Lepidoptera: Thaumetopoeidae) larvae in laboratory conditions. Turkish Journal of Agriculture and Forestry, 31, 255–261.
Fabre, J. H. (1899). Souvenirs Entomologiques, Etudes sur l’instinct et les les moeurs des insectes (p. 420). Paris: Delagrave.
Fitzgerald, T. D. (1995). The tent caterpillars. Ithaca: Cornell University Press, 303 p.
Fitzgerald, T. D. (2003). Role of trail pheromone in foranging and processionary behavior of pine processionary caterpillars Thaumetopoea pityocampa. Journal of Chemical Ecology, 29, 513–532.
Fitzgerald, T. D. (2008). Use of pheromone mimic to cause the disintegration and collapse of colonies of tent caterpillars (Malacosoma spp.). Journal of Applied Entomology, 132, 451–460.
Fitzgerald, T. D., & Webster, F. X. (1993). Identification and behavioral assays of the trail pheromone of the forest tent caterpillar Malacosoma disstria Hubner (Lepidoptera: Lasiocampidae). Canadian Journal of Zoology, 71, 1511–1515.
Franzen, M., & Johannesson, M. (2005). Interesting Macrolepidoptera findings in Sweden 2004. Entomologisk Tidskrift, 126, 55–70.
Frérot, B., & Démolin, G. (1993). Sex pheromone of the processionary moths and biosystematic considerations within the genus Thaumetopoea (Thaumatopoeidae: Thaumetopoeinae). Bollettino di Zoologia Agraria e di Bachicoltura, 25, 33–40.
Frérot, B., Malosse, C., Milat, M. L., Démolin, G., Martin, J. C., Khemici, M., Zamoun, M., & Gachi, M. (1990). Chemical analysis of the sex pheromone glands of Thaumetopoea bonjeani (Powell) (Lep., Thaumatopoeidae). Journal of Applied Entomology, 109, 210–212.
Furth, D., & Halperin, J. (1979). Observations on the phenology and biogeography of Thaumetopoea jordana (Stgr.) (Lep. Thaumetopoeidae). Israel Journal of Entomology, 13, 1–11.
Gabir, İ. (1998). Çamkese tırtılı mücadelesinde helikopter avantajı. Orman Mühendisliği Dergisi, 35, 10–11.
Gäbler, H. (1951). Beobachtungen uber den Kiefernprozessionspinner (Cnethocampa pinivora Tr.). Zeitschrift fur Pflanzenkrankheiten und Pflanzenschutz, 59, 92–96.
Gäbler, H. (1954). Die Prozessionsspinner- Die neue Brehm-Bücherei 137. Wittenberg Lutherstadt: Ziemsen-Verlag, 38p.
Gachi, M., Khémici, M., & Zamoum, M. (1986). Sur la présence en Algérie de la processionnaire du cèdre T. bonjeani Powell (Lepidoptera Thaumetopoeidae). Annales de la Recherche Forestière en Algérie, 1, 53–63.
Gardette, M., Alexakis, A., & Normant, J. F. (1983). Synthesis of (Z)-13-hexadecen-11-yn-1-yl acetate major component of sex pheromone of the processionary moth. Journal of Chemical Ecology, 9, 219–223.
Géri, C. (1983a). Repartition et evolution des populations de la processionnaire du pin, Thaumetopoea pityocampa Schiff, (Lep., Thaumetopoeidae) dans les montagnes corses. I. Regimes d’apparition de l’insecte et dynamique des populations. Acta Oecologica, Oecologia Applicata, 4, 247–268.
Géri, C. (1983b). Dynamique de la processionnaire du pin dans la vallée de Niolo en Corse au cours des cycles 1965–1966, 1967–1968, 1969–1970. Rôle de certains caractères du milieu forestier. Annals of Forest Science, 40, 123–156.
Géri, C., Millier, C., & Xeuxet, D. (1985). Mésure des populations de processionaire du pin (Thaumetopoea pityocampa Schiff. (Lepidoptère Thaumetopoeidae) au Mont Ventoux. Annals of Forest Science, 42, 143–184.
Gindin, G., Kuznetsowa, T., Protasov, A., Saphir, N., Madar, Z., & Mendel, Z. (2008). Susceptibility of the pistachio processionary moth, Thaumetopoea solitaria, to three Bacillus thuringiensis products. Phytoparasitica, 36, 472–482.
Gómez-Bustillo, M. R. (1978). Los Thaumetopoeidae (Aurivillius, 1891) de la peninsula Iberica: nociones de sistematica, ecologia y importancia economica de la familia. (Primera parte). Revista de Lepidopterologia, SHILAP, 5, 283–290.
Gómez-Bustillo, M. R. (1979). Mariposas de la península Ibérica, Volume 4, Heteróceros II. Madrid: Instituto Nacional para la Conservación de la Naturaleza, Ministerio de Agricultura, 280 p.
