Abstract
Microorganisms act as both the source and sink of methane, a potent greenhouse gas, thus making a significant contribution to the environment as an important driver of climate change. The rhizosphere and phyllosphere of plants growing in natural (mangroves) and artificial wetlands (flooded agricultural ecosystems) harbor methane-utilizing bacteria that oxidize methane at the source and reduce its net flux. For several decades, microorganisms have been used as biofertilizers to promote plant growth. However, now their role in reducing net methane flux, especially from flooded agricultural ecosystems is gaining momentum globally. Research in this context has mainly focused on taxonomic aspects related to methanotrophy among diverse bacterial genera, and environmental factors that govern methane utilization in natural and artificial wetland ecosystems. In the last few decades, concerted efforts have been made to develop multifunctional microbial inoculants that can oxidize methane and alleviate greenhouse gas emissions, as well as promote plant growth. In this context, combinations of taxonomic groups commonly found in rice paddies and those used as biofertilizers are being explored. This review deals with methanotrophy among diverse bacterial domains, factors influencing methane-utilizing ability, and explores the potential of novel methane-utilizing microbial consortia with plant growth-promoting traits in flooded ecosystems.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microorganisms belonging to diverse taxa have evolved the ability to utilize single-carbon compounds (such as methane, methanol, and other methylated compounds) as the sole source of carbon for meeting their energy demands and are termed methylotrophs. However, some of the methylotrophs can utilize only methane as the sole C source for their energy metabolism and are referred to as methanotrophs. The phenomenon of methylotrophy was recognized after the isolation of the first methane-oxidizing bacteria, Bacillus methanicus, in the year 1906 (Sohngen 1906). However, research in the field of bacterial methane utilization gained impetus only after 1970, when Whittenbury and co-workers isolated over 100 strains of methane-utilizing bacteria and described their properties, isolation technique, and introduced the Type I, Type II, and Type X classification system (Whittenbury et al. 1970; Whittenbury and Dalton 1981). Methane-utilizing bacteria play an important role in the global carbon cycle, regulating natural and anthropogenic methane emissions. The biochemical conversion of methane to methanol is the first step in methane utilization by methane-utilizing bacteria. The catabolic process is well studied in traditionally known obligate (belonging to the genera Methylomonas, Methylosinus, and Methylococcus) and facultative methanotrophic bacteria (e.g., Methylobacterium, Methylocella, Paracoccus denitrificans). The first step of the reaction in these bacterial genera is either carried out by membrane-bound copper-containing particulate methane monooxygenase (pMMO) or a di-iron center containing soluble methane monooxygenase (sMMO) (Ross and Rosenzweig 2017). The pMMO is ubiquitous among all known obligate methanotrophs, whereas sMMO appears in some obligate and facultative methanotrophs (Nielsen et al. 1997). The sMMO can oxidize wide varieties of alkanes and has structural specificity with other alkane monooxygenases (Ji et al. 2013). This non-specific degradation chemical property of sMMO makes the facultative methanotrophic microorganisms an important candidate for their exploitation in agriculture, bioremediation, and commercial applications (Theisen et al. 2005). The enzyme pMMO exhibits broad substrate specificity and shares functional similarities and evolutionary linkages with ammonia monoxygenases (key enzymes in ammonia oxidizers) (Zheng et al. 2014). In an environment, where methane-utilizing bacteria are abundant, the assimilation of N by these microbes is likely to bring about significant effects on nitrification (Kowalchuk and Stephan 2001). A few pure culture studies have reported that ammonia-oxidizing bacteria also play a role in methane oxidation however, removal of methane in a significant amount from the agricultural fields is yet to be verified (Kowalchuk and Stephen 2001; Zheng et al. 2014). The role of ammonia-oxidizing bacteria in the association with nitrate oxidizing bacteria is more evident in wastewater and activated sludge treatment (Sepehri et al. 2019). For many years, the model microorganisms that shaped our concepts of methanotrophy belonged to the bacterial families- Methylococcaceae, Methylocystaceae, Beijerinckiaceae, Methylobacteriaceae, and Rhodobacteraceae of the phyla Proteobacteria and Verrucomicrobia (Dedysh 2009; Ghashghavi et al. 2017; Kravchenko and Sukhacheva 2017). However, in the last decade, with advances in microbial diversity using next-generation sequencing tools and intensive sampling across distinct ecological niches, various workers have reported the concept of methylotrophy in diverse bacterial and yeast genera such as Methylobacterium, Burkholderia, Hyphomicrobium, Paenibacillus, Rahnella, Meyerozyma and Pseudomonas (Van Aken 2004; Kumar et al. 2012; Jhala et al. 2014; Yang et al. 2019; Rani et al. 2020, 2021c). However, the biochemical and genetic basis of methane utilization in selected species of these genera is not yet elucidated.
Most of the methane-utilizing bacteria have been isolated from a large diversity of plants. Methane-utilizing bacteria have been found to inhabit the rhizosphere and phyllosphere of flooded ecosystems in large numbers where methane is produced, such as natural (mangroves) or man-made (flooded paddy fields) wetland ecosystems (Iguchi et al. 2012). The leaf surfaces of most of the plant species across different agroecological zones are occupied by active methane or methanol utilizing microbes and constitute about 14–20% of the total microbial community of the phyllosphere (Fedorov et al. 2011; Wellner et al. 2011). The high population of different methane-utilizing microbial communities occupying roots, leaves, stems, and internal tissues of plants, with no prevalence of any disease, highlights their important role in sustaining plant growth promotion, mediated through integrated nutrient and abiotic stress management (Wagner et al. 1999; Rani et al. 2021c). Many methane-utilizing and plant growth-promoting bacteria were isolated, belonging to genera Methylobacterium, Burkholderia, Hyphomicrobium, Paenibacillus, Pseudomonas, and Rahnella from the flooded paddy ecosystem of India. Besides, plant growth-promoting attributes, such isolates exhibited a reduction in cumulative methane emissions by 7 to 12% from flooded paddy fields, when used as root and spray inoculants (Rani et al. 2021c).
Methane emission from natural or man-made wetlands is the net balance of methane production by methanogenic archaea and its oxidation by methane-utilizing bacteria (Malyan et al. 2016). Along with suitable agronomic management practices, prospecting methane-utilizing bacteria residing in various plant parts as a significant sink of methane can help and play an important role in managing the threat of methane emissions from the agricultural domain, particularly from flooded paddy ecosystems. This review provides an overview of the diversity of methane-utilizing bacteria, explores their multifaceted roles in agriculture, and proposes future projections leading to the development of next-generation microbial inoculants with the dual ability of crop growth promotion and reduction in methane emission.
