Abstract
Invasive pentatomid Halyomorpha halys recently arrived to the Black Sea region and began damaging agricultural and ornamental plants. We studied the effects of day length and temperature on the pre-adult development and diapause induction in H. halys from Sochi (Russia) under laboratory conditions (20, 24, and 28 °C and several photoperiods). The pre-adult development of H. halys was noticeably faster under L:D 12:12 compared with L:D 15:9. The sum of effective temperatures required for the pre-adult development was ca. 530 and 590 degree days under these two conditions, respectively, whereas the lower developmental thresholds were similar (ca. 13.3 °C). Adults of H. halys demonstrated a typical long-day-type photoperiodic response of facultative winter adult diapause induction: Short days (photophases of 12–15 h) induced diapause in all adults, whereas long days (with photophases longer than 15 h) promoted reproduction. The photoperiodic responses of diapause induction of females and males were very similar. At 24 °C, the threshold of the response was between 15 and 16 h. At 20 °C, even under the very long-day conditions (L:D 18:6) about 50% of adults entered diapause. Field records suggest that H. halys likely produces two generations per year in Sochi. Short days might accelerate nymphal growth of the second generation in August and then induce winter diapause in adults. Phenological studies and monitoring are needed for a better understanding of the adaptation process of this invasive pest to new conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key messages
-
Halyomorpha halys is an invasive pest that arrived to the Black Sea region in 2013 or 2014.
-
Pre-adult growth and induction of adult winter diapause were studied under laboratory conditions.
-
Nymphs grow faster under short-day compared with long-day conditions. This response allows late nymphs to reach the adult stage before autumnal temperature decreases.
-
Females and males enter winter diapause in response to short-day conditions (photophase shorter than 15.5 h).
-
Halyomorpha halys produces two annual generations in the Black Sea region.
Introduction
At the beginning of the twenty-first century, we witnessed a sharp increase in frequencies of pest and pathogen invasions in different parts of the world. The rapid movements of invasive species accompany globalization, often threaten local biodiversity and ecosystem functioning, and cause significant economic loss (Hulme 2009; Pyšek et al. 2010; Zhu et al. 2012; Bertelsmeier and Keller 2018; McPherson 2018). A unique adaptive ability to disperse and naturalize outside of its native range has been demonstrated by the brown marmorated stink bug, Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae). Native to eastern Asia, mostly to China (except Xinjiang and Qinghai Provinces), Korea, Japan (except Hokkaido), Myanmar, Vietnam, and Taiwan, H. halys has recently become one of the most harmful invasive insect pests in North America and Europe (Rice et al. 2014; Haye et al. 2015; Lee 2015; Hamilton et al. 2018). Rapid invasions of H. halys from Asia to both North America and Europe are commonly linked to the increased global trade and travel (Leskey and Nielsen 2018). Recently, H. halys was recorded for the first time in Russia (in 2013 or 2014) and Abkhazia (in 2015) (Gapon 2016; Mityushev 2016; Musolin et al. 2018). However, the invasive North American and European populations differ in their genetic composition, and it is believed that the species invaded North America from the Beijing region (China) and Europe from another (not yet determined) region of Asia (Gariepy et al. 2014; Xu et al. 2014).
In different parts of its native range in Asia, H. halys produces one or two generations per year (Lee et al. 2013). Hoffman (1931) reports that four or even six generations may occur in the Kwangtung (Guangdong) Province of China (23°N, 113°E), but this old record seems doubtful and needs confirmation.
It has been shown in many species with heterodynamic seasonal development that the timing of the commencement of winter dormancy is determined by parameters of the photoperiodic response of facultative diapause induction (Danilevsky 1965; Tauber et al. 1986; Danks 1987). In H. halys, photoperiodic response of adult (often also called reproductive) diapause induction has been experimentally studied only in Japan, and that was before the invasion of this species to North America and Europe. Watanabe (1979) demonstrated that the critical photoperiod for ovarian development was between 13.5 and 14.0 h in the Toyama (Japan) population (36°50′N, 137°35′E). However, a detailed analysis of the mechanism underlying the seasonal development was carried out using only one other local population of this species (from Nagano; 36°40′N, 138°12′E; Yanagi and Hagihara 1980). Essentially, these data from central Japan and recently obtained data from North America have been used in attempts to analyze H. halys phenology and to model its voltinism in different parts of its invasive range (Nielsen and Hamilton 2009; Haye et al. 2014; Nielsen et al. 2016, 2017).
The available body of information on distribution and seasonal development of H. halys in its invasive range is limited and does not allow one to confidently predict the further secondary range changes as well as voltinism of this dangerous invader. Zhu et al. (2012) suggested that H. halys potentially could become established in virtually all parts of Europe with southern Europe being the most favorable. Thus, for example, CLIMEX and other modeling techniques suggest that H. halys will be able to complete up to three generations in southern Greece (e.g., Crete or East Peloponnese; Milonas and Partsinevelos 2014). Models were developed for North America as well (e.g., Nielsen et al. 2016, 2017). Phenological models should be applied with caution as not only temperature, but also local parameters of the photoperiodic response of facultative winter diapause induction often strongly affect voltinism of a population, and to date, photoperiodic responses of H. halys European populations are unknown.
In this paper, we present data on the temperature and day length (i.e., photoperiod) regulation of H. halys pre-adult (i.e., embryonic and nymphal) development and diapause induction obtained under strictly controlled laboratory conditions. We studied a population of H. halys only recently established in Sochi, Russia. The findings are used to analyze the seasonal development of the local population of H. halys in southern Russia, but they could be more broadly applicable for a better understanding and modeling of H. halys voltinism in southern and central Europe.
