Abstract
Biofilm, as a form of the microbial community in nature, represents an evolutionary adaptation to the influence of various environmental conditions. In nature, the largest number of microorganisms occur in the form of multispecies biofilms. The ability of microorganisms to form a biofilm is one of the reasons for antibiotic resistance. The creation of biofilms resistant to various contaminants, on the other hand, improves the biological treatment process in wastewater treatment plants. Heavy metals cannot be degraded, but they can be transformed into non-reactive and less toxic forms. In this process, microorganisms are irreplaceable as they interact with the metals in a variety of ways. The environment polluted by heavy metals, such as wastewater, is also a source of undiscovered microbial diversity and specific microbial strains. Numerous studies show that biofilm is an irreplaceable strategy for heavy metal removal. In this review, we systematize recent findings regarding the bioremediation potential of biofilm-forming microbial species isolated from diverse wastewaters for heavy metal removal. In addition, we include some mechanisms of action, application possibilities, practical issues, and future prospects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The release of heavy metals into the environment has a negative impact not only on human health but also on natural ecosystems. Heavy metals accumulate in food chains, so they pose a serious threat when they are present in higher concentrations. Heavy metals cannot be broken down, although they can be converted into less harmful, non-reactive forms. Microorganisms have proven to be irreplaceable in the restoration of heavy metal-polluted habitats, both ecologically and economically (Shah et al. 2021). The use of microorganisms and their exploitation in waste treatment is today widely accepted as a sustainable alternative to conventional processes such as chemical precipitation, ion exchange, chemical oxidation, adsorption, etc. (Sedlakova-Kadukova 2022). Microbes are used in the bioremediation of environments polluted by various xenobiotics and harmful heavy metals, converting them into non-toxic compounds. Fungi, like bacteria, have a significant potential in the processes of environmental purification and organic pollution removal due to their excellent biosorption capability and ability to create a large number of hydrolytic enzymes (Jakovljević, 2016, Jakovljević and Vrvić 2016). The role of different microorganisms in the remediation of heavy metal pollution is of great importance because some microorganisms show great tolerance and survival in the presence of high metal concentrations (Mishra et al. 2022). Excessive usage of heavy metals selectively promotes antibiotic resistance, which persists and spreads even in the absence of antibiotics. There are at least four potential mechanisms for heavy metal-driven co-selection of AMR in biofilms: co-resistance, cross-resistance, coregulation, and biofilm induction. Metal pollution has an impact on indigenous bacteria, with Firmicutes and Bacteroidota being the most abundant phyla at high metal pollution areas. These bacteria are ubiquitous in metal-contaminated areas and have been found to carry metal resistance gene and antibiotic resistance gene in groups called “gene cassettes,“ which explains why metal exposure can lead to antibiotic resistance. Wastewater treatment plants (WTPs) are thought to be a source of the release of antibiotic-resistant bacteria into the environment (Cesare et al. 2016).
Wastewater treatment plants, especially older ones, often face the problem of large amounts of some heavy metals that create an unbalanced microbial community in the biological treatment basins and in the sewage sludge. The primary reason for this is the release of industrial wastewater with high levels of metals into municipal wastewater without previous treatment. In municipal wastewater, the presence of Cr, Mn, Fe, Co, Ni, Cu, Zn, and Cd was confirmed; in water affected by industry, Ni, Cu, Sr, Pb, Cd, and Hg were confirmed; in water affected by mining, Al, Cr, Pb, Cd, and Hg were confirmed, etc. (Kranthi et al. 2018). Heavy metals significantly affect the composition and spatial variability of the microbial community (Rajeev et al. 2021). In that case, modification of the microflora is achieved by biostimulation, which involves the use of indigenous microorganisms that are already well adapted to the existing environment with the addition of nutrients (Adams et al. 2015). In some industrial and municipal wastewater treatment plants, this is not adequate. That is why bioaugmentation, the introduction of exogenous microorganisms (indigenous or non-indigenous in high concentrations) with the ability to detoxify certain pollutants is used more frequently (Ma et al. 2022). After the application of commercial preparations in wastewater the number of exogenous microorganisms often decreases significantly. Therefore, due to efficiency (but also laws, regulations and public opinion), the most practical use of microorganisms isolated from wastewater that needs to be decontaminated is still the most practical (Younas 2022; Jakovljević et al. 2022a). Mixed microbial cultures have an advantage due to the wider degradation potential of synergism and the possibility of co-metabolism (Jakovljević and Vrvić 2018; Jakovljević 2020; Jakovljević et al. 2022b). For these reasons, there is an ongoing need to isolate and identify new microorganisms with the ability to degrade pollutants, as well as to understand the genetics and biochemistry of the biodegradation process. Microorganisms isolated from wastewater can be opportunistic pathogens or even pathogens. They are removed in a variety of ways: exposure to sunlight and other physicochemical factors (temperature and pH), predation, length of stay in ponds, adsorption on particles and sedimentation filtration, and finally, UV radiation and chlorination.
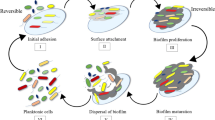
In the presence of pollutants, to improve their survival, bacteria often switch to a biofilm lifestyle (Mahto et al. 2022). A biofilm is an organized community of one or more types of microbes connected by extracellular polymeric compounds and adhered to an abiotic and/or biotic surface. It differs from plankton growth primarily by the vital role of transport and transfer processes that control the rate of growth in the biofilm community (Flemming et al. 2016). The biofilm formation process is a complex phenomenon regulated by intracellular and intercellular signaling systems. Different signaling molecules are involved in complex signaling networks to regulate biofilm development in bacteria (Maddela et al. 2018; Mahto et al. 2022).
How do metals affect biofilm formation and resistance, and how can biofilms help cells resist toxic metals? The organic matrix acts as a barrier that isolates the cells from many environmental stresses, the cell metabolism changes, and a slow-growing or non-growing subpopulation of cells (persisters) appears. In multi-species biofilms, metabolic interactions develop that allow cells to have a greater capacity for survival than in single-species biofilms. The biofilm’s high cell density can promote horizontal gene transfer processes, making new features more easily acquired. These key mechanisms enable microorganisms to survive and colonize toxic environments and likely accelerate ongoing evolutionary processes (Koechler et al. 2015).
Bioremediation involving the use of biofilms is attractive because it is adaptable. Due to its high biomass, it is excellent at absorbing, immobilizing, and remediating pollutants such as heavy metals.
In the current mini-review, we summarize recent discoveries on the bioremediation capability of biofilm-forming microbial species isolated from varied wastewaters for heavy metal removal, covering some mechanisms of action, application possibilities, practical challenges, and future prospects.
Biofilm-based wastewater treatment systems
Today, biofilm-based wastewater treatment systems provide numerous advantages: improved pollutant removal efficiency, minimal sludge formation, shorter hydraulic retention time, the presence of extracellular polymetric substances (EPS), high concentrations of active biomass, and high diversity (Zhao et al. 2019). In fact, these technologies have been widely used for the removal of both organic and inorganic compounds from aqueous media using microorganisms. Microorganisms can be employed in different forms within wastewater treatment systems, as living or dead, suspended or biofilm-immobilized biomass. Saini et al. (2023) listed the types of bioreactors based on biofilms that are currently most commonly used in various wastewater treatment systems (Fig. 1, a). Biofilm reactors primarily consist of five basic parts with certain additional components that are specific to a particular type of reactor (Fig. 1, b), (Asri et al. 2019).