Diversity of methane-oxidizing bacteria
The unique property of aerobic oxidation of methane into methanol and its further assimilation in cells/tissues is widespread among several genera of bacteria, archaea, and yeasts (Dedysh and Knief 2018; Rani et al. 2021b). The reaction step is catalyzed by particulate (pMMO) and soluble methane monooxygenases (sMMO). The methanol formed as a byproduct is further oxidized to formaldehyde by the action of methanol dehydrogenases (MDHs) (Keltjens et al. 2014). The diversity and phylogeny of aerobic methanotrophs have been reviewed extensively earlier by Dedysh and Knief (2018). Whittenbury et al. (1970) categorized methanotrophs into Type I (produce pMMO), Type II (produce both pMMO and sMMO), and Type X (have some features of Types I and II). Later, based on the 16S rRNA gene sequence analysis the methane-utilizing bacteria were taxonomically placed in the phylum Proteobacteria and grouped into Type I (including Type X) and Type II (Chistoserdova et al. 2009). Type I (including Type X) and Type II were further sub-classified into class gamma- and alpha-proteobacteria, respectively (Fei et al. 2014). Some of the traditionally known Type I methane-utilizing bacteria belonging to the gamma-proteobacteria are Methylomicrobium, Methylosphaera, Methylosarcina, Methylothermus, Methylomonas, Methylohalobius, Methylobacter, Methylosoma, Clonothrix, Crenothrix, Methylococcus (Type X) Methylocaldum (Type X). Similarly, some Type II methane-utilizing bacteria belonging to the class alpha-proteobacteria are Methylocystis, Methylocella, Methylosinus, and Methylocapsa (Chistoserdova et al. 2009). Besides alpha- and gamma-proteobacteria, some of the bacteria belonging to beta-proteobacteria have also been identified as methane utilizers viz., Methylophilus (Madhaiyan et al. 2009), Methylovorus (Govorukhina and Trotsenko 1991), Methylibium (Nakatsu et al. 2006), Burkholderia and Methylobacterium (Rani et al. 2021c). Over the last two decades, sulfate-, nitrate- and nitrite-dependent anaerobic methane oxidation has been reported (Guerrero-Cruz et al. 2019). Sulfate-oxidizing anaerobic archaea belonging to the order Methanosarcinales, and Methanomicrobiales have been isolated from anoxic zones of marine sediments, soda lakes, continental margins, methane seeps, and vents (Valentine and Reeburg 2000). The nitrate-dependent anaerobic oxidation of methane is catalyzed by archaea belonging to the ANME-2d clade (Candidatus Methanoperedens nitroreducens) (Ettwig et al. 2016). The archaea carrying out sulfate- and nitrate-dependent anaerobic methane oxidation play an important role in limiting methane emissions from marine sediments. Various mechanisms viz. reverse methanogenesis, acetogenesis, and methylogenesis have been proposed to explain this unique function of anaerobic archaea (Caldwell et al. 2008; Ettwig et al. 2016). The nitrite-dependent anaerobic oxidation of methane was reported in the bacteria belonging to the NC10 phylum (Candidatus Methylomirabilis oxyfera, Candidatus Methylomirabilis lanthanidiphila, and Candidatus Methylomirabilis sinica) (Versantvoort et al. 2018). Despite their anaerobic metabolism, these bacteria oxidize methane using an intra-aerobic pathway and activate methane using oxygen through a pMMO, while reducing nitrite to N2 (Ettwig et al. 2010). Apart from phylum proteobacteria, methane-utilization ability has also been reported in the family Methylacidiphilaceae of the phylum Verrucomicrobia and in the intra-aerobic bacteria of the phylum NC10 candidate (Dunfield et al. 2007; Ettwig et al. 2010). For the utilization of methane and other C1 compounds such as formaldehyde, gamma-proteobacteria (Type I and X), and alpha-proteobacteria (Type II) utilize the ribulose monophosphate cycle and serine cycle, respectively. However, some of the bacteria belonging to the species of Type X, Verrumicrobia, and NC10 (Methylomirabilis spp) phyla can also grow as autotrophs through the Calvin-Benson-Bassham cycle, thus contributing significantly to the global carbon cycle (Sahoo et al. 2021). They possess the advantage of sequestering CO2 and its subsequent enzymatic hydrogenation into methanol (Sahoo et al. 2021). The conversion is carried out in a two-stage process, where the first stage comprises the utilization of CH4 as a carbon substrate to produce biomass and the second stage involves the reduction of CO2 to produce methanol (Sahoo et al. 2021). The role of these CH4 oxidizing autotrophic bacteria in the agricultural ecosystem is still not clear as these microbes are mainly reported from extreme environments such as geothermal vents, volcanoes, low pH levels, and hot springs (van Teeseling et al. 2014; Kim et al. 2021).
Methane-utilizing bacteria belonging to the phylum Proteobacteria are found in a vast variety of natural and extreme ecological niches, including agricultural fields (especially flooded paddy fields), wetlands, thermal springs, volcanic soils, and peatlands (Kolb and Horn 2012; Islam et al. 2020; Hogendoorn et al. 2021; Rani et al. 2021c) (Table 1). Among peatlands, α-proteobacteria dominate oligotrophic and acidic bogs, while γ-proteobacteria dominate nutrient-rich, methanotrophic, and mildly acidic fens (Verbeke et al. 2019). It has been found that among α-proteobacteria prevalent in bogs, Methylocystis are most active, while Methylocella and Methylocapsa species are also common (Verbeke et al. 2019). They even found that Methylobacter, Methylomonas, and Methylomicrobium are the most abundant γ-proteobacteria. In the past few years, various workers have reported the significant presence of methane-utilizing bacteria in agricultural soils, rhizosphere, and phyllosphere of crop plants. Diverse genera of methane-utilizing bacteria and yeast have been isolated from natural wetlands and flooded agroecosystems (paddy fields), where a large quantity of methane is released because of methanogenesis in submerged soil. Metagenomic analysis (V3 region of 16 S rRNA gene) of selected arable and no-tillage soils in the Lublin region of Poland revealed the presence of a 0.1% population of methanotrophs, dominated by the genus Methylocystis (Szafranek-Nakonieczna et al. 2019). The pmoA sequence analysis of forest and agricultural soil revealed the dominance of type I (Methylobacter, Methylocaldum) and type II (Methylocystis, Methylosinus) methanotrophs (Kravchenko and Sukhacheva 2017). They further reported that the differential CH4 oxidation rates between forest and agricultural soils were primarily resulting due to the variation in the composition of the methane-oxidizing communities. Looking at their environmental significance, several researchers focused their attention on demonstrating the presence of methane-utilizing bacteria and the role they play in different wetland, agricultural, and forest ecosystems (Kravchenko and Sukhacheva 2017; Rani et al. 2021c). Further, it is important to recognize that the methanotrophic activity in a flooded agricultural ecosystem such as rice paddies is important in managing net CH4 emissions and promoting plant growth.
Methane-utilizing bacteria: role in agriculture
From an agricultural perspective, flooded agricultural ecosystems (especially rice paddies) and soils rich in organic matter are hotspots of methane emissions and harbor high concentrations of methane in their anoxic, carbon-rich environment. They are the largest source of global atmospheric methane, emitting 142–284 Tg CH4 per year (Kirschke et al. 2013). The methane in such an environment is generated as the end-product of the anaerobic degradation of organic matter under flooded anoxic conditions. The well-known ‘low-affinity’ methanotrophs, which contain the type I (γ-proteobacteria) and type II (α-proteobacteria) subgroups, catalyze methane oxidation at the aerobic–anaerobic interfaces. These interfaces include the oxygenated surface soil layers and the area around the oxygen-releasing roots of wetland plants. It is crucial to recognize the importance of methanotrophic activity in agricultural soils, not just from the perspective of managing the net CH4 emissions, but also other possible benefits to the soil or crop.