Materials and methods
Insects
The laboratory culture originated from about 100 individuals of H. halys (nymphs and adults) as well as ca. 20 egg masses collected in June–August 2017 in the vicinity of Sochi, Krasnodar region of Russia (ca. 43°36′N, 39°35′E). Insects were reared in ventilated transparent plastic containers (28 × 19 × 14 cm) under standard conditions (temperature of 25–28 °C, photoperiod L:D 16:8 h) and fed on peanuts, sunflower seeds, carrots, and broad bean seedlings; water was provided in plastic cylinders plugged with cotton balls. The experiments were conducted using the first and second generations of this laboratory culture.
Experimental design
In the laboratory experiments, we used following 14 treatments (i.e., photo-thermal regimes): temperature of 20 °C combined with photoperiods of L:D 12:12, 14:10, 15:9, and 18:6; temperature of 24 °C combined with photoperiods of L:D 12:12, 13:11, 14:10, 15:9, 16:8, and 18:6; and temperature of 28 °C combined with photoperiods of L:D 12:12, 14:10, 15:9, and 18:6. These combinations of temperature and photoperiod were chosen based on the results of earlier studies (Watanabe 1979; Yanagi and Hagihara 1980; Leskey and Nielsen 2018). It was intended to cover a full scale of natural day length at the optimal temperature of 24 °C plus near-threshold (L:D 14:10, 15:9, and 16:8) as well as short (L:D 12:12) and very long (L:D 18:6) photoperiods under suboptimal temperatures (20 and 28 °C).
Different photoperiods were established in different photoperiodic chambers placed in the same walk-in thermostatic room. To detect possible small differences between photoperiodic chambers within the same thermostatic room, temperature in each photoperiodic chamber was measured twice daily by a laboratory thermometer with an accuracy of 0.1 °C. The differences between the photoperiodic chamber means for the duration of the experiments were always less than 0.2 °C and therefore were not taken into account in subsequent analysis. During the experiments (described below), nymphs and adults were reared in ventilated transparent plastic cylinders (diameter and height of 12 cm) and fed on the same diet as was the laboratory population. Fresh food and water were provided every 2–3 days.
Egg incubation period and development of the first instar nymphs
The egg masses produced by H. halys females of the laboratory population within 24 h were collected and randomly distributed among 14 aforementioned photo-thermal regimes (with a total of ca. 5–8 egg masses per regime). Hatching of the first instar nymphs and their subsequent molting to the second instar were recorded daily, specifically at 4–6 h after the switch on of the light. (The light was switched on simultaneously in all photoperiodic chambers, but switched off at different times.) Four nonsynchronous replicates of each treatment were conducted in this experiment. In each replicate, hatching and first molting of 10–15 nymphs were recorded. The mean egg incubation period (days) and mean duration of the first instar (days) were separately calculated for each replicate in each treatment, and the replicate means were used for further statistical analysis.
Growth and development of the second–fifth instar nymphs
Nymphs molted to the second instar within 24 h in the stock culture were chosen for this experiment. To increase the uniformity of the material, nymphs hatched from one egg mass were distributed among several randomly selected treatments. Five or six replicates of each of the 14 treatments were conducted, and each replicate was started with 20 nymphs of the second instar (with a total of 1460 nymphs). Emergence and sex of adults were recorded every 2–3 days. In total, emergence of 711 adults was recorded (at least 8 adults of each sex per treatment). Based on these data, nymphal survival (a proportion of the second instar nymphs that successfully reached the adult stage) and the mean duration of the cumulative developmental period from the beginning of the second instar to adult emergence were calculated for each replicate in each treatment.
Maturation of male and female reproductive systems and adult diapause induction
Cohorts of 4–10 adults of both sexes emerged within 2–3 days in the aforementioned experiment were placed separately in the same size containers and reared further under the same photo-thermal conditions and on the same diet as during their second–fifth instars. At the end of this experiment (40, 25, and 12 days after adult emergence at 20, 24, and 28 °C, respectively), all adults were killed using low temperature and dissected. The timing of dissection was chosen based on preliminary experiments and constitutes approximately 1.5 times of the period from female emergence to deposition of the first egg masses at the corresponding temperatures under long-day conditions (Watanabe 1980). At dissection, reproductive state of males and females was evaluated based on the criteria used for H. halys and other pentatomids (Watanabe 1980; Nakamura and Numata 1997; Musolin and Numata 2003a; Nielsen et al. 2017; Esquivel et al. 2018; Musolin and Saulich 2018): Females without mature eggs or vitellogenic oocytes in the ovarioles and males without secretory fluids in the ectodermal sacs of the accessory glands were considered to be in diapause in contrast to reproductively active (i.e., nondiapause) adults (Fig. 1). Adults that died during the experiment were discarded. In total, 313 males and 303 females were dissected at the end of this experiment.