Moving bed biofilm reactors (MBBR) are a biofilm-based wastewater treatment method that is currently widely used. MBBR plants are used for the treatment of municipal and industrial wastewater. This technique combines activated sludge and biofiltration processes. In the reactor, aerobic, anoxic and anaerobic processes can take place. Biofilm carriers are free and move within the reservoir. The most commonly used biofilm carrier in MBBR is in the form of a high-density polyethylene cylinder with large effective surfaces (Saini et al. 2023). A well-designed carrier allows for a stable biofilm, so the gap cannot be easily blocked by wastewater particles or large accumulation of biofilm. Efficient mixing/aeration with good carrier design gives good system performance and low maintenance requirements (Wang et al. 2019).
There are also some modified systems based on moving bed biofilm reactors (MBBR) in use today. Combined Fixed Film Activated Sludge (CFAS®/IFAS) utilizes an existing activated sludge process along with MBBR carriers by introducing plastic carriers into the activated sludge process (Sander et al. 2017). The continuous flow intermittent reactor (CFIC®) is a compact and energy-efficient process (20% smaller footprint, 50% lower energy consumption, 80% less waste sludge than MBBR) in normal mode (Ghimire and Wang 2018). The Hybrid Vertical Anaerobic Biofilm Reactor (HiVAB®) integrates both anaerobic and aerobic processes. It has low sludge production, high COD removal, and high methane biogas generation (Wang et al. 2017).
Membrane biofilm reactors (MBR) are a sustainable biotechnology for the removal and/or recovery of pollutants from water. It is effectively used for the purification of urban and industrial wastewater. To support the biofilm, they can be used, e.g., activated carbon in granules (GAC), sponge, plastic media, etc. Biofilm growth is encouraged by adding media for mobile/fixed arrangements, or aerated membranes can be added to the bioreactor. The disadvantage of membranes is frequent clogging, so plant maintenance limits their wide application (Ivanovic and Leiknes 2012).
Fluidized-bed biofilm reactors (FBBRs) use small carriers and build a bed within the column that is maintained by the movement of wastewater and the bed increases as a result. Aeration is done when the wastewater entering the water is combined with the effluent collected from the top of the bed during recycling. Biofilm carriers are based on silica and zeolite (Saini et al. 2023).
Microbial fuel cells (MFCs) reduce energy consumption, excess sludge production, and enable energy recovery. By this method, microorganisms develop a biofilm on the surface of electrodes and generate electrons and protons through the process of oxidation of organic materials (Saini et al. 2023). Electron transport takes place through the external circuit, while proton diffusion towards the cathode takes place through the solution and microorganisms assimilate metal ions into their biomass (Lim et al. 2021). MFC-based electricity generation together with Cr(VI) removal represents a potential future source of sustainable energy (Uddin et al. 2021).
Different types of biological trickling filters (BTFs) are used for wastewater treatment. The removal of suspended solids from TF-treated wastewater requires further liquid-solid separation, since TF treatment produces suspended solids (Saba et al. 2017). The most commonly used filter materials in this system are gravel and stones. Other materials, such as plastic rings, zeolite, sponge, and others, have been used to get around the limitations. Fine particles improve oxygen transport and manage biofilm thickness (Zhang et al. 2016).
Carriers in wastewater treatment systems
Biofilm carriers play an important role in wastewater treatment processes. It has been shown that the maximum efficiency of the removal of certain pollutants depends on the choice of carrier (Sonwani et al. 2019). A well-designed carrier allows for a stable biofilm so that the gap cannot be easily blocked by wastewater particles or excessive biofilm accumulation. Efficient mixing/aeration with good carrier design leads to good system performance and low maintenance requirements (Wang et al. 2019).
Carrier shape, density, protected areas, and carrier void volume are important factors affecting the performance of the MBBR process. Carriers with protected surfaces of 500–1000 m2/m3 are usually applied in wastewater treatment plants (Wang et al. 2019). Jute fibers immobilized with bacteria in fixed film reactors showed maximum immobilization potential (Zimba et al. 2021). In fixed biofilm anaerobic systems, hydrophobic polymeric materials promote initial cell adhesion and biofilm formation. But under longer-term and stable operation, hydrophilic materials show larger amounts of mature biofilm and better wastewater treatment performance (Zhou et al. 2021). Granular anaerobic sludge allows the biofilm to form on its own without the presence of a bio-carrier. Here, in understanding performance, process modeling is very important (Yang 2019).
In addition to the traditional ones, new carriers stand out more and more often due to their characteristics, application performance and mechanisms. Among them, hydrophilic/electrophilic modified carriers to encourage biofilm formation, carriers based on magnetic materials to shorten bioreactor start-up time, and redox mediator carriers to accelerate the biotransformation of some pollutants, such as heavy metals, are particularly important (Zhao et al. 2019).
Some mechanisms of the interaction of microorganisms and heavy metals in biofilms
The interaction of microbes in biofilm-form with heavy metals occurs through different mechanisms. Microorganisms, mostly bacteria, but also fungi and microalgae, have different adaptation mechanisms and the ability to sequester metals, but the most common are biosorption and bioaccumulation (Voica et al. 2016). These interactions are facilitated by the negative charge of extracellular polymeric substances on the biofilm surface, the positive charge of metal ions, high cell density, and high concentrations of cell signaling molecules within the biofilm matrix. The influence of anodic and cathodic redox potentials on the reduction, removal, and recovery of various types of heavy metals provides interesting insight into bacterial biofilm-mediated bioelectroremediation processes (Syed et al. 2022).
Understanding the interaction mechanisms between biofilms and heavy metals contributes to the development of effective biofilm-based heavy metal pollution remediation technologies. By entering the microbial cell, heavy metals can cause intoxication, therefore biosorption is a better method for removing heavy metals than bioaccumulation. (Hansda et al. 2016). Copper was found in the extracellular fraction of Pseudomonas putida CZ1 biofilm at a concentration of 60–67%, with 44.7–42.3% in capsular EPS and 15.5–20.1% and 17.2–21.2% in the cell walls and membranes, respectively (Lin et al. 2020). Pseudomonas aeruginosa RV9 is resistant to high concentrations of Cr(VI) and has a strong bioreduction potential. This strain develops a sophisticated adaptation mechanism based on surface connections, intracellular accumulation, and extracellular sequestration (presence of rhamnolipids), allowing it to achieve an 85% removal capacity (Mat Arisah et al. 2021). Copper is predominantly bound by carboxyl, phosphate, and hydrosulfide ligands within the extracellular polymer matrix, cell walls, and membranes, i.e., the intracellular fraction (Lin et al. 2020). Biosorption of Pb(II) by Arthrobacter viscosus is based on a chemical reaction, and that sorption takes place at the functional groups on the biomass surface (Hlihor et al. 2017).