Role in reducing methane emission from agricultural ecosystem
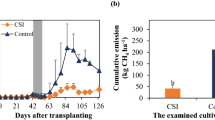
Various researchers have reported the findings of the diversity of methane-utilizing bacteria in a methane-rich environment like a natural wetland, rice field, livestock rumen, peats, and bogs (Dianou et al. 2012; Esson et al. 2016; Auffret et al. 2018; Rani et al. 2021c) (Table 1). However, exploring their potential as inoculants in agriculture for reducing overall methane flux is still in its infancy. In the last few years, various workers have reported a reduction in methane flux using methane-utilizing bacteria in flooded paddies. Inoculation of methanotrophic Ochrobactrum anthropi in combination with nitrogen-fixing Azotobacter and Azospirillum at 25% recommended fertilizer dose decreased the CH4 flux from less than 18.31 mmol m−2 h−1 in control to − 19.57 mmol m−2 h−1 during vegetative phase (Pingak et al. 2014; Sutanto et al. 2014) reported 12.29% lower CH4 emissions from flooded paddy fields (cultivated by applying 2/3rd recommended dose of fertilizer) by inoculating a consortium of methanotrophic bacteria comprising Methylocystis rosea BGM 1, M. rosea BGM 5, Methylococcus capculatus BGM 9, and Methylobacter sp. SKM. Sukmawati et al. (2016) reported a significant decrease in CH4 flux (on the 21st day after transplanting) from less than 74346.45 moles d−1 ha−1 in control to − 24018.80 moles d−1 ha−1 in plots inoculated with Methylocystis rosea BGM 5 and N2O reducing bacteria Ochrobactrum anthropi BL2. Similar findings were reported by Taopan et al. (2018) who reported a decline in CH4 flux from less than 10 mg/m2/d in un-inoculated control to − 23.87 mg/m2/d in rice inoculated with a bacterial consortium, comprising different strains of Methylocystis¸ Methylobacter, and Methylococcus at 60th d after transplanting. Nontji and co-workers (2016) reported a decrease in methane emission by 51–88% in rice fields depending on the type of methane-utilizing bacteria. They observed a significant decline in methane flux till the 5th day of inoculation followed by a gradual decrease in % methane reduction. A similar finding was recorded by Davamani and co-workers (2020) who claimed a 57–68% reduction in CH4 emission during active tillering, flowering, and maturity stage of rice, by the application of methanotrophic bacterial consortium over un-inoculated control. Rani et al. (2021a) investigated the effect of different N fertilizer regimes on cumulative methane emission from paddy fields by performing dual inoculation of paddy by methane-utilizing Methylobacterium oryzae MNL7 (at different growth stages) and plant growth-promoting Paenibacillus polymyxa MaAL70 (seed treatment during nursery sowing). They reported a significant decrease in the cumulative methane flux by 12.03, 11.47, and 6.92% in Urea, DAP + Urea, and FYM fertilized plots, over their respective uninoculated treatments.
In rice paddies, studies have found that methane oxidation activity of methane-utilizing bacteria is affected by oxygen availability under anoxic flood conditions (De Bont et al. 1978). Cyanobacteria are routinely used as biofertilizers in paddy cultivation, and their ability to liberate oxygen due to photosynthetic activity in the flooded ecosystem is known to be beneficial, but their role in complementing or supplementing methane-utilizing bacteria inhabiting rice rhizosphere and soil water-air continuum (Mancinelli 1995; Prasanna et al. 2002) is less investigated. Cyanobacteria and Azolla (an aquatic fern harboring cyanobacterium, Anabaena azollae, often found floating in flooded paddy fields) are among the various biological inputs routinely used in rice fields for providing nitrogen to the plants (Prasanna et al. 2002). However, their role in methane production and oxidation is less documented. Therefore, a study was undertaken using laboratory simulation experiments with rice field soil samples and combinations of cyanobacteria and Azolla (Prasanna et al. 2002). Interesting results in terms of rapid decrease in the headspace concentration of methane were obtained with moist soil cores, taken from treatments involving the application of urea in combination with Azolla microphylla, a cyanobacterial mixture, and cyanobacterial mixture + A. microphylla. These values were distinct, as compared to those from soil cores from plots treated with urea alone (30, 60, 90, and 120 kg N ha−1), illustrating that the application of biofertilizers such as cyanobacteria and Azolla can help in the mitigation of methane emissions, mainly facilitated through the photosynthetic evolution of oxygen in the wetland rice environment. Malyan et al. (2021) studied the effect of 9 different combinations of methane-utilizing bacteria, cyanobacteria biofertilizer, and Azolla on reduction in methane and nitrous oxide flux for the 2 consecutive years in flooded paddy. They observed a maximum significant decrease in cumulative CH4 emission by the application of Methylobacteruim oryzae MNL7 alone (19.9%) followed by treatment of Azolla + cyanobacteria combination (13.2%) as compared to un-inoculated control. Application of Azolla in flooded paddy assists in reducing cumulative CH4 emission due to liberation of oxygen in standing water and an increase in soil Eh (Bharati et al. 2000; Xu et al. 2017). From the limited number of reports in the literature, the idea of managing methane emissions using microorganisms has generated significant interest among the scientific community across the world. However, there are certainly important issues (discussed later in this review) that need to be addressed before developing biofertilizers capable of not only promoting plant growth but also mitigating GHG emissions from agriculture-based ecosystems, especially methane.
Role in plant growth promotion
The plant growth-promoting (PGP) traits exhibited by microorganisms have been reported in several genera of bacteria (Glick 2012), cyanobacteria (Manjunath et al. 2011), archaea (Naitam and Kaushik 2021), and fungi (Hossain et al. 2017). The PGP traits include fixation of atmospheric N, solubilization of P, K, and Zn from complex mineral sources present in the soil, production of phytohormones (auxin, gibberellic acid, abscisic acid, cytokinin, and salicylic acid), induction of systemic resistance to abiotic stress (salinity, moisture, temperature, pH), and biocontrol of pests and diseases (Grover et al. 2011; Glick 2012; Divekar et al. 2022). Among different bacterial genera, the ability to promote plant growth by methane-utilizing bacteria has also been reported by a few researchers. However, obligate methanotrophs have not yet been investigated for the PGP traits. Methane-oxidizing bacteria belonging to the genus Methylophilus, Methylobacillus, Methylovorus, Methylopila, Methylobacterium, Delftia, and Ancyclobacter can solubilize tri-calcium phosphate (Agafonova et al. 2013). These bacteria secrete formic acid and lower the pH of the culture medium converting insoluble tri-calcium phosphate into a soluble form. However, none of them was found to possess the ability to solubilize K and Zn in the growth medium or soil. In another study, Rani et al. (2021c) reported P, K, and Zn solubilization activity in non-traditional methane-utilizing bacteria belonging to genera Methylobacterium, Hyphomicrobium, Paenibacillus, Pseudomonas, and Burkholderia. The P solubilization among different methane-utilizing bacteria ranged from 9.44 to 115.67 mg L−1 (using tri-calcium phosphate as P source), whereas the K solubilization index on potassium aluminum silicate as K source and the Zn solubilization index on ZnO, ZnCO3 and ZnPO4 varied between 1.70 and 2.90, 3.34–6.45, 3.59–6.29, and 3.34–5.73, respectively (Rani et al. 2021c).