Gómez de Aizpúrua, C. (1986). Biología y morfología de las orugas, Lepidoptera; Cossidae, Sphingidae, Thaumetopoeidae, Lymantriidae, Arctiidae (Vol. 2, serie 6). Madrid: Ministerio de Agricultura, Pesca y Alimentación, 239 p.
Gries, R., Reckziegel, A., Bogenschütz, H., Kontzog, H. G., Schlegel, C., Francke, W., Millar, J., & Gries, G. (2004). Z,Z)-11,13-Hexadecadienyl acetate and (Z,E)-11,13,15-hexadecatrienyl acetate: Synergistic sex pheromone components of Oak Processionary moth, Thaumetopoea processionea (Lepidoptera: Thaumetopoeidae). Chemoecology, 14, 95–100.
Grison, P. (1952). La Processionnaire du Chêne (Thaumetopoea processionea L.) dans la région parisienne. Revue de Pathologie Végétale et d’Entomologie Agricole de France, 31, 103–114.
Groenen, F. (2010). Variation of Thaumetopoea processionea (Notodontidae: Thaumetopoeinae) in Europe and the Middle East. Entomologische Berichte, 70, 77–83.
Groenen, F., & Meurisse, N. (2012). Historical distribution of the Oak Processionary moth Thaumetopoea processionea in Europe suggests recolonization instead of expansion. Agricultural and Forest Entomology, 14, 147–155.
Guerrero, A., Camps, F., Coll, J., Riba, M., Einhorn, J., Descoins, C., & Lallemand, J. Y. (1981). Identification of a potential sex pheromone of the processionary moth, Thaumetopoea pityocampa (Lepidoptera, otodontidae). Tetrahedron Letters, 22, 2013–2016.
Hajduk, Z. (1963). Thaumetopoea pinivora in Poland. Przedlag Zoologiczny, 7, 55–57 [in Polish].
Halperin, J. (1983). Thaumetopoea solitaria Freyer (Lepidoptera, Thaumetopoeidae) in Israel. Phytoparasitica, 11, 71–82.
Halperin, J. (1990a). Natural enemies of Thaumetopoea sp. in Israel. Journal of Applied Entomology, 109, 425–435.
Halperin, J. (1990b). Life history of Thaumetopoea spp. (Lep., Thaumetopoeidae) in Israel. Journal of Applied Entomology, 110, 1–6.
Halperin, J., & Sauter, W. (1999). The occurrence of Thaumetopoea processionea L. (Lepidoptera, Thaumetopoeidae) on Mt. Hermon. Phytoparasitica, 27, 211.
Hellrigl, K. (1995). Der Kiefernprozessionspinner (Thaumetopoea pityocampa Denis & Schiff.) in Südtirol. Schriftenreihe für Wissenschaftliche Studien, Landesabteilung Forstwirtschaft der Autonome Provinz Bozen/Südtirol, Bolzano/Bozen, Italy, 1, 1–80.
Hering, F. (1970). Regelmäßiger Wechsel von Fraß- und Flugjahren bei Thaumetopoea pinivora Treitschke schon seit mindestens 1910 bekannt (Lepidoptera, Thaumetopoeidae). Anzeiger für Schädlingskunde und Pflanzenschutz, 43, 112.
Hill, J. K., Thomas, C. D., & Blakeley, D. S. (1999). Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia, 121, 165–170.
Hill, J. K., Griffiths, H. M., & Thomas, C. D. (2011). Climate change and evolutionary adaptations at species’ range margins. Annual Review of Entomology, 56, 143–159.
Hoch, G., Verucchi, S., & Schopf, A. (2008). Microsporidian pathogens of the Oak Processionary moth, Thaumetopoea processionea. Mitteilungen der Deutsche Gesellschaft für allgemeine und angewandte Entomologie, 16, 225–228.
Hódar, J. A., & Zamora, R. (2004). Herbivory and climatic warming, a Mediterranean outbreaking caterpillar attacks a relict, boreal pine species. Biodiversity and Conservation, 13, 493–500.
Hódar, J. A., Castro, J., & Zamora, R. (2003). Pine processionary caterpillar Thaumetopoea pityocampa as a new threat for relict Mediterranean Scots pine forests under climatic warming. Biological Conservation, 110, 123–129.
Hódar, J. A., Zamora, R., Castro, J., & Baraza, E. (2004). Feast and famine, previous defoliation limiting survival of pine processionary caterpillar Thaumetopoea pityocampa in Scots pine Pinus sylvestris. Acta Oecologica, 26, 203–210.
Hódar, J. A., Cayuela, L., & Zamora, R. (2012). Climate change and the incidence of a forest pest in Mediterranean ecosystems, can the North Atlantic Oscillation be used as a predictor? Climatic Change, 113, 699–711.