A number of halotolerant and moderately halophilic bacteria possess tolerance to heavy metals. Microorganisms that live in salty habitats have developed an antioxidant defense mechanism that allows them to survive. The response of microorganisms to salinity is focused on the regulation of osmotic potential and the transport of cations and anions that regulate homeostasis. Increased salt concentration triggers an enhanced defense mechanism against other limiting factors, such as heavy metals, through a co-resistance mechanism. The main mechanisms include extracellular sequestration of metals by biopolymers, efflux of metals mediated by specific transporters, and enzymatic detoxification (Voica et al. 2016). In bacterial biofilms, enzymatic induction of the remediation process takes place through electrostatic interaction or metal chelation (Jasu and Ray 2021). Fungi biosorb and accumulate metals, either by complexation or ion exchange in their fruiting bodies or various polymeric substances such as EPS (Geetha et al. 2021). Removal efficiency is determined by fecundity, age of the mycelium, and duration of metal exposure (Jasu and Ray 2021).
Whether it is diverse and/or specific biofilm-forming species, some properties will always influence the degree of their biofilm formation and heavy metal removal capacity, e.g., pH, temperature, and heavy metal concentration (Bhattacharya et al. 2019; Maurya et al. 2021). Abiotic factors such as low molecular weight organic acids, temperature, pH and humic acids can alter the transport, transformation, and valence state of heavy metals, thus changing the bioavailability of heavy metals to microorganisms (Jasu and Ray 2021). The intermittent aeration regime has a significant effect on the secretion of EPS and the microbial community of MBBR. In this mode, microbes tend to secrete polysaccharides primarily after attachment. The high protein/polysaccharide ratio and certain genera are the main reasons for the highest concentration of biofilm biomass in the 3 h/3 h regime (Gu et al. 2018). The presence of titanium dioxide nanoparticles (TiO2 NPs) increases the surface binding energy between Cd2+ and functional groups of the biofilm (Wang et al. 2022). In monometallic systems, the biosorption capacity is two to three times higher than in the presence of bimetallic and multimetallic solutions (Díaz et al. 2022).
Extracellular polymeric substances (EPS)
Biofilms acquire resistance to heavy metal stress most commonly by forming an EPS barrier that can absorb, trap, or immobilize metals surrounding their cell population (Teitzel and Parsek 2003). Glycoproteins, polysaccharides, humic and uronic acids, proteins, nucleic acids, lipids, organic and inorganic compounds, and metal ions such as Mn, Mg, Fe, and K, are common EPS secreted by bacteria (Sardar et al. 2018). The composition and configuration of EPS are influenced by environmental factors (Henagamage et al. 2022) and extreme temperatures, acidic or alkaline conditions, and other physiological challenges simultaneously increase the efficiency of metal removal (Khosravi et al. 2020).
The adsorption capacity of EPS depends on the properties of heavy metals as well as on the components of EPS. Different components of EPS contribute differently to the adsorption of heavy metals. There are significant electrostatic binding interactions of heavy metals to proteins/humic acid (except for Cu2+). Carbonyl C = O and aromatic C = C stretching within humic acid contribute to the chemisorption of metal ions, and amide I and II groups are necessary for chemisorption in proteins (Wei et al. 2019). Metal binding is enabled by the ionizable functional groups of EPS, and the anionic sites are metal binding sites (Wei et al. 2017).
Exopolysaccharides are well-studied high molecular weight by-products of microorganisms and are well known for their effectiveness in the bioremediation of water with heavy metals. Exopolysaccharides support growth and provide self-defense in case of negative external influences such as pH, temperature, and starvation conditions. Circumstances that lead to the progress of its production also increase the capacity of bioremediation in heavy metal contamination (Kranthi et al. 2018). Exopolysaccharides facilitate the biosorption mechanism. Electrostatic attraction at specific sites allows the anionic composition of exopolysaccharides to help sequester positively charged heavy metal ions (Gupta and Divan 2017). Fungi, bacteria, and archaea play important roles in this process. Polysaccharides have the ability to chelate heavy metals from contaminated sites, forming organo-metallic complexes (Dey and Paul 2018). Bioremediation based on EPS for the removal of heavy metals has recently had several successful case studies (Maddela et al. 2021).
It has been proven that even without the presence of biofilm, EPS in sludge samples plays a major role in the removal of heavy metals during wastewater treatment. Extracellular polymeric substances produced by bacterial isolates with a high level of resistance to heavy metals play an important role in metal sorption and represent a passive method in which metal cations bind to the negative charge of acid groups from exopolysaccharides. The protein/polysaccharide ratio in EPS can be used to evaluate EPS capacity and heavy metal adsorption capacity (Wei et al. 2017). The excellent biosorption performance of EPS is closely related to the large concentrations of carboxyl and hydroxyl groups located on protein surfaces. But the extracted EPS, both hydrophobic and hydrophilic, contains amphiphilic material, which can also capture metal ions via electrostatic attraction and ion exchange (Liu et al. 2015). Isolates from industrial wastewater, Exiguobacterium profundum PT2 and Ochrobactrum ciceri SW1, show increased EPS production, especially protein and carbohydrate content, in the presence of arsenic. Increased production of bacterial EPS with a large number of polyanionic functional groups on its surface tends to sequester arsenic through electrostatic or covalent interactions (Saba et al. 2019).
Examination of polluted aquatic ecosystems showed that functional groups on the surface of calcite, together with periphyton biofilm, were responsible for the removal and binding of As(III) at the contaminated site (Zhu et al. 2018).
Biofilms of indigenous microorganisms in recent research on heavy metal removal
The use of biofilms has shown synergistic effects with multiple increases in heavy metal removal as a sustainable environmental technology in the near future (Igiri et al. 2018). Many bacterial biofilms have been used for the elimination of heavy metals. The largest number of microorganisms that were most successful in this were isolates from various wastewaters. An overview of recent research on the ability to remove heavy metal isolates from contaminated environments in the form of biofilms on different substrates is given in Table 1.
Single-species biofilms
In recent years, a large number of different microorganisms isolated from polluted environments have been investigated for their ability to form biofilms, tolerate the presence and efficiently remove various heavy metals. In the presence of various heavy metals, isolates from polluted environments in the form of biofilms showed high tolerances. In the presence of Cu(II) and Zn(II), E. coli and R. mucilaginosa (Grujić et al. 2017b), Enterobacter asburiae ENSD102, Vitreoscilla sp. ENSG301, Acinetobacter lvoffii ENSG302 (Mosharaf et al. 2018), Acinetobacter sp. IrC1, Acinetobacter sp. IrC2, Cupriavidus sp. IrC4 (Irawaiti et al. 2018) and Halobacterium salinarum R1 (Völkel et al. 2020). In the presence of Cr(VI), Methylobacterium organophylum (Bharagava and Mishra 2018), E. coli and Staphylococcus epidermidis (Quiton et al. 2018), Ochrobactrum anthropi (Tandon et al. 2020) show marked tolerance. In the presence of mercury and lead, tolerance is shown by Bacillus pumilus (Pepi et al. 2016), Pseudomonas sp. (Meliani and Bensoltane 2016), R. mucilaginosa and E. coli (Buzejić et al. 2016; Grujić et al. 2017a), Mucor hiemalis (Hoque and Fritscher 2016), Pseudochrobactrum saccharoliticum LY10 (Long et al. 2015), Rhizobium-MAP7 (Alfadaly et al. 2021), in the presence of Cd(II) and Ni(II) E. asburiae (Bhagat et al. 2016) R. mucilaginosa (Grujić et al. 2018) E. faecium (Maurya et al. 2021) E. cloacae MC9 (Syed et al. 2021). Pseudomonas aeruginosa (AK2) (Tariq et al. 2019), and Leptospirillum ferrooxidans (Liu et al. 2019) tolerate the presence of arsenic and thallium.