Earlier, N2-fixation ability was thought to be limited to type II methanotrophs except for the type I genus Methylococcus (Murrell and Dalton 1983). However, results from DNA hybridization studies and acetylene reduction assays suggest that some members of the type I genus Methylomonas and the type I strain Methylobacter marinus A45 may fix nitrogen (Oakley and Murrell 1988). Methylomonas-like nifH fragments have been amplified from rice roots and freshwater lakes (Zani et al. 2000). Reports are available on the role and localization of type II methanotrophic bacteria of the family Methylocystaceae in rice crops. They are known to fix N2 and oxidize CH4 in the rice plant as they inhabit the vascular bundles and epidermal cells of the roots and thus play a major role in reducing methane emissions besides providing fixed nitrogen (Bao et al. 2014). Among non-traditional methane-utilizing bacteria viz. Burkholderia, Hyphomicrobium, Paenibacillus, Methylobacterium, and Rahnella were isolated from the flooded paddy ecosystem, and the nitrogen fixation in terms of acetylene reduction assay ranged from 122.72 ± 21.91 to 768.86 ± 25.89 nmoles of C2H2 mg protein−1 h−1 (Rani et al. 2021c). It has been hypothesized that nitrogen fixation may reduce the activity of methane oxidation due to competition in terms of energy consumption, therefore it is essential to investigate the role of N2-fixing and methane-utilizing bacteria in detail before using them for the dual purpose of N2 fixation and methane oxidation in the natural environment (Kruistum et al. 2018).
Indole-3-acetic acid (IAA) is one of the most widespread auxins and plays a major role in determining root growth. IAA production in a few genera capable of oxidizing methane such as Methylobacillus, Methylomonas, and Methylobacter in the range of 6–8 µg mL−1 has been reported (Doronina et al. 2002). Similarly, methanotrophs isolated from the rhizosphere region of the paddy could produce IAA (28.15 µg mL−1 of culture filtrate) and gibberellic acid (70.84 µg mL−1 of culture filtrate) (Davamani et al. 2020). They found that using a consortium of these methanotrophic bacteria increased paddy grain and straw yields by 34.61 and 11.46%, respectively, over the un-inoculated control. Similarly, inoculation of a methanotrophic bacterial consortium comprising Methylocystis rosea BGM 1, Methylobacter sp. SKM 14, Methylocystis palvus BGM 3, and Methylococcus capsulatus BGM 9) significantly increased the paddy yield by 4.9 t ha−1 (without fertilizer) to 6.6 t ha−1 (Taopan et al. 2018).
Among different genera of methane-utilizing bacteria, the most widely studied genus is Methylobacterium. Different species of Methylobacterium are known for their ability to promote the growth of various crops by the production of different phytohormones such as IAA, gibberellic acid, and cytokinins (Ivanova et al. 2001; Lidstrom and Chistoserdova 2002; Siddikee et al. 2010), and by alleviating abiotic stress of heat, drought, and salinity (Egamberdieva et al. 2015; Jorge et al. 2019; Grossi et al. 2020; Rani et al. 2021c) reported IAA production by methane-utilizing isolates belonging to 7 bacteria and 1 yeast genera obtained from a flooded paddy ecosystem. Among these 7 bacterial (Hyphomicrobium, Burkholderia, Methylobacterium, Pseudomonas, Paenibacillus, Curtobacterium, and Rahnella) and 1 yeast (Meyerozyma guilliermondii) genera, the IAA production ranged from 13.37 to 82.02 µg mL−1 and 26.94 to 132.99 µg mL−1 in the absence and presence of tryptophan, respectively (Rani et al. 2021c). Field evaluation of methane-utilizing and plant growth-promoting bacterial consortium, comprising Methylobacterium oryzae MNL7 and Paenibacillus polymyxa MaAL70 in different fertilizer treatments showed a significant increase in the crop yield by 11.08–14.04% over un-inoculated control and reduced cumulative net methane flux by 6.92 to 12.03% (Rani et al. 2021a). Methylobacterium species also hold great potential as a biocontrol agent for plant disease management and are effective against fungal (Phytophthora infestans, Botrytis cinerea, and Fusarium graminearum) and bacterial (Pectobacterium atrosepticum and Pseudomonas syringae) pathogens (Ardanov et al. 2012; Grossi et al. 2020). They are effective against Ralstonia solanacearum causing bacterial wilt in tomatoes by synthesizing ACC (aminocyclopropane-1-carboxylic acid) deaminase enzyme and pathogenesis-related proteins (β-1,3-glucanase, phenylalanine ammonia-lyase, polyphenol oxidase, peroxidase) leading to low ethylene levels in plants (Yim et al. 2013). Research exploring the biocontrol potential of methanotrophic isolates against Xanthomonas oryzae pv. oryzae causing bacterial leaf blight in rice has shown positive results (Nontji and Amra 2019).
The above studies show that methane-utilizing bacteria at the aerobic–anaerobic niche in a flooded agricultural ecosystem play a crucial role in reducing the net methane flux and stimulating plant growth by producing plant growth-promoting substances. However, various factors influencing the growth and proliferation of methane-utilizing bacteria in the agricultural ecosystem are poorly understood. These factors play a major role in determining the net methane flux of any ecosystem, essential to be considered while cultivating crops to combat climate change.
Factors affecting microbial utilization of methane in the agricultural ecosystem
The net CH4 emission from the paddy field is governed by the abundance of methane-utilizing bacteria and three major processes viz. CH4 production, CH4 oxidation, and its transport through diffusion, ebullition, and aerenchymal routes (Cai et al. 2007). The abundance of methane-utilizing bacteria and their methane oxidation ability in paddy fields is influenced by a variety of soil physicochemical factors such as temperature, pH, nitrogenous fertilizer application, and rice varieties. Among these factors, no detailed reports are available about the effect of temperature and soil pH on the methane oxidation ability of methane-utilizing bacteria in agricultural soils. The pH requirement for the growth and oxidation of methane by these bacteria is influenced by their habitat. In agricultural soils, these bacteria are generally mesophilic and grow at an optimum temperature of 25–35 °C (Sadasivam and Reddy 2014). Methane-utilizing bacteria are adapted to a wider range of pH (Reddy et al. 2020). The alkalophilic methane-utilizing bacteria Methylomicrobium alcaliphilum was isolated from saline Tuva soda lakes with an optimum pH requirement of 9.0-9.5 (Khmelenina et al. 1997). The acidophilic or acid-tolerant methane utilizing bacteria has been reported in the families Methylocystaceae, Beijerinckiaceae, and Methylococcaceae (Nguyen et al. 2018). The effect of nitrogenous fertilizer and different rice varieties is discussed below (Fig. 1).
-
(a)
Effect of different nitrogenous fertilizers on the activity of methane-utilizing bacteria
The application of N fertilizers affects CH4 transport as plant biomass responds positively to the dose of fertilizer and provides a channel for the release of CH4 into the atmosphere (Le Mer and Roger 2001). Researchers suggest that the application of N-fertilizer will increase plant biomass and root exudate formation, thus providing more substrate for the growth of methanogenic archaea and a channel for the release of CH4 causing increased emission (Xu et al. 2004; Jia et al. 2006). Simultaneously, at the microbial level, the application of N-fertilizer will augment the activities of both methanogens and methanotrophs depending on the soil moisture content and existing CH4 and O2 concentration in the paddy field (Bodelier et al. 2000a, b). Studies showing both stimulation and repression of CH4 flux on the use of N- fertilizer are documented, which highlights that nitrogenous fertilizer may show a varying effect on CH4 emission based on several factors (Liu and Greaver 2009; Bin-feng et al. 2016).