State of gonadal development in diapause and nondiapause adults of Halyomorpha halys: a nondiapause female (reproductive; mature, i.e., chorionated, eggs in ovarioles; loose fat body; expanded and filled spermatheca); b nondiapause male (reproductive; expanded ectodermal sacs containing milky white secretion); c diapause female (nonreproductive; transparent and small ovarioles without oocytes; developed and dense fat body; shrunken and empty spermatheca); d diapausing male (nonreproductive; shrunken and empty ectodermal sacs; developed and dense fat body). Photograph by Dr. K. Samartsev
Statistical analysis
The differences between the treatments were evaluated, and the interactions of the factors were tested using ANOVA and the Tukey’s HSD test. (See figure and table legends for details.) Nonparametric data (nymphal survival, sex ratio, and proportion of diapausing individuals) were transformed (i.e., ranked) before the analysis. To avoid empty cells in Table 1 and lost degrees of freedom, two-way and multi-way ANOVAs were conducted for the four photoperiods that were used at all three temperature regimes (L:D 12:12, 14:10, 15:9, and 18:6). Studying growth and development of nymphs (Table 2), to avoid the issue of pseudoreplication, the analysis was conducted with replicate means of the pooled data for males and females rather than the data for individual bugs. To estimate the dependence of development on temperature, linear regression analysis was used. First, the rate of development was calculated as a reciprocal value of the duration of development RD = 100/D, where R is the rate of development (%/day) and D is the duration of development (days). Then, based on these data, the equation of linear regression RD = a*T + b was calculated, where T is temperature (°C), whereas a and b are experimentally determined coefficients. Finally, based on these coefficients, the lower thermal threshold for development (LDT) and the sum of effective temperatures (SET) were calculated as LDT = b/a (°C) and SET = 100/b (degree days), correspondingly. All calculations were made with SYSTAT 10.2 software (SYSTAT 10.2 2018).
Results
Egg incubation period and development of the first instar nymphs
Two-way ANOVA demonstrated that the egg incubation period and duration of the first instar nymphs were significantly affected only by temperature; influence of photoperiod and the temperature * photoperiod interaction were not statistically significant (Table 1). The rate of development of embryos and the first instar nymphs calculated together based on the pooled data from all the photoperiods linearly depended on temperature (Fig. 2). The total duration of development from oviposition to the molting to the second instar was ca. 19, 12, and 9 days at 20, 24, and 28 °C, correspondingly.
Effect of temperature on the rate of development of Halyomorpha halys embryos and first instar nymphs. Means (± S.E.M.) shown on the graph are means of the replicate means. Regression lines correspond to the equations RD = 1.265 T − 15.35, n = 56, R2 = 0.87 (eggs) and RD = 1.865 T − 26.01, n = 56, R2 = 0.85 (the first instar nymphs)
Growth and development of the second–fifth instar nymphs
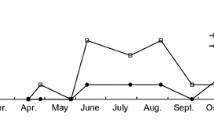
The developmental period from the beginning of the second instar to the end of the fifth (the final) instar strongly depended not only on temperature, but also on photoperiod with significant interaction of these two factors. The difference between males and females as well as interaction of the sex with temperature and photoperiod was not statistically significant (Table 2). The analysis demonstrated that the interaction of photoperiod and temperature was also (although marginally) not statistically significant (Table 1). Thus, at all temperatures, the duration of the major part of nymphal development (i.e., second–fifth instars) was the shortest at L:D 12:12 and the longest at L:D 15:9 (Fig. 3).
Effects of temperature and photoperiod on the duration of Halyomorpha halys developmental period from the beginning of the second instar to the adult stage. Means (± S.D.) shown on the graph are means of the replicate means. Different letters along the same line indicate significant statistical differences between photoperiods at the same temperature (P < 0.05; the Tukey’s HSD test). Some symbols are slightly shifted to avoid overlap
The effect of temperature on the rate of development from the beginning of the second instar to the end of the fifth instar under two photoperiods (L:D 12:12 and 15:9) was clearly evident and significantly different (Fig. 4). However, based on the linear regressions, the relative photoperiodically induced increase in the rates of development at L:D 12:12 was almost the same at 20 and 28 °C (16.1 and 17.5%, respectively).
Effects of temperature and photoperiod on the rate of Halyomorpha halys development from the beginning of the second instar to the end of the fifth instar. Means (± S.E.M.) shown on the graph are means of the replicate means. Regression lines correspond to the equations RD = 0.254 T − 3.40, n = 16, R2 = 0.98 (L:D = 12:12) and RD = 0.214 T − 2.84, n = 15, R2 = 0.97 (L:D = 15:9)
The nymphal survival (calculated as a proportion of the second instar nymphs that successfully reached the adult stage) also depended only on temperature (Table 1). Based on the pooled data, it was the highest at 24 °C (63.3%), somewhat lower at 28 °C (49.7%) and significantly lower at 20 °C (27.7%). The sex ratio was very close to 1:1 (48.9% of females) and did not significantly depend on temperature or photoperiod (Table 1).
The total pre-adult development
Combined, the total duration of H. halys pre-adult development (from the oviposition to the emergence of adults; mean ± S.D.) under the conditions of L:D 15:9 constituted 91.9 ± 8.7, 53.9 ± 3.4, and 41.5 ± 3.3 days (i.e., the rate of development was 1.09, 1.86, and 2.41%/day) at 20, 24, and 28 °C, correspondingly. However, under the short-day conditions (L:D 12:12), the pre-adult development was noticeably faster: 79.2 ± 5.7, 48.6 ± 3.3, and 35.9 ± 2.1 days (i.e., the rate of development was 1.26, 2.06, and 2.78%/day) at 20, 24, and 28 °C, respectively. Linear regression analysis gave the following two equations: RD = 0.169 T − 2.252 and RD = 0.189 T − 2.508 for L:D 15:9 and L:D 12:12, correspondingly. Based on these data, the SET required for pre-adult development was about 590 degree days under the conditions of L:D = 15:9 and 530 degree days under the short-day conditions (L:D = 12:12), whereas the LDT was approximately the same (ca. 13.3 °C) at these photoperiods.