The biofilm always proves to be more tolerant to the presence of heavy metals than planktonic cells (Grujić et al. 2017a, 2018; Völkel et al. 2018). The biofilm of E. cloacae, K. oxytoca, S. odorifera and S. cerevisiae was more than 10 times resistant to various heavy metals compared to its planktonic form (Jakovljević et al. 2022a; Radojević et al. 2023). The R. mucilaginosa biofilm is tolerant to all tested concentrations of Cd2+, Zn2+, and Ni2+ metals and removes over 90% of them after 48 h, which the planktonic form is not able to do (Grujić et al. 2018). R. mucilaginosa also shows a marked tolerance to the presence of Hg2+, Cu2+, and Pb2+, whereby the metal removal efficiency is 4.79–10.25% for planktonic cells and 91.71–95.39% for biofilm (Grujić et al. 2017a). The biofilm of R. mucilaginosa is the most resistant, even in the presence of solvents (Radojevic et al. 2019).
Bacteria isolated from a mercury-contaminated environment were resistant to mercury and able to form a biofilm at a dose of 25/50 ppm mercury. Biofilms of Bacillus toionensis (JGT-F1), Burkholderia cepacia (PJT-K), and Microbacterium chocolatum (PJT-D) had the highest mercury adsorption values (Nurfitriani et al. 2022). Biofilms of E. cloacae, K. oxytoca, S. odorifera and S. cerevisiae had MBEC values > 100,000 mg/mL. E. cloacae showed the highest mercury removal efficiency (97.81% in 10 days) (Radojević et al. 2023).
Some concentrations of heavy metals activate the formation of biofilms. Isolates from wastewater E. asburiae ENSD102, Vitreoscilla sp. ENSG301, and Acinetobacter lvoffii ENSG302 form biofilms when exposed to different concentrations of heavy metals (0-2000 mg/L copper, zinc, lead, nickel and dichromate). Biofilm formation is dependent on a particular metal, the concentration of the metal, and bacterial strain (Mosharaf et al. 2018). Mean value of biofilm biomass of E. asburiae ENSD102, E. ludvigii ENSH201, Vitreoscilla sp. ENSG301, A. lvoffii ENSG302, and Bacillus thuringiensis ENSV401 was higher in different concentrations of copper and nikl compared to different concentrations of lead (Haque et al. 2021). E. faecium, isolated from wastewater, requires a certain content of heavy metals such as cadmium, chrome (VI), and nikl (0.25–0.5 mM) in order to form a strong biofilm (Maurya et al. 2021). Thiomonas sp. in the presence of arsenic, secretes biomolecules to form surface biofilms (Gupta and Divan 2017). Different metals show different effects on biofilm H. salinarum R1 and induce a metal-specific protective response of cells in biofilms (Völkel et al. 2018).
Bacillus pumilus in the presence of Pb(II) forms a brown, compact biofilm with lead, suggesting its active uptake (Pepi et al. 2016). Strain EM2 forms a pellicle-like biofilm (floating biofilm) that is resistant to Pb(II) up to 800 mg/L. Metal uptake is characterized by the occurrence of multilayer adsorption of Pb(II) (Jeong et al. 2019). A. viscosus biofilm removes 96% of 100 mg/L Pb(II) (Hlihor et al. 2017), while individual K. oxytoca biofilms remove Ni2+ (98.47%) and E. cloacae Zn2+ (98.06%) (Jakovljević et al. 2022a).
Different isolates of Pseudomonas sp. (P. aeruginosa and P. fluorescens) produce a greasy-looking biofilm that varies in thickness depending on the presence of zinc and lead (Meliani and Bensoltane 2016). E. cloacae MC9 forms a biofilm in the concentration range of 25–200 mg/mL chromium, cadmium, nickel, and lead. Cadmium has the greatest harmful effect on the bacteria’s physiology and ability to form biofilms. With an increase in the concentration of heavy metals, the activities of bacteria decrease, e.g., 200 mg/mL chromium significantly reduced EPS synthesis by E. cloacae MC9 (Syed et al. 2021).
Indigenous microorganisms that form a biofilm can significantly improve the process of color removal (Radojevic et al. 2019). Some of them, for example, the biofilm of the fungus Cunninghamella elegans together with the degradation of different colors, can effectively and repeatedly remove Cr(VI) from liquid cultures even in the presence of high concentrations (40 g/L) of NaCl and other metal ions (Hussain et al. 2017).
Multi-species biofilms
There is a significant difference in tolerance and heavy metal removal potential between single-species biofilms and consortia (Herath et al. 2014; Jakovljević et al. 2022a; Radojević et al. 2023).
The great success of the use of indigenous wastewater microorganisms in the form of a consortium of biofilms, which have a great ability to remove zinc, lead, cadmium, copper, nickel, and mercury, has been demonstrated. A significant difference in tolerance and heavy metal removal potential was observed between single-species biofilms and consortia (Jakovljević et al. 2022a; Radojević et al. 2023). The minimum inhibitory concentration for lead for the biofilm consortium of E. coli LM1 and R. mucilaginosa was 4 times higher than individual biofilms (Buzejić et al. 2016). Biofilm consortia composed of three species showed the best ability to remove mercury (Radojević et al. 2023). Compared to its monocultures, the bacterial-fungal biofilm removed significantly more Cr(VI), approximately 90% in 10 days (Herath et al. 2014).
The presence of TiO2 NPs significantly increased the adsorption capacity of Cd2+ in natural periphytic biofilms, but this effect was significantly less pronounced at high concentrations of TiO2 NPs. The optimal pH for Cd2+ adsorption increased with increasing Cd2+ and TiO2 NP content (Wang et al. 2022). A biofilm of a consortium of microorganisms isolated from wastewater removes significant lead, cadmium, and copper, and the success depends primarily on the metal concentration and exposure time (Ogbuagu et al. 2018).
The formation of biofilms of R. mucilaginosa and P. expansum in media with different auto stains is positively related to the secretion of hydrolytic enzymes such as alkaline phosphatase activity (ALP), which indicates their role in the hydrolysis of these pollutants (Jakovljević et al. 2022b). In this case too, the mixed biofilm (consortium) proved to be the most productive and, in most cases, the most resistant (Jakovljević et al. 2022b). A mixed-species biofilm showed better removal efficiency for all tested metals than a single-species biofilm. Metal removal efficiency was in the range of 81.56-97.85% for single species biofilm and 94.99-99.88% for mixed species biofilm (Grujic et al. 2017b).