Dose and time of fertilizer application
The effect of nitrogenous fertilizer on CH4 emissions is said to be dose-dependent with higher emissions on the application of a small dose of fertilizer and vice-versa (Banger et al. 2012; Linquist et al. 2012). However, the opposite trend was observed by Aronson and Helliker (2010). Linquist and coworkers (2012) reported that application of N-fertilizer at low rates (≈ 79 kg N ha−1) increased the CH4 emission by 18%, whereas emissions were reduced by 15% at high N application rate (≈ 249 kg N ha−1). Their findings suggest that excess NH4+-N formed in soil due to the application of higher doses of N fertilizer increases the CH4 oxidation activity, which decreases CH4 emissions. N fertilization at the tillering stage, when anaerobic conditions are prevalent in flooded paddy fields with ample CH4 supply, increases the activity of methanotrophs, which results in lower net methane flux (Cai et al. 2007). However, N fertilizer application during panicle initiation and grain filling stages increases the root exudation and methanogenesis, resulting in higher CH4 emissions (Cai et al. 2007). The findings suggest that the effect of the application of N-fertilizer on net CH4 emission is governed by the prevailing CH4 concentration in the rice ecosystem, as well as the growth stage of plants, which regulate stimulation in the activity of methanogens or methanotrophs (Bin-feng et al. 2016).
Type of fertilizer used in rice crops
Nitrate and ammonium-based fertilizer differ in terms of their effect on net CH4 emission. Nitrate-based fertilizers are usually not recommended for paddy crops due to their high mobility, low use efficiency, and stimulatory effect on N2O emissions which is another potent GHG (Gaihre et al. 2020). But the application of nitrate-based fertilizer show lower CH4 emission as denitrification of nitrate consumes electrons and H2 required by CH4 producers (Kluber and Conrad 1998). Nitrate and its denitrified product are toxic to acetate-utilizing methanogens resulting in lower CH4 emissions (Lindau et al. 1990). However, this effect is reported to be short-lived as an increase in CH4 production is observed after the denitrification process is over (Lu et al. 2000). Moreover, nitrate has been found inhibitory to the activity of methane-utilizing bacteria at very high concentrations mainly because of the osmotic effect (Bodelier and Laanbroek 2004). Therefore, the use of nitrate-based fertilizer cannot be recommended as a remedy to mitigate CH4 emission due to its short-term effect, low fertilizer use efficiency, and enhanced N2O emission.
The effect of ammonium fertilizers on CH4 emission is determined by their impact on the activity of methanogens and methanotrophs. Enhanced activity of methanogens is observed with the addition of NH4+ fertilizers due to the increased plant growth and availability of substrate (Bodelier et al. 2000a). It has been reported that the application of ammoniacal fertilizers inhibits the methane oxidation ability of methane-utilizing bacteria in soil (Le Mer and Roger 2001; Bodelier and Laanbroek 2004). Short-term inhibition in CH4 oxidation on the addition of NH4+ fertilizer is observed which may be due to competitive inhibition of MMO by ammonia (Dunfield and Knowles 1995; Hooper et al. 1997). Moreover, the intermediates and end products of ammonia oxidation i.e., hydroxylamine and nitrite, can be toxic to the methanotrophic bacterial community and thus inhibits CH4 consumption (Schnell and King 1994). A higher dose of NH4+ fertilizer may cause osmotic stress and inhibit the activity of the methane-utilizing bacteria (Whalen 2000). The diverse varying effect of NH4+ fertilizer on CH4 emission may be due to change in the community composition, either by a shift between ammonium tolerant and ammonium-intolerant CH4-oxidizing species or by a relative increase of ammonia oxidizers consuming CH4 (Bodelier and Laanbroek 2004).
One of the earliest reports on the positive impact of urea-based fertilizers on methane oxidation was given by Bodelier et al. (2000a) who observed stimulation of methane oxidation in a microcosm planted with rice along with the application of 200 or 400 kg N ha−1 urea or (NH4)2SO4. Using molecular tools and radioactive fingerprinting, this was attributed to the proliferation and activity of type I methanotrophs. Fertilizer containing sulfate may affect net CH4 emission either by suppressing methanogenesis or by promoting anaerobic CH4 oxidation (Segers 1998; Pennock et al. 2010). The use of ammonium sulfate in the paddy field is found to reduce CH4 emissions by up to 40% as compared to plots treated with urea (Linquist et al. 2012; Malyan et al. 2016). An in-depth analysis shows that the effect of sulfate on CH4 emission is influenced by the dose of sulfate fertilizer used. A higher dose of sulfate fertilizers @ 208 and 992 kg S ha−1 can reduce CH4 emission by 28% and 53%, respectively (Linquist et al. 2012; Traore et al. 2017) compared the effect of different ammonium nitrate-based fertilizers on CH4 emission. They reported higher emission of CH4 in urea treated pots followed by ammonium sulfate, ammonium chloride, and sodium nitrate, which was 2, 1.5, 1.3, and 0.2 times, respectively greater than that of control (pots without N-fertilizer). Their findings indicate that urea can also be substituted by other NH4+ based fertilizers like ammonium sulfate in flooded ecosystems.
Organic amendments such as FYM, straw, and green manure are said to increase CH4 emissions due to the increase in the activity of methanogenic archaea (Cai et al. 2007; Kim et al. 2014; Ho et al. 2015). High organic matter content in the soil activates microbial activities, consumes O2, lowers soil redox potential, and creates an environment conducive to the growth and proliferation of methanogens (Yang et al. 2010; Zhang et al. 2018). About 46% higher CH4 emissions were reported on the application of organic amendments as compared to control with a balanced fertilizer dose of NPK (Yang et al. 2010). Statistical analysis shows that the organic amendment and water regime play a major role in determining CH4 flux as compared to climate and soil properties (Yan et al. 2005). Therefore, the choice of organic substrate for amendment in the rice field will play a major role in determining CH4 flux. The use of slow-release fertilizer along with safe organic amendments such as biochar has been recommended to increase crop yield, maintain soil health, and reduce CH4 emission (Miao et al. 2016; Ly et al. 2015). Being recalcitrant, biochar does not provide an ideal organic substrate for the growth of methanogens (Kuzyakov et al. 2009). In addition, it increases soil aeration, thereby reducing methanogenesis (Karhu et al. 2011). This slow-release fertilizer reduces the plant biomass, and thus decreases the amount of methane transported by the plants and the amount of carbon substrate in the plant debris and root exudates (Kim et al. 2017). In a study, a significant reduction in the cumulative methane emission from paddy fields was reported due to the application of biochar and slow-release fertilizer. The cumulative methane emissions were significantly reduced by the combined application of biochar and slow-release fertilizers (8916 mg CH4 m−2 growing season−1), as compared to using only slow-release fertilizers (13,858 mg CH4 m−2 growing season−1) and urea (15,864 mg CH4 m−2 growing season−1) (Kim et al. 2017).
Another alternative is to use controlled-release nitrogen fertilizer such as neem oil-coated urea and, polymer-coated urea in rice fields as their application results in lower emission of CH4 giving additional benefits of enhanced yield and lower disease incidence (Ankita and Bindu 2016; Wang et al. 2016).
The above findings suggest that N-fertilizer influences the CH4 production, oxidation, and transport process in the paddy field, resulting in variable effects on CH4 flux. The application of slow-release fertilizers with recalcitrant organic material (biochar) can cause a significant reduction in the net CH4 emission. The use of sulfate-containing fertilizers like ammonium sulfate along with in-depth placement can be another strategy to reduce net CH4 emissions. These studies indicate the strong impact of nitrogenous fertilizer on CH4 emission, and therefore, should be an important consideration, while preparing the fertilization schedule for paddy cultivation.