Holm, G., & Larsson, S. (2006). Allergi mot tallprocessionsspinnarlarver – ökad larvtäthet ger ökad risk. Läkartidningen, 103, 2217–2219.
Houri, A., & Doughan, D. (2006). Behavior patterns of the pine processionary moth (Thaumetopoeawilkinsoni, Tams; Lepidoptera: Thaumetopoeidae). American Journal of Agriculture and Biological Sciences, 1, 1–5.
Huchon, H., & Démolin, G. (1970). La bioécologie de la processionnaire du pin. Dispersion potentielle – Dispersion actuelle. Revue Forestière Française (N° spécial “La lutte biologique en forêt”), 220–234.
Huchon, H., & Démolin, G. (1971). La bioécologie de la processionaire du pin. Dispersion potentielle – Dispersion actuelle. Phytoma, 23, 11–20.
İpekdal, K. (2012). Delimitation and phylogeography of the pine processionary moth species, Thaumetopoea pityocampa and T. wilkinsoni. Ph.D. dissertation, Hacettepe University, Institute of Science, Ankara, Turkey, 194 p. [in Turkish].
İpekdal, K., & Çağlar, S. S. (2012). Effects of temperature on the host preference of pine processionary caterpillar Thaumetopoea wilkinsoni Tams, 1924 (Lepidoptera, Notodontidae). Turkish Journal of Zoology, 36, 319–328.
İpekdal, K., & Beton, D. (2013). Modelling future distribution of the pine processionary moth. Proceedings of the international Caucasian forestry symposium, pp. 402–407.
Jans, H. W. A., & Franssen, A. E. M. (2008). The urticating hairs of the Oak Processionary caterpillar (Thaumetopoea processionea L.), a potential problems for animals? Tijdschrift Voor Diergeneeskunde, 133, 424–429.
Kara, K., & Tschorsnig, H. P. (2003). Host catalogue for the Turkish Tachinidae (Diptera). Journal of Applied Entomology, 127, 465–476.
Karadağ, S., Er, M., Mart, C., & Tursun, A. (2007). Thaumetopoea solitaria Frey. (Lepidoptera, Thaumetopoeidae)’ nin biyolojisi ve morfolojisine dliskin gözlemler. KSÜ Fen ve Mühendislik Dergisi, 10, 132–137.
Kerdelhué, C., Battisti, A., Burban, C., Branco, M., Cassel-Lundhagen, A., Ipekdal, K., Larsson, S., Lopez-Vaamonde, C., Magnoux, E., Mateus, E., Mendel, Z., Negrisolo, E., Paiva, M. R., Pivotto, I., Rocha, S., Ronnås, C., Roques, A., Rossi, J.-P., Rousselet, J., Salvato, P., Santos, H., Simonato, M., & Zane, L. (2014). Genetic diversity and structure at different spatial scales in the processionary moths. In A. Roques (Ed.), Processionary moths and climate change: An update. Dordrecht: Springer.
Kiriakoff, S. G., & Talhouk, A. S. (1975). Thaumetopoealibanotica spec. nov. (Lepidoptera Thaumetopoeidae). Opuscula Zoologica, 137, 1–5.
Klapwijk, M. J., Csóka, G., Hirka, A., & Björkman, C. (2013). Forest insects and climate change: Long-term trends in herbivore damage. Ecology and Evolution, 3, 4183–4196.
Koch, M. (1953). Zur Biologie des Kiefernprozessionsspinners, Thaumetopoea pinivora Tr. Beitrage Entomologie, 4, 423–427.
Krehan, H. (1993). Outbreaks of caterpillar forest pests in oak forests of eastern Austria. Forstschutz Aktuell, 12, 1–4.
Kugler, J. (1979). New taxa of Tachinidae (Diptera) with a list of the species from Israel and adjacent territories. Israel Journal of Entomology, 8, 27–60.
Lamy, M. (1990). Contact dermatitis (erucism) produced by processionary caterpillars (genus Thaumetopoea). Journal of Applied Entomology, 110, 425–437.
Lamy, M., & Novak, F. (1987). The oak Processionary caterpillar (Thaumetopoea processionea L), an urticating caterpillar related to the pine processionary caterpillar (Thaumetopoea pityocampa Schiff) (Lepidoptera, Thaumetopoeidae). Experientia, 43, 456–458.
Larsson, S., Aimi, A., Ronnås, C., Battisti, A. (2006). A local outbreak of the northern pine processionary moth Thaumetopoea pinivora on Gotland, south Sweden. In Proceedings of the IUFRO WP 7.03.10 Symposium, 11–14 September 2006, Gmunden, Austria (pp. 219–224).