A reactor system with a modified biofilm layer (presence of a large surface carrier and a high microbial population up to 10,000 mg/L in the treatment of industrial wastewater can effectively treat 20 mg/L concentrations of combined heavy metals at an optimal hydraulic retention time (2 h), while above this strength there should be a substantial negative impact on the effectiveness of the treatment (Azizi et al. 2016).
Conclusion
New research shows that biofilm-mediated bioremediation is widely represented both in research and in practice. Research shows full momentum in terms of effectiveness, conditions of application, and cost-effectiveness of the application of autochthonous isolates in the form of biofilms. Because of their simpler use and economic profitability, extracellular polymeric substances of biofilms are widely researched as biosorbents of heavy metals. The focus of research is still largely focused on single-species biofilms of isolates, but research is increasingly directed towards proven more effective consortia of the most effective combinations or natural biofilms. Some of the heavy metal removal techniques by biofilm of microorganisms are not functional while others have limitations in terms of efficiency. Some may work well in laboratory and pilot scale but are not yet suitable for practical application. Furthermore, for the practical application of biofilms safety requirements for human health and the environment must be met. Complicated procedures are often required, so the costs are of consideration. Regardless of the limitations, the potential of single-species and biofilm consortia is increasingly highlighted, so it is necessary to work further to overcome the problems mentioned.
Data Availability
This mini-review contains data from cited manuscripts (list of references).
References
Adams GO, Tawari-Fufeyin P, Okoro SE, Ehinomen I (2015) Bioremediation, Biostimulation and Bioaugmention: a review. Int J Environ Bioremediat Biodegrad 3:28–39. https://doi.org/10.12691/ijebb-3-1-5
Alfadaly RA, Elsayed A, Hassan RYA, Noureldeen A, Darwish H, Gebreil AS (2021) Microbial sensing and removal of heavy metals: bioelectrochemical detection and removal of chromium(VI) and cadmium(II. Molecules 26(9):2549. https://doi.org/10.3390/molecules26092549
Asri M, Elabed S, Koraichi SI, ElGhachtouli N (2019) Biofilm-Based Systems for Industrial Wastewater Treatment. In: Hussain C (ed) Handbook of environmental materials management. Springer, Cham, pp 1767–1787
Azizi S, Kamika I, Tekere M (2016) Evaluation of heavy metal removal from Wastewater in a modified packed Bed Biofilm Reactor. PLoS ONE 11(5):e0155462. https://doi.org/10.1371/journal.pone.0155462
Bhagat N, Vermani M, Bajwa HS (2016) Characterization of heavy metal (cadmium and nickle) tolerant gram negative enteric bacteria from polluted Yamuna River, Delhi. Afr J Microbiol Res 10:127–137. https://doi.org/10.5897/AJMR2015.7769
Bharagava RN, Mishra S (2018) Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol Environ Saf 147:102–109. https://doi.org/10.1016/j.ecoenv.2017.08.040
Bhattacharya A, Gupta A, Kaur A, Malik D (2019) Alleviation of hexavalent chromium by using microorganisms: insight into the strategies and complications. Water Sci Technol 79(3):411–424. https://doi.org/10.2166.wst.2019.060
Buzejić A, Grujić S, Radojević I, Ostojić A, Čomić L, Vasić S (2016) Pb and hg metal tolerance of single and mixed-species biofilm (Rhodotorula mucilaginosa and Escherichia coli). Kragujev J Sci 38:115–124. https://doi.org/10.5937/KgJSci1638115B
Cesare AD, Eckert EM, D’Urso S, Bertoni R, Gillan DC, Wattiez R, Corno G (2016) Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants. Water Res 94:208–214. https://doi.org/10.1016/j.watres.2016.02.049
Dey S, Paul AK (2018) Influence of metal ions on biofilm formation by Arthrobacter sp. SUK 1205 and evaluation of their cr(VI) removal efficacy. Int Biodeter Biodegr 132:122–131. https://doi.org/10.1016/j.ibiod.2018.02.015
Díaz A, Marrero J, Cabrera G, Coto O, Gómez JM (2022) Biosorption of nickel, cobalt, zinc and copper ions by Serratia marcescens strain 16 in mono and multimetallic systems. Biodegradation 33:33–43. https://doi.org/10.1007/s10532-021-09964-9
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575. https://doi.org/10.1038/nrmicro.2016.94
Focardi S, Pepi M, Landi G, Gasperini S, Ruta M, Di Biasio P, Focardi SE (2012) TA-04. Hexavalent chromium reduction by whole cells and cell free extract of the moderate halophilic bacterial strain Halomonas sp. Int Biodeterior Biodegrad 66:63–70. https://doi.org/10.1016/j.ibiod.2011.11.003
Geetha N, Bhavya G, Abhijith P, Shekhar R, Dayananda K, Jogaiah S (2021) Insights into Nanomycoremediation: Secretomics and Mycogenic Biopolymer Nanocomposites for Heavy Metal Detoxification. J Hazard Mater 409:124541. https://doi.org/10.1016/j.jhazmat.2020.124541
Ghimire N, Wang S (2018) Biological treatment of petrochemical wastewater. In: Zoveidavianpoor M (ed) Petroleum Chemicals—Recent Insight, IntechOpen https://doi.org/10.5772/intechopen.79655
Grujić S, Vasić S, Radojević I, Čomić L, Ostojić A (2017a) Comparison of the Rhodotorula mucilaginosa Biofilm and Planktonic Culture on Heavy Metal susceptibility and removal potential. Water Air Soil Pollut 228:73. https://doi.org/10.1007/s11270-017-3259-y
Grujić S, Vasić S, Čomić L, Ostojić A, Radojević I (2017b) Heavy metal tolerance and removal potential in mixed-species biofilm. Water Sci Technol 76(4):806–812. https://doi.org/10.2166/wst.2017.248
Grujić MS, Radojević DI, Vasić MS, Čomić RL, Ostojić MA (2018) Heavy metal tolerance and removal efficiency of the Rhodotorula mucilaginosa and Saccharomyces boulardii planktonic cells and biofilm. Kragujev J Sci 40:217–226. https://doi.org/10.5937/KgJSci1840217G
Gu Y-Q, Li T-T, Li H-Q (2018) Biofilm formation monitored by confocal laser scanning microscopy during startup of MBBR operated under different intermittent aeration modes. Process Biochem 74:132–140. https://doi.org/10.1016/j.procbio.2018.08.032
Gupta P, Diwan B (2017) Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep 13:58–71. https://doi.org/10.1016/j.btre.2016.12.006