Maturation of male and female reproductive systems and adult diapause induction
Adults of H. halys clearly demonstrated typical photoperiodic response of facultative winter adult diapause induction. The proportion of adults that were in the state of diapause at the day of dissection markedly depended on photoperiod (Fig. 5); the dependence on temperature was weak and only marginally significant, but the effect of the interaction of the two factors was relatively strong (Table 2). The sex as well as its interactions with both photoperiod and temperature did not have any statistically significant impact on the proportion of diapausing individuals. Thus, the photoperiodic responses of diapause induction in males and females were very similar (Fig. 5). Halyomorpha halys demonstrated a very clear long-day-type photoperiodic response of diapause induction: Short days (with photophases from 12 to 15 h) induced adult diapause in all individuals, whereas long days (with longer photophases) promoted reproductive maturation. The results obtained at 24 °C demonstrated that the threshold of the photoperiodic response was between 15 and 16 h. At the relatively low temperature of 20 °C, however, even under the very long-day conditions (L:D 18:6) about 50% of adults of both sexes had reproductive organs in the undeveloped state 40 days after the emergence suggesting formation of diapause or at least a substantial delay of reproductive maturation (Fig. 5).
Discussion
Success of adaptations to new environmental conditions in invasive insects is determined, among other factors, by ecophysiological peculiarities of these insects (Tauber et al. 1986; Musolin and Numata 2003b; Musolin and Saulich 2018). Thus, the potential to establish in a new area differs from species to species. Available and satisfactory food resources as well as local thermal conditions sufficient for complete development of at least one generation per year (except for very rare special cases of species with semivoltine development) are the major factors that determine the very possibility of establishment of an invader in a new region. In temperate and cool climates, winter diapause or another seasonal adaptation that ensures overwintering is also a necessary condition of success (Danilevsky 1965; Tauber et al. 1986; Danks 1987; Musolin and Saulich 2018). When all three components are present, invasion and initial establishment might be successful.
Halyomorpha halys has a well-pronounced facultative winter adult diapause (Watanabe 1979; Yanagi and Hagihara 1980; Niva and Takeda 2003; Cira et al. 2018; Hamilton et al. 2018), its nymphs and adults are very polyphagous (Nielsen and Hamilton 2009; Lee et al. 2013; Rice et al. 2014; Hamilton et al. 2018; Leskey and Nielsen 2018), and thermal conditions of the Black Sea region are mild enough to allow realization of at least one or two generations per year of this pentatomid. Halyomorpha halys most likely arrived to the Black Sea coast in 2013 or 2014 (Musolin et al. 2018). How well has it adapted to the new region by now, just three or four years after the arrival? How do the local environmental conditions shape its seasonal cycle in the new land?
Experimental studies of the thermal norms of development for the H. halys Sochi population demonstrate that the total duration of its pre-adult development under conditions of L:D 15:9 constitutes ca. 92, 53, and 41 days at 20, 24, and 28 °C, respectively. This total duration of its pre-adult development is longer than that under the short-day (L:D 12:12) conditions (ca. 79, 49, and 36 days at the same temperatures). Based on these data, the sum of effective temperatures necessary to complete the pre-adult development is ca. 530 and 590 degree days at the day length of 12 and 15 h, correspondingly, whereas the lower developmental threshold is approximately the same (ca. 13.3 °C) at both photoperiods (the adult sexual maturation period is not included). These data are close to those reported earlier for native (Asian) and invasive (central European and North American) populations of H. halys (Watanabe 1980; Yanagi and Hagihara 1980; Kiritani 2007; Nielsen et al. 2008, 2016; Haye et al. 2014).
In Sochi, ca. 1220–1455 degree days can be accumulated above 13.3 °C from mid-April to early November (data for 2013–2017 from Sochi Agro-Meteorological Station analyzed in the All-Russian Research Institute of Floriculture and Subtropical Cultures, Sochi). Basic calculations suggest that these thermal conditions are quite sufficient for development of two complete generations of H. halys per year. However, for successful realization of a bivoltine seasonal cycle in this species, two following conditions should be met: (1) Nymphs of the first generation should reach the adult stage before day length becomes shorter than its critical value (i.e., threshold) for diapause induction, and (2) adults of the 2nd generation should get fully prepared for overwintering by accumulation of sufficient fat body (and this must occur under favorable temperatures). Ecophysiological responses such as photoperiodic response of diapause induction ensure exact adaptation of local populations to local climate. The same adaptations, on the other hand, often limit migration or relocation of individuals even within the species’ range.
Each environmental factor can function as a seasonal signal and a trigger inducing a particular physiological state (Tyshchenko 1980). Both of these functions are ecologically important. Day length performs these functions in many insect species: It acts as a trigger of a shift between alternative physiological states such as diapause and reproduction and it also plays a key role in synchronization of biological events (between different species and/or between a species and its environmental conditions). These two functions are well coordinated under stable conditions, but in the cases of inter-zonal movements or distant relocations, this coordination might be disturbed, and diapause induction, for example, might take place too early or too late. In such case, day length still acts as a trigger inducing diapause, but appropriate synchronization with local environmental changes is lost (or rather not yet established; Tyshchenko 1980).