Lehmann, M. (2009). Reaktionen lästiger und humanhygienisch bedenklicher Organismen an Pflanzen auf Klimaerwärmung. In Klimawandel und Gesundheit. Welche Probleme verursachen Wärme liebende Schadorganismen? Abschlussgespräch zum Internationalen UBA/BMU- Fachgespräch, 9–10 November 2009, Umweltbundesamt Berlin-Dahlem, 17–22.
Lempke, B. J. (1989). Interesting captures and observations of Macrolepidoptera in the period 1985–1987. Entomologische Berichten (Amsterdam), 49, 89–95.
Lobinger, G. (2010). Der Eichenprozessionsspinner und die Eichenfraßgesellschaft 2009/2010. LWF aktuell, 75, 54–55.
Lobinger, G. (2011). Schadpotenzial des Eichenprozessionsspinners in den Wäldern des Freistaates Bayern. Retrieved September 3, 2013, from http://www.jki.bund.de/fileadmin/dam_uploads/_GF/FG_EPS/3_Schadpotenzial_in_Waeldern_BayernsV.pdf
Lobinger, G. (2012). Pheromongestutzte Prognoseverfahren bei schadlichen Schmetterlingen im Forst, Eingefurhrte Praxisverfahrenm Forschung und Entwickelung. Retrieved September 3, 2013, from http://anmeldung.fowita.de/Presse/Vortraege/Freising/Forstschutz/Lobinger_Prognoseverfahren%20bei%20sch%C3%A4dlichen%20Schmetterlingen.pdf
Lovgren, R., & Dalsved, B. (2005). Thaumetopoea processionea L. (Lepidoptera, Thaumetopoeidae) found in Sweden. Entomologisk Tidskrift, 126, 93–94.
Maier, H., Spiegel, W., Kinaciyan, T., Krehan, H., Cabaj, A., Schopf, A., & Hönigsmann, H. (2003). The Oak Processionary caterpillar as the cause of an epidemic airborne disease, survey and analysis. British Journal of Dermatology, 149, 990–997.
Maksymov, J. K. (1978). Thaumetopoeidae, Prozessionsspinner. In W. Schwenke (Ed.), Die Forstschädlinge Europas, Band 3 (pp. 391–404). Hamburg: Paul Parey.
Mart, C., Uygun, N., Altın, M., Erkılıc, L., & Bolu, H. (1995). General review on the injurious and beneficial species and pest control methods used in pistachio orchards of Turkey. Acta Horticulturae, 419, 379–385.
Martin, J. C. (2014). Development of environment-friendly management strategies of processionary moths. In A. Roques (Ed.), Processionary moths and climate change: An update. Dordrecht: Springer.
Mendel, Z. (1988). Host selection by the pine processionary caterpillar Thaumetopoea wilkinsoni. Phytoparasitica, 16, 101–108.
Meurisse, N., Hoch, G., Schopf, A., Battisti, A., & Grégoire, J. C. (2012). Low temperature tolerance and starvation ability of the Oak Processionary moth, implications in a context of increasing epidemics. Agricultural and Forest Entomology, 14, 239–250.
Mirchev, P., Schmidt, G. H., & Tsankov, G. (1998). The egg parasitoids of the pine processionary moth Thaumetopoea pityocampa (Den. and Schiff.) in the Eastern Rhodopes, Bulgaria. Bollettino di Zoologia Agraria e di Bachicoltura, 30, 131–140.
Mirchev, P., Tsankov, G., & Petrov, Y. (2003). Study on some aspects of the bioecology of the Oak Processionary moth (Thaumetopoea processionea (Linnaeus, 1758) (Lepidoptera, Notodontidae) in North-East Bulgaria. Silva Balcanica, 3, 5–10.
Mirchev, P., Schmidt, G. H., Tsankov, G., & Avci, M. (2004). Egg parasitoids of Thaumetopoea pityocampa (Den. and Schiff.) (Lep., Thaumetopoeidae) and their impact in SW Turkey. Journal of Applied Entomology, 128, 533–542.
Mirchev, P., Tsankov, G., & Matova, M. (2006). Aspects of bioecology of pistachio but moth, Thaumetopoea solitaria (Freyer, 1838) (Lepidoptera, Thaumetopoeidae) in Bulgaria. Forest Science, 3, 95–106.
Mirchev, P., Georgiev, G., & Draganova, S. (2012). Disease caused by Beauveria bassiana (Bals.-Criv.) Vuill. on newly hatched larvae of Thaumetopoea solitaria Frey. Silva Balcanica, 13, 61–65.
Mol, T., & Küçükosmanoğlu, A. (2002). Control methods used against Thaumetopoea pityocampa (Den. and Schiff.) in Turkey. In M. Kanat (Ed.), Proceedings of the Symposium for Pine Processionary Moth Problem in Turkey and Solutions 24–25 April 2002, Kahramanmaras, Turkey (pp. 135–147) [in Turkish].