Hansda A, Kumar V, Anshumali (2016) A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J Microbiol Biotechnol 32:170. https://doi.org/10.1007/s11274-016-2117-1
Haque MM, Mosharaf MK, Haque MA, Tanvir MZH, Alam MK (2021) Biofilm formation, production of matrix compounds and biosorption of copper, nickel and lead by different bacterial strains. Front Microbiol 12:615113. https://doi.org/10.3389/fmicb.2021.615113
Henagamage AP, Peries CM, Seneviratne G (2022) Fungal-bacterial biofilm mediated Heavy Metal Rhizo-Remediation. World J Microbiol Biotechnol 38(5):85. https://doi.org/10.1007/s11274-022-03267-8
Herath HMLI, Rajapaksha AU, Vithanage M, Seneviratne G (2014) Developed fungal–bacterial biofilms as a novel tool for bioremoval of hexavelant chromium from wastewater. Chem Ecol 30(5):418–427. https://doi.org/10.1080/02757540.2013.861828
Hlihor RM, Rosca M, Tavares T, Gavrilescu M (2017) The role of Arthrobacter viscosus in the removal of pb(II) from aqueous solutions. Water Sci Technol 76:1726–1738. https://doi.org/10.2166/wst.2017.360
Hoque E, Fritscher J (2016) A new mercury accumulating Mucor hiemalis strain EH8 from cold sulfidic spring water biofilms. MicrobiologyOpen 5:763–781. https://doi.org/10.1002/mbo3.368
Hussain S, Quinn L, Li J, Casey E, Murphy CD (2017) Simultaneous removal of malachite green and hexavalent chromium by Cunninghamella elegans biofilm in a semi-continuous system. Int Biodeterior Biodegradation 125:142–149. https://doi.org/10.1016/j.ibiod.2017.09.003
Igiri BE, Okoduwa SIR, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK (2018) Toxicity and bioremediation of heavy Metals contaminated ecosystem from Tannery Wastewater: a review. J Toxicol 2568038. https://doi.org/10.1155/2018/2568038
Irawaiti W, Yuwono T, Ompusunggu NP (2018) Growth characteristics and copper accumulation of bacterial consortium Acinetobacter sp. and Cupriavidus sp. isolated from a wastewater treatment plant. Biodivers J 19(5):1884–1890. https://doi.org/10.13057/biodiv/d190540
Ivanovic I, Leiknes T (2012) The biofilm membrane bioreactor (BF-MBR)-a review. Desalin Water Treat 37:288–295. https://doi.org/10.1080/19443994.2012.661283
Jakovljević V (2016) Metabolic activity of Aspergillus niger and Fusarium lateritium induced by ethoxylated oleyl cetyl alcohol and their bioremediation and biotechnological potential. Appl Biochem Microbiol 52(4):406–412. https://doi.org/10.1134/S0003683816040074
Jakovljević VD (2020) Synergistic effect of Fusarium lateritium LP7 and Trichoderma viride LP5 promotes ethoxylated oleyl-cetyl alcohol biodegradation. J Environ Sci Health A 55(4):438–447. https://doi.org/10.1080/10934529.2019.1706334
Jakovljević V, Vrvić M (2016) The effect of ethoxylated oleyl-cetyl alcohol on metabolism of some fungi and their potential application in mycoremediation. Hem ind 70(3):277–286. https://doi.org/10.2298/HEMIND141229034J
Jakovljević VD, Vrvić MM (2018) Potential of pure and mixed cultures of Cladosporium cladosporioides and Geotrichum candidum for application in bioremediation and detergent industry. Saudi J Biol Sci 25(3):529–536. https://doi.org/10.1016/j.sjbs.2016.01.020
Jakovljević V, Grujić S, Simić Z, Ostojić A, Radojević I (2022a) Finding the best combination of autochthonous microorganisms with the most effective biosorption ability for heavy metals removal from wastewater. Front Microbiol 13:1017372. https://doi.org/10.3389/fmicb.2022.1017372
Jakovljević V, Radojević I, Grujić S, Ostojić A (2022b) Response of selected microbial strains and their consortia to the presence of automobile paints: Biofilm growth, matrix protein content and hydrolytic enzyme activity. Saudi J Biol Sci 29:103347. https://doi.org/10.1016/j.sjbs.2022.103347
Jasu A, Ray RR (2021) Biofilm mediated strategies to mitigate heavy metal pollution: a critical review in metal bioremediation. Biocatal Agric Biotechnol 37:102183. https://doi.org/10.1016/j.bcab.2021.102183
Jeong SW, Kim HK, Yang JE, Choi YJ (2019) Removal of pb(II) by Pellicle-Like Biofilm-Producing Methylobacterium hispanicum EM2 strain from aqueous media. https://doi.org/10.3390/w11102081. Water 11:2081
Khosravi A, Javdan M, Yazdanpanah G, Malakootian M (2020) Removal of Heavy Metals by Escherichia Coli (E. Coli) Biofilm placed on Zeolite from Aqueous Solutions (Case Study: the Wastewater of Kerman Bahonar Copper Complex). Appl Water Sci 10:167. https://doi.org/10.1007/s13201-020-01257-5
Koechler S, Farasin J, Cleiss-Arnold J, Arsène-Ploetze F (2015) Toxic metal resistance in biofilms: diversity of microbial responses and their evolution. Res Microbiol 166(10):764–773. https://doi.org/10.1016/j.resmic.2015.03.008
Kranthi KR, Sardar UR, Bhargavi E, Devi I, Bhunia B, Tiwari ON (2018) Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: a critical review. Carbohydr Polym 199:353–364. https://doi.org/10.1016/j.carbpol.2018.07.037
Lim SS, Fontmorin JM, Pham HT, Milner E, Abdul PM, Scott K, Head I, Yu EH (2021) Zinc removal and recovery from Industrial Wastewater with a Microbial fuel cell: experimental investigation and theoretical prediction. Sci Total Environ 776:145934. https://doi.org/10.1016/j.scitotenv.2021.145934
Lin H, Wang C, Zhao H, Chen G, Chen X (2020) A subcellular level study of copper speciation reveals the synergistic mechanism of microbial cells and EPS involved in copper binding in bacterial biofilms. Environ Pollut 263(Pt A):114485. https://doi.org/10.1016/j.envpol.2020.114485
Liu W, Zhang J, Jin Y, Zhao X, Cai Z (2015) Adsorption of pb(II), cd(II) and zn(II) by extracellular polymeric substances extracted from aerobic granular sludge: efficiency of protein. J Environ Chem Eng 3(2):1223–1232. https://doi.org/10.1016/j.jece.2015.04.009
Liu J, Yin M, Zhang W, Tsang D, Wei X, Zhou Y, Xiao T, Wang J, Dong Y, Li H, Hou L (2019) Response of microbial communities and interactions to thallium in contaminated sediments near a pyrite mining area. Environ Pollut 248:916–928. https://doi.org/10.1016/j.envpol.2019.02.089
Long D, Zou L, Hashmi MZ, Cai K, Tang X, Chen G, Shi J (2015) Determination of the accumulation, spatial distribution and reduction of cr in unsaturated Pseudochrobactrum saccharolyticum LY10 biofilms by X-ray fluorescence and absorption methods. Chem Eng J 280:763–770. https://doi.org/10.1016/j.cej.2015.06.013