The most detailed analysis of seasonal development of H. halys was conducted in Japan and was based on the experiments performed under both laboratory and quasi-natural conditions in Nagano (36°40′N, 138°12′E; Yanagi and Hagihara 1980; reviewed in Saulich and Musolin 2018). Said studies demonstrated that the facultative winter adult diapause in that population is controlled by a long-day-type photoperiodic response with a critical day length being slightly less than 15 h at 25 °C. In accordance with this critical day length, adults emerging after July 15–25 do not start reproduction, but directly enter winter diapause. Thus, in Nagano, this species is univoltine (Yanagi and Hagihara 1980).
A few studies of native Japanese populations of H. halys demonstrated geographic variation in the critical day length of diapause induction in females: At 25 °C, it was between 13.5 and 14.0 h in Toyama Prefecture (36°50′N, 137°35′E; Watanabe 1979), between 14 and 15 h in Chiba Prefecture (35°33′N, 140°11′E; Fujiie 1985), and about 14.5 h in Hyogo Prefecture (34°40′N, 135°15′E; Niva 2003). Some of these populations of H. halys and those in China (Lee et al. 2013) are reported to be bivoltine at least in some years. In such populations, adults of the first generation emerge in late June or early July when local day-length conditions determine nondiapause development thereby allowing for a second generation. In contrast, adults of the second generation emerging in these regions in late August or later do not reproduce, but enter facultative winter adult diapause because the natural day length at that time is already short.
The seasonal cycle of H. halys has also been studied within its invasive range. In northeast USA (e.g., Pennsylvania and New Jersey), only one generation per year is recorded (Nielsen et al. 2008; Nielsen and Hamilton 2009). The calculated sum of effective temperatures for a full generation of this species is approximately 700 degree days with the lower developmental threshold of 14 °C. The peak of the field abundance of H. halys was recorded in August, when 800–1000 degree days are accumulated suggesting a univoltine seasonal development in this part of the species’ invasive range (Nielsen and Hamilton 2009; Leskey et al. 2012). In many other locations in North America, populations of H. halys are reported or predicted to be bivoltine (Nielsen et al. 2016, 2017).
Field records in Sochi (Musolin et al. 2018) suggest that overwintered adults of H. halys start to lay eggs in early May, nymphs of the first generation complete their development in late July, and adults of the first generation can produce eggs for a second generation starting from late July or early August. Nymphs of the second generation likely reach the adult stage and go through the pre-overwintering feeding during rather warm and mild autumn when mean daily temperatures are close to 15–20 °C (ClimaTemps.com 2018). During this period, days are short and nymphal development of H. halys is likely accelerated (Fig. 3). Such short-day acceleration of nymphal development has been found in many heteropteran species and considered a quantitative photoperiodic response allowing insects to reach the only possible overwintering stage and prepare for overwintering before temperature drops dramatically in autumn (Musolin and Saulich 1997, 1999, 2018).
Parameters of the second photoperiodic response found in the same species, namely photoperiodic response of diapause induction, have never been experimentally studied in any invasive population of H. halys. In the current study, it was demonstrated that adults of the Sochi population have very clear long-day-type photoperiodic response of diapause induction with a critical day length of ca. 15.5 h at 24 °C (Fig. 5). It is hard to say how strongly temperature can affect photoperiodic response in this species because not all the photoperiods were tested at 20 and 28 °C, but it is obvious that at the lower temperature (20 °C) ca. 50% of individuals entered diapause even under very long-day conditions (L:D 18:6) and, even at high temperature of 28 °C, almost all adults entered diapause at the near-threshold, but still short-day conditions (L:D 15:9; Fig. 5).
Shapes of the photoperiodic responses of two sexes almost completely coincided (Fig. 5) strongly suggesting that males and females respond to the day length similarly, which is considered characteristic of Pentatomidae (Niva 2003; Musolin and Saulich 2018), but not of some other heteropterans (e.g., Anthocoridae; Ruberson et al. 1998; Saulich and Musolin 2009).
The resulting critical day length of ca. 15.5 h at 24 °C (Fig. 5) in the Caucasian invasive population is somewhat longer than the critical day length in the Japanese populations of H. halys. Such critical day length ensures earlier induction of facultative winter adult diapause. Most probably, this situation reflects the ongoing adaptation of the invasive population to the cooler climate in central Europe from where the European invasion most likely started (Lee 2015; Hamilton et al. 2018; Musolin et al. 2018). On the other hand, it is long known that ovarian development and diapause induction in H. halys as well as in many other polyphagous insects can markedly depend on the diet (Watanabe 1979; Tauber et al. 1986; Saulich and Musolin 2012; Musolin and Saulich 2018). Unfortunately, there are no data on photoperiodic responses in any other European and North American invasive populations of H. halys. The lack of information suggests the necessity of a special comparative study.
Day length of 15.5 h (if civil twilights are taken into consideration) arrives in Sochi in early August when mean temperature is close to 22–24 °C (ClimaTemps.com 2018). The data obtained suggest that at this period of the summer adults should enter diapause and prepare for overwintering.
It has been demonstrated that in H. halys not only adults, but also nymphs of late instars are sensitive to the day length (Niva 2003; Niva and Takeda 2003); thus, the further developmental pathway (reproductive maturation or diapause) is determined during these developmental stadia. As mentioned above, adults of the first generation emerge in Sochi in late July or early August (Musolin et al. 2018), which coincides with the period when natural day length is shortening and approaching the threshold of diapause induction, whereas nymphal development of these adults takes place earlier when the natural day length is clearly longer than the critical one. Thus, the majority of adults of this first generation should be destined to reproduce.