Moneo, I., Battisti, A., Dufour, B., García-Ortiz, J. C., González-Muñoz, M., Moutou, F., Paolucci, P., Petrucco Toffolo, E., Riviere, J., Rodríguez-Mahillo, A. I., Roques, A., Roques, L., Vega, J. M., Vega, J., & Vega, J. M. (2014). Medical and veterinary impact of the urticating processionary larvae. In A. Roques (Ed.), Processionary moths and climate change: An update. Dordrecht: Springer.
Montoya, R., & Robredo, F. (1972). Thaumetopoea pinivora Tr., la procesionaria de verano. Boletin Estacion Central Ecologia, 1, 43–56.
Mourikis, P., Tsourgianni, A., & Chitzanidis, A. (1998). Pistachio nut insect pests and means of control in Greece. Acta Horticulturae, 470, 604–611.
Offenberg, K. (2000). The calamity of the oak procession moth (Thaumetopoea processionea L) during the last century in Westfalen. Forst und Holz, 55, 424–426.
Özkazanç, O. (2002). Bio-ecology of Thaumetopoea pityocampa Schiff. (Lepidoptera, Thaumetopoeidae) in the Mediterranean Region. In M. Kanat (Ed.), Proceedings of the symposium for pine processionary moth problem in Turkey and solutions, 24–25 April 2002, Kahramanmaras, Turkey (pp. 1–11) [in Turkish].
Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution and Systematics, 37, 637–669.
Pascual, J. (1988). Biologia de la Procesionaria del roble (Thaumetopoea processionea L.) (Lep. Thaumetopoeidae) en el centro-oeste de la Peninsula Iberica. Boletin de Sanidad Vegetal Plagas, 14, 383–404.
Pekel, N. (1999). Investigations on the biology and natural enemies of the pine processionary moth, Thaumetopoea pityocampa (Den. & Schiff.) in forests of the Forest General Directorate in Isparta. M.Sc. dissertation, Süleyman Demirel University Institute of Graduate Studies in Science, Isparta, Turkey, 58 p. [in Turkish].
Petrucco Toffolo, E., Zovi, D., Perin, C., Paolucci, P., Roques, A., Battisti, A., & Horvath, H. (2014). Size and dispersion of urticating setae in three species of processionary moths. Integrative Zoology, 9, 320–327.
Pimentel, C., & Nilsson, J.-Å. (2009). Response of passerine birds to an irruption of a pine processionary moth Thaumetopea Pityocampa population with a shifted phenology. Ardeola, 56, 189–203.
Pimentel, C., Calvão, T., Santos, M., Ferreira, C., Neves, M., & Nilsson, J.-A. (2006). Establishment and expansion of a Thaumetopoea pityocampa (Den. & Schiff.) (Lep. Notodontidae) population with a shifted life cycle in a production pine forest, Central-Coastal Portugal. Forest ecology and Management, 233, 108–115.
Pimentel, C., Ferreira, C., & Nilsson, J.-Å. (2010). Latitudinal gradients and the shaping of life-history traits in a gregarious caterpillar. Biological Journal of the Linnean Society, 100, 224–236.
Pimentel, C., Calvão, T., & Ayres, M. P. (2011). Impact of climatic variation on populations of pine processionary moth Thaumetopoea pityocampa in a core area of its distribution. Agricultural and Forest Entomology, 13, 273–281.
Pimentel, C., Santos, M., Ferreira, C., & Nilsson, J.-Å. (2012). Temperature, size, reproductive allocation, and life-history evolution in a gregarious caterpillar. Biological Journal of the Linnean Society, 105, 340–349.
Quero, C., Camps, F., & Guerrero, A. (1995). Behavior of processionary males (Thaumetopoea pityocampa) induced by sex pheromone and analogs in a wind tunnel. Journal of Chemical Ecology, 21, 1957–1969.
Quero, C., Malo, E., Fabriàs, G., Camps, F., Lucas, P., Renou, M., & Guerrero, A. (1997). Reinvestigation of female sex pheromone of processionary moth (Thaumetopoea pityocampa, no evidence for minor components. Journal of Chemical Ecology, 23, 713–726.
Quero, C., Bau, J., Guerrero, A., Breuer, M., De Loof, A., Kontzog, H.-G., & Camps, F. (2003). Sex pheromone of the Oak Processionary moth Thaumetopoea processionea. Identification and biological activity. Journal of Agricultural and Food Chemistry, 51, 2987–2991.
Robinet, C. (2006). Modélisation mathématique des phénomènes d’invasion en écologie, exemple de la chenille processionnaire du pin. Thèse de doctorat, spécialité Mathématiques et Applications aux Sciences de l’Homme, Ecole des Hautes Études en Sciences Sociales (E.H.E.S.S.), Paris, France, 208 p.