-
(b)
Effect of different rice cultivars on the activity of methane-utilizing bacteria
The rice plant harbors a diverse group of methane producers and consumers that play a vital role in determining CH4 flux in a particular area. About 90% of CH4 generated in a paddy field by the methanogens gets consumed by aerobic methanotrophs even before it is released into the atmosphere (Holzapfel-Pschorn et al. 1986; Hanson and Hanson 1996). A comprehensive study determining the role of rice cultivar in influencing root exudation, aerenchymatous space, the population of methanogenic archaea and methanotrophic bacteria, and its impact on CH4 oxidation has been carried out by a few workers (Wang and Adachi 2000; Liechty et al. 2020). They observed significant differences in the population of methanogenic archaea at booting and ripening stages with different rice cultivars. Similarly, the population of methane-utilizing bacteria also varied significantly in the root samples collected from different rice cultivars. The population and diversity of both methane-producing and methane-utilizing microbes residing in the rice phyllosphere and rhizosphere in a particular variety can play a major role in determining the net CH4 flux in that area. CH4 emission is reported to be positively correlated to tiller number, culm biomass, soil organic matter, dissolved soil organic carbon, and total carbon content in the rice field (Qin et al. 2015). Varieties with high yield and low CH4 emission have been identified by researchers (Gogoi et al. 2008; Qin et al. 2015; Islam et al. 2019). A significant difference in the microbial community of both methanogens and methanotrophs in low and high-emitting rice cultivars has been demonstrated by Liechty and coworkers (2020). It is reported that hybrid rice as compared to Indica and Japonica cultivars stimulates the growth of methane-utilizing bacteria in the rice rhizosphere, and hence enhances CH4 oxidation which limits CH4 emissions from the paddy soil (Ma et al. 2009). Besides affecting the population of methanogenic archaea and methane-utilizing bacteria, high CH4 emitting rice cultivars are also associated with a higher population of sulfate-reducing and iron-reducing taxa responsible for lowering soil oxidation-reduction potential to a point where methanogenesis can occur (Liechty et al. 2020). The greater abundance of fermentative taxa which produces methanogenesis precursors (acetate, CO2, and H2) along with microorganisms associated with acetogenesis which compete with methanogens for CO2 and H2 is reported in high CH4 emitting cultivars (Liechty et al. 2020). Thus, it is quite evident that CH4 emission from rice fields is not only influenced by the population of methanogens and methanotrophs, but also by other microbial taxa involved in upstream and downstream processes of CH4 production.
Rice cultivars differ widely in terms of growth-related parameters like the number of tillers and plant biomass (Wang et al. 1997). A higher number of tillers and plant biomass are found to be positively correlated to the CH4 exchange rate during the vegetative phase of the rice plant (Aulakh et al. 2001). Root exudates along with plant debris act as an important source of nutrients for the growth and proliferation of diverse groups of microorganisms residing in the soil. They play an important role in determining the complex microbial dynamics in environmental samples (Bakker et al. 2018; Olanrewaju et al. 2019). By way of its exudate pattern, they alter the diversity of both methanogens and methanotrophs residing in the soil. Studies conducted show that the community distribution and abundance of both methanogens and methanotrophs vary with the oxic condition of the soil along with total organic carbon content mainly contributed by the root exudate and decomposition of plant debris (Lee et al. 2015). Transgenic high starch and low CH4 rice developed by introducing a single transcription factor gene SUSIBA2 from barley favors allocation of photosynthates to aboveground biomass over-allocation to roots (Su et al. 2015). Transfer of this gene resulted in reduced CH4 emission by altering root exudate composition and increased plant biomass and starch content in the seeds and stems.
The aerenchymal architecture of the rice plant determines the passage for the emission of CH4 into the atmosphere. Moreover, they act as a conducting duct for the transport of oxygen from the atmosphere into the plant root zone determining the redox level of the paddy soil. The architecture of aerenchymatous tissue is governed by several genes and the positive impact of ethylene levels and hydrogen peroxide on the development of aerenchyma tissue has been reported (Fukao et al. 2006; Hattori et al. 2009; Steffens et al. 2010). Research shows that treatment with ethephon, an ethylene releasing compound promotes aerenchyma development across all rice varieties. It promotes the formation of O2− radicals, and H2O2 which directly promotes aerenchyma formation. The finding was confirmed by the downregulation of the MT2b gene involved in scavenging H2O2 which directly affects aerenchyma formation (Steffens et al. 2010). Genes like SNORKEL1, SNORKEL2, and ethylene response factor (ERF) influence ethylene levels in plant and thus indirectly determines the air space in aerenchymatous tissue (Fukao et al. 2006; Hattori et al. 2009). Therefore, genes involved in the upregulation and downregulation of ethylene levels can play an important role in determining the air space in aerenchymatous tissue directly influencing gaseous exchange including CH4 and O2. The CH4 transport capacity of rice plants can be an important factor when choosing a rice variety for cultivation. Its impact on the net CH4 emission should not be undermined. The use of high-yielding cultivars with low CH4 transport capacity could be an economically feasible, environmentally sound, and promising approach to mitigate CH4 emissions from rice fields.
Interaction of other microbial forms with methane-utilizing bacteria that aid in methane mitigation
Methanotrophic bacteria can grow with other organisms and aid in the removal of other greenhouse gases (Singh et al. 2019). Co-culture of a methanotrophic bacterial consortium with cyanobacteria or microalga can lead to complete CH4 and CO2 uptake (Hill et al. 2017; Ruiz-Ruiz et al. 2020) and thus, is a promising strategy for greenhouse gases mitigation in a single step (Fig. 2). Earlier research suggested that inoculation of Synechocystis sp. (cyanobacteria) in a laboratory simulation experiment using soil samples from rice fields can cause a significant reduction in the headspace concentration of methane. Moreover, co-inoculation of cyanobacteria with Azolla microphylla could further enhance the methane removal rate (Prasanna et al. 2002). The cyanobacteria can consume CO2 creating an oxygen-rich environment via oxygenic photosynthetic in the root zone thereby promoting the growth and activity of methane-utilizing bacteria. Oxygen released by photosynthesis can provide dual benefits as it enhances the growth and activity of methane-oxidizing bacteria as well as limit the growth of anaerobic methanogenic archaea culminating in reduced methane emission. Formulating the ratios of the partners, stage of growth, the C–N dynamics, and media constituents would be interesting as such consortia would be a fructuous model to decipher the feedback and cross-feeding mechanisms.
Synergistic interaction occurs between methanotrophs and non-methane utilizing methylotrophs (NUM) where the intermediates produced by the methanotrophs (methanol, formaldehyde, and formate) can be used as a C-source by the NUM and supports its existence. On the other hand, NUM consumes these toxic intermediates of the methanotrophs by cross-feeding and aid in the methane removal process (Rani et al. 2021d). Besides algae, growth stimulation of methane utilizing Methylovulum miyakonense in presence of cobalamin secreting Rhizobium has been documented (Iguchi et al. 2011). Removal of toxic intermediates like organic acids can also support the growth and proliferation of methanotrophic partners (Singh et al. 2019). Synergistic interactions occur between the methanotrophs and heterotrophs where one provides the other with a carbon source and the other produces a growth factor or removes toxic intermediates from the environment and allows them to thrive in natural environments (Stock et al. 2013; Ho et al. 2014; Veraart et al. 2018; Singh et al. 2019).