However, other parameters of the photoperiodic response of diapause induction are important as well in this context. Thus, for proper manifestation of the photoperiodic response, insects need to accumulate so-called required day number, i.e., a particular species-specific number of short-day or long-day cycles (Goryshin and Tyshchenko 1972; Saunders 1976). This important parameter determines how many days are required for proper photoperiodic induction of diapause or reproduction after the arrival of critical day length. It is experimentally estimated only in a few heteropteran species (Musolin and Saulich 2018) and is not studied in H. halys. It is also not yet known how H. halys responds to decreasing and increasing day length and how such changing photoperiodic conditions affect the species’ threshold for diapause induction. And finally, we do not know the threshold of light intensity above which H. halys will perceive twilights as a light part of the daily cycle. If the species is responding to dim light, then twilights might postpone diapause induction by a few days. This lack of information makes modeling of the species’ phenology imprecise. However, the already available data suggest that environmental conditions in Sochi are sufficient for development of two full generations of H. halys per year not only taking into consideration the thermal conditions (i.e., the SET), but also interrelationships of day-length and temperature conditions during the period of diapause induction and preparation for overwintering. Based on the experimental data, it is likely that the bivoltine seasonal pattern of the species will fit well the climatic conditions of the region and, thus, will allow H. halys to establish in the region, produce two generations every year, smoothly build up its population, and become widespread.
Whether the voltinism pattern of H. halys in this region remains the same or changes in the future is difficult to confirm based on the currently available knowledge. On the one hand, as previously discussed, the bivoltine pattern fits the local environmental conditions well. On the other hand, adult diapause seems to be induced in the beginning of August, i.e., somewhat early in the season. As autumns are warm and mild in the Sochi region, theoretically, an additional full generation might be produced in August–October, at least in some years. In general, diapause induced too early in the season means not only that adults can have longer pre-diapause feeding, but also that diapause itself is longer for them and they might (and actually do; NN Karpun, unpublished data) suffer high winter mortality. All this being said, it might be expected that under the pressure of the local environmental conditions the critical day length of the photoperiodic response of H. halys will change in the near future: Shortening of the critical day length might allow longer diapause-free period in summer and shorter overwintering period. Such changes are possible as critical day length of photoperiodic response is known to be a flexible and selectable physiological trait, at least in some insect species (Gomi 1997; Bradshaw and Holzapfel 2001, 2008; Musolin and Numata 2003b; Gomi et al. 2007; Urbanski et al. 2012; Shintani et al. 2018). Further close monitoring and additional studies of H. halys in its invasive range are needed to clarify these issues and develop management recommendations.
Author contributions
DLM, SYaR, and AKhS conceived and designed the study. NNK and VYeP collected material and created culture. MYuD and SYaR conducted laboratory experiment. SYaR performed statistical analysis. All authors discussed the data, wrote, and approved the manuscript.
References
Bertelsmeier C, Keller L (2018) Bridgehead effects and role of adaptive evolution in invasive populations. Trends Ecol Evol 33(7):527–534. https://doi.org/10.1016/j.tree.2018.04.014
Bradshaw WE, Holzapfel CM (2001) Genetic shift in photoperiodic response correlated with global warming. Proc Natl Acad Sci USA 98:14509–14511. https://doi.org/10.1073/pnas.241391498
Bradshaw WE, Holzapfel CM (2008) Genetic response to rapid climate change: it’s seasonal timing that matters. Mol Ecol 17(1):157–166. https://doi.org/10.1111/j.1365-294X.2007.03509.x
Cira TM, Koch RL, Burkness EC, Hutchison WD, Venette RC (2018) Effects of diapause on Halyomorpha halys (Hemiptera: Pentatomidae) cold tolerance. Environ Entomol. https://doi.org/10.1093/ee/nvy064
ClimaTemps.com (2018) http://www.soci.climatemps.com/ Accessed 09 May 2018
Danilevsky AS (1965) Photoperiodism and seasonal development of insects. Oliver & Boyd, Edinburgh
Danks HV (1987) Insect dormancy: an ecological perspective. Biological survey of Canada, Ottawa (Monograph ser. N 1)
Esquivel JF, Musolin DL, Jones WA, Rabitsch W, Greene JK, Toews MD, Schwertner CF, Grazia J, McPherson RM (2018) Nezara viridula (L.). In: McPherson JE (ed) Invasive stink bugs and related species (Pentatomoidea): biology, higher systematics, semiochemistry, and management. CRC Press, Boca Raton, pp 351–423
Fujiie A (1985) Seasonal life cycle of Halyomorpha mista. Bull Chiba Agric Exp Stn 26:87–93