Robinet, C., Baier, P., Pennerstorfer, J., Schopf, A., & Roques, A. (2007). Modelling the effects of climate change on the potential feeding activity of Thaumetopoea pityocampa (Den. & Schiff.) (Lep., Notodontidae) in France. Global Ecology and Biogeography, 16, 460–471.
Robinet, C., Imbert, C. E., Rousselet, J., Sauvard, D., Garcia, J., Goussard, F., & Roques, A. (2011). Human-mediated long-distance jumps of the pine processionary moth in Europe. Biological Invasions, 14, 1557–1569.
Robinet, C., Rousselet, J., Pineau, P., Miard, F., & Roques, A. (2013). Are heatwaves susceptible to mitigate the expansion of a species progressing with global warming? Ecology and Evolution, 3, 2947–2957.
Ronnås, C., Larsson, S., Pitacco, A., & Battisti, A. (2010). Effects of colony size on larval performance in a processionary moth. Ecological Entomology, 35, 436–445.
Ronnås, C., Cassel-Lundhagen, A., Battisti, A., Wallén, J., & Larsson, S. (2011). Limited emigration from an outbreak of a forest pest insect. Molecular Ecology, 20, 4606–4617.
Roques, A., Rousselet, J., Avci, M., Avtzis, D. N., Basso, A., Battisti, A., Ben Jamaa, M., Bensidi, A., Berardi, L., Berretima, W., Branco, M., Chakali, G., Çota, E., Dautbašić, M., Delb, H., El Alaoui El Fels, M. A., El Mercht, S., El Mokhefi, M., Forster, B., Garcia, J., Georgiev, G., Glavendekić, M. M., Goussard, F., Halbig, P., Henke, L., Hernandez, R., Hodar, J. A., İpekdal, K., Jurc, M., Klimetzek, D., Laparie, M., Larsson, S., Mateus, E., Matošević, D., Meier, F., Mendel, Z., Meurisse, N., Mihajlović, L., Mirchev, P., Nasceski, S., Nussbaumer, C., Paiva, M. R., Papazova, I., Pino, J., Podlesnik, J., Poirot, J., Protasov, A., Rahim, N., Sanchez Peña, G., Santos, H., Sauvard, D., Schopf, A., Simonato, M., Tsankov, G., Wagenhoff, E., Yart, A., Zamora, R., Zamoum, M., & Robinet, C. (2014). Climate warming and past and present distribution of the processionary moths (Thaumetopoea spp.) in Europe, Asia Minor and North Africa. In A. Roques (Ed.), Processionary moths and climate change: An update. Dordrecht: Springer.
Ruf, C., Costa, J. T., & Fieldler, K. (2000). Trail-based communication in social caterpillars of Eriogaster lanestris (Lepidoptera, Lasiocampidae). Journal of Insect Behavior, 14, 231–245.
Salvato, P., Battisti, A., Concato, S., Masutti, L., Patarnello, T., & Zane, L. (2002). Genetic differentiation in the winter pine processionary moth (Thaumetopoea pityocampa-wilkinsoni complex), inferred by AFLP and mitochondrial DNA markers. Molecular Ecology, 11, 2435–2444.
Santos, H., Rousselet, J., Magnoux, E., Paiva, M. R., Branco, M., & Kerdelhué, C. (2007). Genetic isolation through time, allochronic differentiation of a phenologically atypical population of the pine processionary moth. Proceedings of the Royal Society of London Series B, 274, 935–941.
Santos, H., Burban, C., Rousselet, J., Rossi, J.-P., Branco, M., & Kerdelhué, C. (2011a). Incipient allochronic speciation in the pine processionary moth Thaumetopoea pityocampa (Lepidoptera, Notodontidae). Journal of Evolutionary Biology, 24, 146–158.
Santos, H., Paiva, M. R., Tavares, C., Kerdelhué, C., & Branco, M. (2011b). Temperature niche shift observed in a Lepidoptera population under allochronic divergence. Journal of Evolutionary Biology, 24, 1897–1905.
Santos, H., Paiva, M. R., Rocha, S., Kerdelhué, C., & Branco, M. (2013). Phenotypic divergence in reproductive traits of a moth population experiencing a phenological shift. Ecology and Evolution, 3, 5098–5108.
Sbabdji, M., & Kadik, B. (2011). Effects of Atlas cedar (Cedrus atlantica) defoliation on performance of the pine processionary moth (Thaumetopoea pityocampa). Journal of Pest Science, 84, 213–217.
Schimitschek, E. (1944). Forstinsekten der Turkei und ihre Umwelt (pp. 242–247). Prague: Volk und Reich Verlag.
Serafimovski, A. (1975). Thaumetopoea solitaria var. iranica Bang-Haas. Eine morphologo-biologische Analyse. Bulletin of Forest Institute of Macedonia, Skopje, 2, 59–86.