Limitations and possible solutions of using methane-utilizing bacteria in agriculture
The potential of methane-utilizing bacteria in reducing the overall CH4 emission by the process of oxidizing the CH4 released from the paddy ecosystem has not been explored and needs to be integrated into the biofertilizer demonstration, popularization, and commercialization programs at the policy level. Blue-green algae, Azotobacter, and Azospirillum are still the popular bioinoculants recommended for rice cultivation (Ojha et al. 2018). The effect of other microorganisms like Burkholderia, Gluconacetobacter, Azoarcus, Herbaspirillum, Alcaligenes, Pantoea, Bacillus, and Stenotrophomonas are known for their promise in growth promotion and yield of the crop, under field condition (Egener et al. 1999; Duangpaeng et al. 2012; Adnan et al. 2016; Gholamalizadeh et al. 2017). But the use of methane-utilizing bacteria for CH4 mitigation and plant growth promotion in the flooded ecosystem of paddy is still in its infancy. One of the major limitations of using methane-utilizing bacteria is the fluctuating CH4 concentration which acts as a C source for the growth and proliferation of these microorganisms (Jain et al. 2004). CH4 concentration in the paddy field varies with the growth stage as well as the water level (Tyagi et al. 2009; Khosa et al. 2011). Higher emission of CH4 at the tillering to the flowering stage followed by a gradual decrease till maturity has been observed (Islam et al. 2019; Rani et al. 2021a). Some researchers report maximum emission at the tillering stage (Oda and Nguyen 2019), whereas others observed an increase in CH4 emission till the flowering stage (Gaihre et al. 2011). Slight variation may be observed due to the standing water regime in the rice field determining the redox potential of the soil and the growth and activity of methanogenic archaea responsible for biogenic CH4 production (Epule et al. 2011). The changing CH4 concentration in the rice field leads to a fluctuating population of methane-utilizing bacteria in the field (Macalady 2002; Ma et al. 2013). Methane-utilizing bacteria-based bioinoculant when used will face a constant problem of maintaining the microbial load under in vivo conditions for optimum performance. A higher reduction in CH4 emission was observed till the 5th day of inoculation with methane-utilizing bacteria in a field experiment followed by a gradual decrease signifying the inability of the isolate to sustain their population under natural conditions (Nontji et al. 2016). A possible solution to this problem is the recurrent use of bioinoculant at regular time intervals viz. at the time of sowing, transplanting, tillering, and followed by flowering if required (Rani et al. 2021a). Another alternative is to use methane-utilizing bacteria which can form some resting structures like cysts and spores and can maintain their population under field conditions (Thirumurugan and Asha 2010). Members of type I methanotrophs (Methylobacter) along with type II methanotrophs of genera Methylosinus and Methylocystis can form resting structures (Bowman 2006) and thus can be preferred while choosing methane-utilizing bacteria for field application. The strain selected for field application should also have a good competitive ability and outgrow the natural microflora already present in the soil (Thomas and Singh 2019). Facultative CH4 oxidizers (Methylocystis, Methylocapsa, and Methylacidiphilum) that can utilize only one or two alternative substrates viz. acetate, ethanol, or H2, depending on the strain with high affinity for CH4 can be selected under field condition so that in the absence of CH4 they can sustain on other available carbon sources (Dedysh and Dunfield 2010). However, their preference for CH4 over other carbon sources should be ascertained as Methylocella can grow on a range of alternative substrates like acetate, pyruvate, succinate, malate, ethanol, propane, ethane, propanol, propanediol, acetone, methyl acetate, acetol, glycerol, propionate, tetrahydrofuran, and gluconate and thus, cannot be considered for mitigation of CH4 emission under natural environment (Dedysh and Dunfield 2010). Based on these limitations and precautions, research findings of field experiments on the large-scale application of methane-utilizing bacteria in paddy are limited.
With global warming and climate change being recognized as major challenges, it is time to rethink our approaches to agricultural cultivation and livestock production. Experts recommend that staple crops such as rice, alternate wetting, and drying or direct seeding approaches be popularized that could halve emissions and require one-third less water, making it more economical. They also advocate that paddy be irrigated and drained two to three times throughout the growing season, rather than continuous flooding limiting methane production without impacting yield. Alternatively, the use of methane-utilizing bacteria in agriculture for mitigating CH4 emissions and promoting crop productivity is an environment-friendly option, which can benefit crop and soil productivity. This requires directed and concentrated focused efforts supported by biologists, farmers, policy makers, and administrators at the village/farm level.
References
Adnan M, Patel M, Reddy MN, Khan S, Alshammari E, Abdelkareem AM, Hadi S (2016) Isolation and characterization of effective and efficient plant growth-promoting rhizobacteria from rice rhizosphere of diverse paddy fields of Indian soil. ARPN J Agric Biol Sci 11(9):373–379
Agafonova NV, Kaparullina EN, Doronina NV, Trotsenko YA (2013) Phosphate-solubilizing activity of aerobic methylobacteria. Microbiology 82(6):864–867. https://doi.org/10.1134/s0026261714010020
Ankita K, Bindu G (2016) Effect of Controlled-Release Nitrogen Fertilizer on Methane. Appl Ecol Environ Res 3(2):1–27. https://doi.org/10.1016/j.protcy.2016.05.027
Ardanov P, Sessitsch A, Häggman H, Kozyrovska N, Pirttilä AM (2012) Methylobacterium-Induced Endophyte Community Changes Correspond with Protection of Plants against Pathogen Attack. PLoS ONE 7(10):e46802. https://doi.org/10.1371/journal.pone.0046802
Aronson EL, Helliker BR (2010) Methane flux in non-wetland soils in response to nitrogen addition: A meta-analysis. Ecology 91:3242–3251. https://doi.org/10.1890/09-2185.1
Auffret MD, Stewart R, Dewhurst RJ, Duthie CA, Rooke JA, Wallace RJ, Freeman TC, Snelling TJ, Watson M, Roehe R (2018) Identification, Comparison, and Validation of Robust Rumen Microbial Biomarkers for Methane Emissions Using Diverse Bos Taurus Breeds and Basal Diets. Front Microbiol 8:2642. https://doi.org/10.3389/fmicb.2017.02642
Aulakh MS, Wassmann R, Rennenberg H (2001) Methane emission from rice fields—quantification, mechanisms, role of management and mitigation options. Adv Agron 70:193–260. https://doi.org/10.1016/s0065-2113(01)70006-5