Gapon DA (2016) First records of the brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera, Pentatomidae) in Russia, Abkhazia, and Georgia. Entomol Rev 96:1086–1088. https://doi.org/10.1134/S001387381608011X
Gariepy TD, Fraser H, Scott-Dupree CD (2014) Brown marmorated stink bug (Hemiptera: Pentatomidae) in Canada: recent establishment, occurrence, and pest status in southern Ontario. Can Entomol 146:579–582. https://doi.org/10.4039/tce.2014.4
Gomi T (1997) Geographic variation in critical photoperiod for diapause induction and its temperature dependence in Hyphantria cunea Drury (Lepidoptera: Arctiidae). Oecologia 111:160–165. https://doi.org/10.1007/s004420050220
Gomi T, Nagasaka M, Fukuda T, Nagihara H (2007) Shifting of the life cycle and life-history traits the fall webworm in relation to climate change. Entomol Exp Appl 125:179–184. https://doi.org/10.1111/j.1570-7458.2007.00616.x
Goryshin NI, Tyshchenko GF (1972) Experimental analysis of photoperiodic induction of diapause in insects. Trudy Biologicheskogo instituta Leningraskogo gosudarstvennogo universiteta [Proc of the Biol Inst Leningrad State Univ] 21:68–89
Hamilton GC, Ahn JJ, Bu W, Leskey TC, Nielsen AL, Park Y-L, Rabitsch W, Hoelmer KA (2018) Halyomorpha halys (Stål). In: McPherson JE (ed) Invasive stink bugs and related species (Pentatomoidea): biology, higher systematics, semiochemistry, and management. CRC Press, Boca Raton, pp 243–292
Haye T, Abdallah S, Gariepy T, Wyniger D (2014) Phenology, life table analysis, and temperature requirements of the invasive brown marmorated stink bug, Halyomorpha halys, in Europe. J Pest Sci 87:407–418. https://doi.org/10.1007/s10340-014-0560-z
Haye T, Gariepy T, Hoelmer K, Rossi J-P, Streito J-C, Tassus X, Desneux N (2015) Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: an increasing threat to field, fruit and vegetable crops worldwide. J Pest Sci 88(4):665–673. https://doi.org/10.1007/s10340-015-0670-2
Hoffman WE (1931) A pentatomid pest of growing beans in south China. Peking Nat Hist Bull 5:25–27
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46(1):10–18. https://doi.org/10.1111/j.1365-2664.2008.01600.x
Kiritani K (2007) The impact of global warming and land use change on the pest status of rice and fruit bugs (Heteroptera) in Japan. Global Change Biol 13:1586–1595. https://doi.org/10.1111/j.1365-2486.2007.01397.x
Lee D-H (2015) Current status of research progress on the biology and management of Halyomorpha halys (Hemiptera: Pentatomidae) as an invasive species. Appl Entomol Zool 50(3):277–290. https://doi.org/10.1007/s13355-015-0350-y
Lee D-H, Short BD, Joseph SV, Bergh JC, Leskey TC (2013) Review of the biology, ecology and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan and the Republic of Korea. Environ Entomol 42:627–641. https://doi.org/10.1603/EN13006
Leskey TC, Nielsen AL (2018) Impact of the invasive brown marmorated stink bug in North America and Europe: History, biology, ecology, and management. Annu Rev Entomol 63:599–618. https://doi.org/10.1146/annurev-ento-020117-043226
Leskey TC, Hamilton GC, Nielsen AL et al (2012) Pest status of the brown marmorated stink bug, Halyomorpha halys (Stål), in the USA. Outlooks on Pest Management 23(5):218–226
McPherson JE (ed) (2018) Invasive stink bugs and related species (Pentatomoidea): biology, higher systematics, semiochemistry, and management. CRC Press, Boca Raton, pp. 497–564
Milonas PG, Partsinevelos GK (2014) First report of brown marmorated stink bug Halyomorpha halys Stål (Hemiptera: Pentatomidae) in Greece. EPPO Bull 44(2):183–186. https://doi.org/10.1111/epp.12129
Mityushev IM (2016) The first record of Brown marmorated stink bug in Russia. Zashita i Karantin Rasteniy [Prot Quar Plants] 3:48
Musolin DL, Numata H (2003a) Photoperiodic and temperature control of diapause induction and colour change in the southern green stink bug Nezara viridula. Physiol Entomol 28(2):65–74. https://doi.org/10.1046/j.1365-3032.2003.00307.x
Musolin DL, Numata H (2003b) Timing of diapause induction and its life-history consequences in Nezara viridula: is it costly to expand the distribution range? Ecol Entomol 28(6):694–703. https://doi.org/10.1111/j.1365-2311.2003.00559.x
Musolin DL, Saulich AKh (1997) Photoperiodic control of nymphal growth in true bugs (Heteroptera). Entomol Rev 77(6):768–780
Musolin DL, Saulich AH (1999) Diversity of seasonal adaptations in terrestrial true bugs (Heteroptera) from the temperate zone. Entomol Sci 2(4):623–639
Musolin DL, Saulich AKh (2018) Diapause in Pentatomoidea. In: McPherson JE (ed) Invasive stink bugs and related species (Pentatomoidea): biology, higher systematics, semiochemistry, and management. CRC Press, Boca Raton, pp 497–564
Musolin DL, Konjević A, Karpun NN, Procenko VYe, Ayba LYa, Saulich AKh (2018) Invasive brown marmorated stink bug Halyomorpha halys (Stål) (Heteroptera: Pentatomidae) in Russia, Abkhazia, and Serbia: history of invasion, range expansion, early stages of establishment, and first records of damage to local crops. Arthropod-Plant Interact 12(4):517–529. https://doi.org/10.1007/s11829-017-9583-8
Nakamura K, Numata H (1997) Seasonal life cycle of Aelia fieberi (Heteroptera: Pentatomidae). Ann Entomol Soc Am 90:625–630. https://doi.org/10.1093/aesa/90.5.625