Shani, A., Klug, J. T., & Skorka, J. (1983). Stereoselective synthesis of (Z)-13-hexadecen-11-yn-1-yl acetate, the major component of the sex pheromone of the pine processionary moth (Thaumetopoea pityocampa). Journal of Chemical Ecology, 9, 863–867.
Simonato, M., Mendel, Z., Kerdelhué, C., Rousselet, J., Magnoux, E., Salvato, P., Roques, A., Battisti, A., & Zane, L. (2007). Phylogeography of the pine processionary moth Thaumetopoea wilkinsoni in the Near East. Molecular Ecology, 16, 2273–2283.
Skule B., & Vilhelmsen F. (1997). Thaumetopoea processionea L., found in Denmark [Online document]. Retrieved March 14, 2013, from www.lepidoptera.dk/process.htm
Sliwa, E. (2002). Thaumetopoea pinivora in Poland. Las Polski, 9, 12–13.
Solt, I., & Mendel, Z. (2002). The pine processionary caterpillar. Harefuah (Medicine), 141, 810–814 [in Hebrew].
Spiegel, W., Maier, H., & Maier, M. (2004). A non-infectious airborne disease. Lancet, 363, 1438.
Stigter, H., & Romeijn, G. (1992). Thaumetopoea processionea locally observed in large numbers in the Netherlands after more than 100 years (Lepidoptera, Thaumetopoeidae). Entomologische Berichte (Amsterdam), 52, 66–69.
Stigter, H., Geraedts, W., & Spijkers, H. (1997). Thaumetopoea processionea in the Netherlands: Present status and management perspectives (Lepidoptera, Notodontidae). In Proceedings of the section experimental and applied entomology of the Netherlands Entomological Society (N.E.V.) (pp. 3–16).
Tamburini, G., Marini, L., Hellrigl, K., Salvadori, C., & Battisti, A. (2013). Effects of climate and density-dependent factors on population dynamics of the pine processionary moth in the Southern Alps. Climatic Change, 121, 701–712.
Tams, W. H. T. (1925). A new processionary month (Notodontidae) injurious to pine trees in Cyprus. Bulletin of Entomological Research, 15, 293–294.
Thomas, C. D., Bodsworth, E. J., Wilson, R. J., Simmons, A. D., Davies, Z. G., Musche, M., & Conradt, L. (2001). Ecological and evolutionary processes at expanding range margins. Nature, 411, 577–581.
Tiberi, R., & Bin, F. (1988). Parasitism and other mortality factors in Thaumetopoea processionea L. egg masses in central Italy (Hymenoptera, Chalcidoidea; Lepidoptera, Thaumetopoeidae). Redia, 71, 299–312.
Tiberi, R., Roversi, P., & Bin, F. (1991). Egg parasitoids of pine and Oak Processionary caterpillars in central Italy. Redia, 74, 249–250.
Tomiczek, C., & Krehan, H. (1996). Occurrence of oak processionaries and winter moths in Vienna. Forstschutz Aktuell, 17/18, 23.
Townsend, M. (2009). Report on survey and control of Oak Processionary Moth Thaumetopoea processionea (Linnaeus) (Lepidoptera, Thaumetopoeidae) (OPM) in London in 2008 [Online]. Retrieved September 3, 2013, from www.forestry.gov.uk/pdf/fcopmsurvey07.pdf
Triggiani, O., & Tarasco, E. (2002). Efficacy and persistence of entomopathogenic nematodes in controlling larval populations of Thaumetopoea pityocampa (Lepidoptera, Thaumetopoeidae). Biocontrol Science and Technology, 12, 747–752.
Trougth, T. (1954). The life history of Thaumetopoea jordana Staudinger. Entomologist’s Record, 66, 188–191.
Tsankov, G., Schmidt, G. H., & Mirchev, P. (1998). Studies on the egg parasitism in Thaumetopoea pityocampa over a period of four years (1991–1994) at Marikostino/Bulgaria. Anzeiger für Schadlingskunde Pflanzenschutz Umweltschutz, 71, 1–7.
Tsankov, G., Mirchev, P., & Matova, M. (2006). Egg parasitoids, rate of parasitism and structure of egg batches of Thaumetopoea pityocampa (Den. and Schiff.) (Lep., Thaumetopoeidae) from the region of Ochrid (Republic of Macedonia). Silva Balcanica, 7, 77–87.
Tschorsnig, H.-P. (1993). Parasitoide aus dem Eichenprozessionsspinner Thaumetopoea processionea. Mitteilungen des Entomologischen Vereins Stuttgart, 31, 105–107.
Tschorsnig, H.-P., & Wagenhoff, E. (2012). On the oviposition of Phorocera grandis (Tachinidae). The Tachinid Times, 25, 3–7.
Vago, C. (1959). Sur le mode d’infection de la virose intestinale de Thaumetopoea pityocampa Schiff. Entomophaga, 4, 311–314.