Ma H, Zhao Y, Yang K, Wang Y, Zhang C, Ji M (2022) Application oriented bioaugmentation processes: mechanism, performance improvement and scale-up. Bioresour Technol 344 (Part B) 126192. https://doi.org/10.1016/j.biortech.2021.126192
Maddela NR, Zhou Z, Yu Z, Zhao S, Meng F (2018) Functional determinants of extracellular polymeric substances in membrane Biofouling: experimental evidence from pure-cultured sludge Bacteria. Appl Environ Microbiol 84(15). https://doi.org/10.1128/AEM.00756-18
Maddela NR, Scalvenzi L, Radice M (2021) Novel insights of Microbial Exopolysaccharides as bio-adsorbents for the removal of Heavy Metals from Soil and Wastewater. In: Nadda AK, KVS Sharma S (eds) Microbial Exopolysaccharides as Novel and significant biomaterials. Springer Series on Polymer and Composite Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-75289-7_10
Mahto KU, Kumari S, Das S (2022) Unraveling the complex regulatory networks in biofilm formation in bacteria and relevance of biofilms in environmental remediation. Crit Rev Biochem Mol Biol 57(3):305–332. https://doi.org/10.1080/10409238.2021.2015747
Mat Arisah F, Amir AF, Ramli N, Ariffin H, Maeda T, Hassan MA, Mohd Yusoff MZ (2021) Bacterial resistance against Heavy Metals in Pseudomonas aeruginosa RW9 Involving Hexavalent Chromium removal. Sustainability 13:9797. https://doi.org/10.3390/su13179797
Maurya A, Kumar R, Singh A, Raj A (2021) Investigation on biofilm formation activity of Enterococcus faecium under various physiological conditions and possible application in bioremediation of tannery effluent. Bioresour Technol 339:125586. https://doi.org/10.1016/j.biortech.2021.125586
Meliani A, Bensoltane A (2016) Biofilm-mediated heavy Metals Bioremediation in PGPR Pseudomonas. J Bioremediat Biodegrad 7:370. https://doi.org/10.4172/2155-6199.1000370
Mishra S, Huang Y, Li J, Wu X, Zhou Z, Lei Q, Bhatt P, Chen S (2022) Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants. Chemosphere 294:133609. https://doi.org/10.1016/j.chemosphere.2022.133609
Mosharaf MK, Tanvir MZH, Haque MM, Haque MA, Khan MAA, Molla AH, Alam MZ, Islam MS, Talukder MR (2018) Metal-adapted Bacteria isolated from wastewaters produce Biofilms by expressing Proteinaceous Curli Fimbriae and Cellulose Nanofibers. Front Microbiol 9:1334. https://doi.org/10.3389/fmicb.2018.01334
Naeem A, Batool R, Jamil N (2013) Cr(VI) reduction by Cellulosimicrobium sp. isolated from tannery effluent. Turk J Biol 37:315–322. https://doi.org/10.3906/biy-1209-18
Nurfitriani S, Arisoesilaningsih E, Nuraini Y, Handayanto E (2022) Biofilm formation by mercury resistant bacteria from polluted soil small-scale gold mining waste. Biodiversitas 23:992–999. https://doi.org/10.13057/biodiv/d230242
Ogbuagu DH, Nwachukwu IN, Ejike OJ (2018) Application of biofilms in removal of heavy metals from wastewater in static condition. Biotechnol Acta 11(3):47–55. https://doi.org/10.15407/biotech11.03.047
Pani T, Das A, Osborne JW (2017) Bioremoval of zinc and manganese by bacterial biofilm: a bioreactor-based approach. J Photochem Photobiol B: Biol 175:211–218. https://doi.org/10.1016/j.jphotobiol.2017.08.039
Pepi M, Borra M, Tamburrino S, Saggiomo M, Viola A, Biffali E, Balestra C, Sprovieri M, Casotti R (2016) A Bacillus sp. isolated from sediments of the Sarno River mouth, Gulf of Naples (Italy) produces a biofilm biosorbing pb(II). Sci Total Environ 562:588–595. https://doi.org/10.1016/j.scitotenv.2016.04.097
Quiton KG, Doma JB, Futalan CM, Wan MW (2018) Removal of chromium (VI) and zinc(II) from aqueous solution using kaolin-supported bacterial biofilms of Gram-negative E. coli and Gram-positive Staphylococcus epidermidis. Sustainable Environ Res 28(5):206–213. https://doi.org/10.1016/j.serj.2018.04.002
Radojevic I, Grujic S, Rankovic B, Comic Lj, Ostojic A (2019) Single-species biofilms from autochthonous microorganisms: biotechnological potential in automotive wastewater treatment. Int J Environ Sci Technol 16(10):6189–6198. https://doi.org/10.1007/s13762-019-02265-y
Radojević I, Jakovljević V, Grujić S, Ostojić A, Ćirković K (2023) Biofilm formation by selected microbial strains isolated from wastewater and their consortia: mercury resistance and removal potential. Res Microbiol 104092. https://doi.org/10.1016/j.resmic.2023.104092
Rajeev M, Sushmitha TJ, Aravindraja C, Toleti SR, Pandian SK (2021) Exploring the impacts of heavy metals on spatial variations of sediment-associated bacterial communities. Ecotoxicol Environ Saf 209:111808. https://doi.org/10.1016/j.ecoenv.2020.111808
Saba B, Christy AD, Yu Z, Co AC, Islam R, Tuovinen OH (2017) Characterization and performance of anodic mixed culture biofilms in submersed microbial fuel cells. Bioelectrochemistry 113:79–84. https://doi.org/10.1016/j.bioelechem.2016.10.003
Saba YR, Mehboob A, Sabri AN (2019) Potential role of bacterial extracellular polymeric substances as biosorbent material for arsenic bioremediation. Bioremediat J 23:72–81. https://doi.org/10.1080/10889868.2019.1602107
Saini S, Tewari S, Dwivedi J, Sharma V (2023) Biofilm-mediated wastewater treatment: a comprehensive review. Mater Adv 4:1415–1443. https://doi.org/10.1039/D2MA00945E
Sander S, Behnisch J, Wagner M (2017) Energy, cost and design aspects of coarse-and fine-bubble aeration systems in the MBBR IFAS process. Water Sci Technol 75:890–897. https://doi.org/10.2166/wst.2016.571
Sardar UR, Bhargavi E, Devi I, Bhunia B, Tiwari ON (2018) Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: a critical review. Carbohydr Polym 199:353–364. https://doi.org/10.1016/j.carbpol.2018.07.037
Sedlakova-Kadukova J (2022) Microorganisms in metal recovery—Tools or teachers? In: Singh RP, Manchanda G, Bhattacharjee K, Panosyan H (eds) Developments in applied microbiology and biotechnology, microbial syntrophy-mediated eco-enterprising, Academic Press, pp 71–86
Shah MP, Rodrigez Couto S, Kumar V (2021) New Trends in removal of Heavy Metals from Industrial Wastewater. Elsevier Inc
Sonwani RK, Swain G, Giri BS, Singh RS, Rai BN (2019) A novel comparative study of modified carriers in moving bed biofilm reactor for the treatment of wastewater: process optimization and kinetic study. Bioresour Technol 281:335–342. https://doi.org/10.1016/j.biortech.2019.02.121