Nielsen AL, Hamilton GC (2009) Life history of the invasive species Halyomorpha halys (Hemiptera: Pentatomidae) in Northeastern United States. Ann Entomol Soc Am 102(4):608–616. https://doi.org/10.1603/008.102.0405
Nielsen AL, Hamilton GC, Matadha D (2008) Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environ Entomol 37:348–355. https://doi.org/10.1093/ee/37.2.348
Nielsen AL, Chen S, Fleischer SJ (2016) Coupling developmental physiology, photoperiod, and temperature to model phenology and dynamics of an invasive heteropteran, Halyomorpha halys. Front Physiol 7:165. https://doi.org/10.3389/fphys.2016.00165
Nielsen AL, Fleischer S, Hamilton GC et al (2017) Phenology of brown marmorated stink bug described using female reproductive development. Ecol Evol 7:6680–6690. https://doi.org/10.1002/ece3.3125
Niva CC (2003) Molecular and neuroendocrine mechanisms of photoperiodism in Halyomorpha halys (Heteroptera: Pentatomidae). Ph.D. Dissertation, Kobe University
Niva CC, Takeda M (2003) Effects of photoperiod, temperature and melatonin on nymphal development, polyphenism and reproduction in Halyomorpha halys (Heteroptera: Pentatomidae). Zool Sci 20:963–970. https://doi.org/10.2108/zsj.20.963
Pyšek P, Bacher S, Chytrý M et al (2010) Contrasting patterns in the invasions of European terrestrial and freshwater habitats by alien plants, insects and vertebrates. Glob Ecol Biogeogr 19(3):317–331. https://doi.org/10.1111/j.1466-8238.2009.00514.x
Rice KB, Bergh JC, Bergmann EJ et al (2014) Biology, ecology, and management of brown marmorated stink bug (Halyomorpha halys). J Integr Pest Manag 5:1–13. https://doi.org/10.1603/IPM14002
Ruberson JR, Kring TJ, Elkassabany N (1998) Overwintering and the diapause syndrome of predatory Heteroptera. In: Coll M, Ruberson JR (eds) Predatory Heteroptera: their ecology and use in biological control. Thomas Say Publications in Entomology, Entomological Society of America, Lanham, pp 49–69
Saulich AKh, Musolin DL (2009) Seasonal development and ecology of anthocorids (Heteroptera, Anthocoridae). Entomol Rev 89(5):501–528. https://doi.org/10.1134/S0013873809050017
Saulich AKh, Musolin DL (2012) Diapause in the seasonal cycle of stink bugs (Heteroptera, Pentatomidae) from the temperate zone. Entomol Rev 92(1):1–26. https://doi.org/10.1134/S0013873812010010
Saulich AKh, Musolin DL (2018) Seasonal cycles of Pentatomoidea. In: McPherson JE (ed) Invasive stink bugs and related species (Pentatomoidea): biology, higher systematics, semiochemistry, and management. CRC Press, Boca Raton, pp 565–607
Saunders DS (1976) Insect clocks. Pergamon Press, Oxford
Shintani Y, Kato Y, Saito T, Oda Y, Terao M, Nagamine K (2018) Maladaptive photoperiodic response in an invasive alien insect, Milionia basalis pryeri (Lepidoptera: Geometridae), in southern Kyushu, Japan. Appl Entomol Zool 53(3):343–351. https://doi.org/10.1007/s13355-018-0562-z
SYSTAT 10.2 (2018) https://systat.informer.com/10.2/ Accessed 22 Dec 2018
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, New York
Tyshchenko VP (1980) Signal and vital modes of action of ecological factors. Zhurnal Obshey Biologii [J General Biol] 41:655–667
Urbanski J, Mogi M, O’Donnell D, DeCotiis M, Toma T, Armbruster P (2012) Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am Nat 179:490–500. https://doi.org/10.1086/664709
Watanabe M (1979) Ecology and extermination of Halyomorpha halys. 4. The relationship between day length and ovarian development. Ann Rep Toyama Inst Health 3:33–37
Watanabe M (1980) Study of the life cycle of the brown marmorated stink bug, Halyomorpha mista. Insectarium 17:168–173
Xu J, Fonseca DM, Hamilton GC et al (2014) Tracing the origin of US brown marmorated stink bugs, Halyomorpha halys. Biol Invasion 16:153–166. https://doi.org/10.1007/s10530-013-0510-3
Yanagi T, Hagihara Y (1980) Ecology of the brown marmorated stink bug. Plant Prot 34:315–321
Zhu G, Bu W, Gao Y, Liu G (2012) Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PLoS ONE 7:e31246. https://doi.org/10.1371/journal.pone.003124
Acknowledgements
The present study was partially supported by the Russian Foundation for Basic Research (Grant No. 17-04-01486 for DLM), the EU COST Action FP1401 Global Warning (A global network of nurseries as early warning system against alien tree pests; http://www.cost.eu/COST_Actions/fps/FP1401 for DLM), the Russian state research project AAAA-A17-117030310205-9 (for MYuD and SYaR), and The Innessa Charitable Foundation (for AKhS). We sincerely thank Dr. J.F. Esquivel (USDA, ARS, SPARC), Dr. K. Tsytsulina and anonymous reviewers for critical reading of the MS and helpful comments, Dr. K. Samartsev for photographs, and T.Yu. Moskaleva for assistance with the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals (vertebrate) performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by D.C. Weber.
Rights and permissions
About this article
Cite this article
Musolin, D.L., Dolgovskaya, M.Y., Protsenko, V.Y. et al. Photoperiodic and temperature control of nymphal growth and adult diapause induction in the invasive Caucasian population of the brown marmorated stink bug, Halyomorpha halys. J Pest Sci 92, 621–631 (2019). https://doi.org/10.1007/s10340-019-01080-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-019-01080-1