Van Handel, E., & Day, J. F. (1988). Assay of lipids, glycogen and sugars in individual mosquitoes, correlations with wing length in field collected Aedes vexans. Journal of the American Mosquito Control Association, 4, 549–550.
Wagenhoff, E., & Delb, H. (2011). Aktuelles vom Eichenprozessionsspinner. Forstliche Versuchs- und Forschungsanstalt Baden-Württemberg, 2, 3–6.
Wagenhoff, E., & Veit, H. (2011). Five years of continuous Thaumetopoea processionea monitoring, Tracing population dynamics in an arable landscape of South-Western Germany. Gesunde Pflanzen, 63, 51–61.
Wagenhoff, E., Blum, R., Engel, K., Veit, H., & Delb, H. (2012). Temporal synchrony of Thaumetopoea processionea egg hatch and Quercus robur budburst. Journal of Pest Science, 86, 1–10.
Waring, P., Townsend, M., & Lewington, R. (2003). Field guide to the moths of Great Britain and Ireland. Hook: British Wildlife Publishing, 432 p.
Way, M. J., Paiva, M. R., & Cammell, M. E. (1999). Natural biological control of the pine processionary moth Thaumetopoea pityocampa (Den. & Schiff.) by the Argentine ant (Linepithema humile) in Portugal. Agricultural and Forest Entomology, 1, 27–31.
Wedd, D. (2002). Short note. British Journal of Entomology and Natural History, 15, 161.
Wilkinson, D. S. (1926). The Cyprus processionary caterpillar (Thaumetopoea wilkinsoni, Tams). Bulletin of Entomological Research, 18, 163–182.
Williams, D. T., Straw, N., Townsend, M., Wilkinson, A. S., & Mullins, A. (2013). Monitoring oak processionary moth Thaumetopoea processionea L. using pheromone traps, the influence of pheromone lure source, trap design and height above the ground on capture rates. Agricultural and Forest Entomology, 15, 126–134.
Wiltshire, E. P. (1982). Insects of Saudi Arabia. Lepidoptera, Fam. Cossidae, Zygaenidae, Sesiidae, Lasiocampidae, Bombycidae, Sphingidae, Thaumetopoeidae, Thyretidae, Notodontidae, Geometridae, Lymantriidae, Noctuidae, Ctenuchidae (Part 2). Fauna of Saudi Arabia, 4, 271–332.
Zamoum, M. (1998). Données sur la bioécologie, les facteurs de mortalité et la dynamique de populations de Thaumetopoea pityocampa Denis et Schiffermuller (Lep. Thaumetopoeidae) dans les pineraies subsahariennes de la région de Djelfa (Algérie), Thèse de doctorat Rennes I France.
Zamoum, M., & Démolin, G. (2005). The life cycle of the pine processionary caterpillar in the bioclimatic conditions of a sub-Saharan region. In F. Lieutier & D. Ghaioule (Eds.), Entomological Research in Mediterranean Forest Ecosystems (pp. 107–116). Paris: INRA.
Zamoum, M., Démolin, G., & Bensidi, A. (2006). Complexe des ennemis naturels de la processionnaire du pin Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae) au sud de son aire de répartition. Actes Congrès d’entomologie et de nématologie (pp. 140–152). Alger: Inst. Nat. Agro.
Zamoum, M., Guendouz, H., & Deia, D. (2007). Structure des communautésd’ennemisnaturels de Thaumetopoeapityocampa (Lep., Thaumetopoeidae) sur pin d’AlepenAlgériesubsaharienne. Entomologica Bari, 40, 139–151.
Zeegers, T. (1997). Tachinid flies (Diptera, Tachinidae) from Dutch Thaumetopoea processionea. Entomologische Berichten (Amsterdam), 57, 73–78.
Zhang, Q. H., & Paiva, M. R. (1998). Female calling behaviour and male response to the sex pheromone in Thaumetopoea pityocampa (Den. and Schiff.) (Lep., Thaumetopoeidae). Journal of Applied Entomology, 122, 353–360.
Zovi, D., Stastny, M., Battisti, A., & Larsson, S. (2008). Ecological costs on local adaptation of an insect herbivore imposed by host plants and enemies. Ecology, 89, 1388–1398.
Zwakhals, C. (2005). Pimpla processioneae and P. rufipes, specialist versus generalist (Hymenoptera, Ichneumonidae, Pimplinae). Entomologische Berichten (Amsterdam), 65, 14–16.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Éditions Quæ
About this chapter
Cite this chapter
Battisti, A. et al. (2015). Natural History of the Processionary Moths (Thaumetopoea spp.): New Insights in Relation to Climate Change. In: Roques, A. (eds) Processionary Moths and Climate Change : An Update. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9340-7_2
Download citation
DOI: https://doi.org/10.1007/978-94-017-9340-7_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9339-1
Online ISBN: 978-94-017-9340-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)