Syed A, Zeyad MT, Shahid M, Elgorban AM, Alkhulaifi MM, Ansari IA (2021) Heavy Metals Induced Modulations in Growth, Physiology, Cellular viability, and Biofilm formation of an identified bacterial isolate. ACS Omega 6(38):25076–25088. https://doi.org/10.1021/acsomega.1c04396
Syed Z, Sogani M, Rajvanshi J, Sonu K (2022) Microbial Biofilms for Environmental Bioremediation of Heavy Metals: a review. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-022-04276-x
Tandon S, Jha M, Dudhwadkar S (2020) Study on Ochrobactrum pseudintermedium ADV31 for the removal of hexavalent chromium through different immobilization techniques. SN Appl Sci 2:296. https://doi.org/10.1007/s42452-020-2103-y
Tariq A, Ubaid U, Asif M, Sadiq I (2019) Biosorption of arsenic through bacteria isolated from Pakistan. Int Microbiol 22(1):59–68. https://doi.org/10.1007/s10123-018-0028-8
Teitzel GM, Parsek MR (2003) Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl Environ Microbiol 69:2313–2320. https://doi.org/10.1128/AEM.69.4.2313-2320.2003
Uddin MJ, Jeong Y, Lee W (2021) Microbial fuel cell for bioelectricity generation through reduction of hexavalent chromium in wastewater: a review. Int J Hydrog Energy 46(20):11458–11481. https://doi.org/10.1016/j.ijhydene.2020.06.134
Voica DM, Bartha L, Banciu HL, Oren A (2016) Heavy metal resistance in halophilic Bacteria and Archaea. FEMS Microbiol Lett 363(14):fnw146. https://doi.org/10.1093/femsle/fnw146
Völkel S, Fröls S, Pfeifer F (2018) Heavy metal ion stress on Halobacterium salinarum R1 planktonic cells and biofilms. Front Microbiol 9:3157. https://doi.org/10.3389/fmicb.2018.03157
Völkel S, Hein S, Benker N, Pfeifer F, Lenz C, Losensky G (2020) How to cope with heavy metal ions: cellular and proteome-level stress response to divalent copper and nickel in Halobacterium salinarum R1 planktonic and biofilm cells. Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.03056
Wang S, Ghimire N, Xin G, Janka E, Bakke R (2017) Efficient high strength petrochemical wastewater treatment in a hybrid vertical anaerobic biofilm (HyVAB) reactor: a pilot study. Water Pract Technol 12:501–513. https://doi.org/10.2166/wpt.2017.051
Wang S, Parajuli S, Sivalingam V, Bakke R (2019) Biofilm in moving Bed Biofilm process for Wastewater Treatment. In: Dincer S Sümengen Özdenefe M Arkut A (eds) Bacterial Biofilms IntechOpen. https://doi.org/10.5772/intechopen.88520
Wang W, Zhu S, Li N, Xie S, Wen C, Luo X (2022) Enhanced Cd2+ adsorption and toxicity for microbial biofilms in the presence of TiO2 nanoparticles. Environl Pollut 314:120239. https://doi.org/10.1016/j.envpol.2022.120239
Wei L, Li Y, Noguera DR, Zhao N, Song Y, Ding J, Zhao Q, Cui F (2017) Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism. J Hazard Mater 321:473–483. https://doi.org/10.1016/j.jhazmat.2016.05.016
Wei L, Li J, Xue M, Wang S, Li Q, Qin K, Jiang J, Ding J, Zhao Q (2019) Adsorption behaviors of Cu2+, Zn2+ and Cd2+ onto proteins, humic acid, and polysaccharides extracted from sludge EPS: Sorption properties and mechanisms. Bioresour Technol 291:121868. https://doi.org/10.1016/j.biortech.2019.121868
Wen X, Du C, Zeng G, Huang D, Zhang J, Yin L, Tan S, Huang L, Chen H, Yu G, Hu X, Lai C, Xu P, Wan J (2018) A novel biosorbent prepared by immobilized Bacillus licheniformis for lead removal from wastewater. Chemosphere 200:173–179. https://doi.org/10.1016/j.chemosphere.2018.02.078
Yang J (2019) Approaches for modeling anaerobic granule-based reactors. In: Dincer S Sümengen Özdenefe M Arkut A (eds) Bacterial Biofilms IntechOpen. https://doi.org/10.5772/intechopen.90201
Younas H, Nazir A, Latif Z, Thies JE, Shafiq M, Firdaus-e-Bareen (2022) Biosorption potential and molecular characterization of metal resistant autochthonous microbes from tannery solid waste. Arch Microbiol 204(10):651. https://doi.org/10.1007/s00203-022-03238-5
Zhang X, Li J, Yu Y, Xu R, Wu Z (2016) Biofilm characteristics in natural ventilation trickling filters (NVTFs) for municipal wastewater treatment: comparison of three kinds of biofilm carriers. Biochem Eng J 106:87–96. https://doi.org/10.1016/j.bej.2015.11.009
Zhao Y, Liu D, Huang W, Yang Y, Ji M, Nghiem LD, Trinh QT, Tran NH (2019) Insights into biofilm carriers for biological wastewater treatment processes: current state-of-the-art, challenges, and opportunities. Bioresour Technol 288:121619. https://doi.org/10.1016/j.biortech.2019.121619
Zhou Y, Kiely PD, Kibbee R, Ormeci B (2021) Effect of polymeric support material on biofilm development, bacterial population, and wastewater treatment performance in anaerobic fixed-film systems. Chemosphere 264(Pt 1):128477. https://doi.org/10.1016/j.chemosphere.2020.128477
Zhu N, Zhang J, Tang J, Zhu Y, Wu Y (2018) Arsenic removal by periphytic biofilm and its application combined with biochar. Bioresour Technol 248:49–55. https://doi.org/10.1016/j.biortech.2017.07.026
Zimba S, Kumar TS, Mohan N, Rao PH (2021) Evaluation of various waste substrates for biofilm formation and subsequent use in aerobic packed-bed reactor for secondary treatment of domestic wastewater. World J Microbiol Biotechnol 37(2):25. https://doi.org/10.1007/s11274-020-02992-2
Acknowledgements
This work was supported by the Serbian Ministry of Science, Technological Development and Innovation (Agreement No. 451-03-47/2023-01/ 200122) and COST Action 18113 (STSM grant ECOSTSTSM-Request-CA18113-45768), EuroMicropH Understanding and exploiting the impacts of low pH on micro-organisms.
Author information
Authors and Affiliations
Contributions
Article idea: [Ivana Radojević]Literature search: [Ivana Radojević, Violeta Jakovljević, Aleksandar Ostojić]Data analysis: [Ivana Radojević, Violeta Jakovljević, Aleksandar Ostojić]Work done by: [Ivana Radojević]Critical revision of work: [Violeta Jakovljević, Aleksandar Ostojić]
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Radojević, I.D., Jakovljević, V.D. & Ostojić, A.M. A mini-review on indigenous microbial biofilm from various wastewater for heavy-metal removal - new trends. World J Microbiol Biotechnol 39, 309 (2023). https://doi.org/10.1007/s11274-023-03762-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03762-6