Bakker PAHM, Pieterse CMJ, Jonge R, Berendsen RL (2018) The soil-borne legacy. Cell 172(6):1178–1180. https://doi.org/10.1016/j.cell.2018.02.024
Banger K, Tian HQ, Lu CQ (2012) Do nitrogen fertilizers stimulate or inhibit methane emissions from rice fields? Glob Change Biol 18:3259–3267. https://doi.org/10.1111/j.1365-2486.2012.02762.x
Bao Z, Okubo T, Kubota K, Kasahara Y, Tsurumaru H, Anda M, Ikeda S, Minamisawa K (2014) Metaproteomic Identification of Diazotrophic Methanotrophs and Their Localization in Root Tissues of Field-Grown Rice Plants. Appl Environ Microbiol 80(16):5043–5052. https://doi.org/10.1128/aem.00969-14
Bharati K, Mohanty SR, Singh DP, Rao VR, Adhya TK (2000) Influence of incorporation or dual cropping of Azolla on methane emission from a flooded alluvial soil planted to rice in eastern India. Agric Ecosyst Environ 79:73–83. https://doi.org/10.1016/S0167-8809(99)00148-6
Bin-feng S, Hong Z, Yi-Zhong L, Fei L, Xiao-ke W (2016) The effects of nitrogen fertilizer application on methane and nitrous oxide emission/uptake in Chinese croplands. J Integr Agric 15(2):440–450. https://doi.org/10.1016/s2095-3119(15)61063-2
Bodelier PLE, Laanbroek HJ (2004) Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol Ecol 47:265–277. https://doi.org/10.1016/s0168-6496(03)00304-0
Bodelier PL, Hahn AP, Arth IR, Frenzel P (2000a) Effects of ammonium-based fertilization on microbial processes involved in methane emission from soils planted with rice. Biogeochemistry 51:225–257
Bodelier PL, Roslev P, Henckel T, Frenzel P (2000b) Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 43:421–424. https://doi.org/10.1038/35000193
Bowman J (2006) The methanotrophs—the families Methylococcaceae and Methylocystaceae. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes, 3rd edn. Springer, Cham, pp 266–289
Cai Z, Shan Y, Xu H (2007) Effects of nitrogen fertilization on CH4 emissions from rice fields. Soil Sci Plant Nutr 53:353–361. https://doi.org/10.1111/j.1747-0765.2007.00153.x
Caldwell SL, Laidler JR, Brewer EA, Eberly JO, Sandborgh SC, Colwell FS (2008) Anaerobic Oxidation of Methane: Mechanisms, Bioenergetics, and the Ecology of Associated Microorganisms. Environ Sci Technol 42(18):6791–6799. https://doi.org/10.1021/es800120b
Chistoserdova L, Kalyuzhnaya MG, Lidstrom ME (2009) The expanding world of methylotrophic metabolism. Annu Rev Microbiol 63:477–499. https://doi.org/10.1146/annurev.micro.091208.073600
Davamani V, Parameswari E, Arulmani S (2020) Mitigation of methane gas emissions in flooded paddy soil through the utilization of methanotrophs. Sci Total Environ 726:138570. https://doi.org/10.1016/j.scitotenv.2020.138570
De Bont JAM, Lee KK, Bouldin DF (1978) Bacterial oxidation and emission of methane in rice paddies. Ecol Bull 26:91–96
Dedysh SN (2009) Exploring methanotroph diversity in acidic northern wetlands: Molecular and cultivation-based studies. Microbiology 78:655–669. https://doi.org/10.1007/978-3-319-74866-5_2
Dedysh SN, Dunfield PF (2010) Facultative methane oxidizers. Handbook of hydrocarbon and lipid microbiology. Springer, Berlin. https://doi.org/10.1007/978-3-540-77587-4_144
Dedysh SN, Knief C (2018) Diversity and phylogeny of described aerobic methanotrophs. Methane biocatalysis: paving the way to sustainability. Springer, Cham, pp 17–42
Dianou D, Ueno C, Ogiso T, Kimura M, Asakawa S (2012) Diversity of cultivable methane-oxidizing bacteria in microsites of a rice paddy field: investigation by cultivation method and fluorescence in situ hybridization (FISH). Microbes Environ 27(3):278–287. https://doi.org/10.1264/jsme2.me11327
Divekar PA, Narayana S, Divekar BA, Kumar R, Gadratagi BG, Ray A, Singh AK, Rani V, Singh V, Singh AK, Kumar A (2022) Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int J Mol Sci 23(5):2690. https://doi.org/10.3390/ijms23052690
Doronina NV, Ivanova EG, Trotsenko YA (2002) New Evidence for the Ability of Methylobacteria and Methanotrophs to Synthesize Auxins. Microbiology 71(1):116–118
Duangpaeng A, Phetcharat P, Chanthapho S, Boonkantong N, Okuda N (2012) The study and development of endophytic bacteria for enhancing organic rice growth. Proc Eng 32:172–176. https://doi.org/10.1016/j.proeng.2012.01.1253
Dunfield PF, Knowles R (1995) Kinetics of inhibition of methane oxidation by nitrate, nitrite, and ammonium in a humisol. Appl Environ Microbiol 61:3129–3135. https://doi.org/10.1128/aem.61.8.3129-3135.1995
Dunfield PF, Yuryev A, Senin P, Smirnova AV, Stott MB, Hou S, Ly B, Saw JH, Zhou Z, Ren Y, Wang J (2007) Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia. Nature 450(7171):879–882. https://doi.org/10.1038/nature06411
Egamberdieva D, Wirth S, Alqarawi AA, Allah EFA (2015) Salt tolerant Methylobacterium mesophilicum showed viable colonization abilities in the plant rhizosphere. Saudi J Biol Sci 22(5):585–590. https://doi.org/10.1016/j.sjbs.2015.06.029
Egener T, Hurek T, Reinhold–Hurek B (1999) Endophytic expression of nif genes of Azoarcus sp. strain BH72 in rice roots. Mol Plant Microbe Interact 12:813–819. https://doi.org/10.1094/mpmi.1999.12.9.813
Epule TE, Peng C, Mafany NM (2011) Methane emissions from paddy rice fields: strategies towards achieving a win-win sustainability scenario between rice production and methane emission reduction. J Sustain Dev 4(6):188–196. https://doi.org/10.5539/jsd.v4n6p188
Esson KC, Lin X, Kumaresan D, Chanton JP, Murrell JC, Kostka JE (2016) Alpha- and Gammaproteobacterial Methanotrophs Codominate the Active Methane-Oxidizing Communities in an Acidic Boreal Peat Bog. Appl Environ Microbiol 82(8):2363–2371. https://doi.org/10.1128/aem.03640-15
Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MMM, Schreiber F, Dutilh BE, Zedelius J, de Beer D, Gloerich J, Wessels HJCT, van Alen T, Luesken F, Wu ML, van de Pas-Schoonen KT, den Op HJM, Janssen-Megens EM, Francoijs K-J, Stunnenberg H, Weissenbach J, Jetten MSM, Strous M (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548. https://doi.org/10.1038/nature08883
Ettwig KF, Zhu B, Speth D, Keltjens JT, Jetten MSM, Kartal B (2016) Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc Natl Acad Sci USA 113:12792–12796. https://doi.org/10.1073/pnas.1609534113
Fedorov DN, Doronina NV, Trotsenko YA (2011) Phytosymbiosis of aerobic methylobacteria: new facts and views. Microbiology 80:443–454. https://doi.org/10.1134/s0026261711040047
Fei Q, Guarnieri MT, Tao L, Laurens LM, Dowe N, Pienkos PT (2014) Bioconversion of natural gas to liquid fuel: opportunities and challenges. Biotechnol Adv 32(3):596–614. https://doi.org/10.1016/j.biotechadv.2014.03.011
Fukao T, Xu K, Ronald PC, Serres JB (2006) A variable cluster of ethylene response factor like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18:2021–2034
Gaihre YK, Padre A, Wassmann R, Aquino E, Villegas-Pangga G, Sta-Cruz PC (2011) Spatial and temporal variations in methane fluxes from irrigated lowland rice fields. Philipp Agric Sci 94:335–342
Gaihre YK, Singh U, Bible WD, Fugice J Jr, Sanabria J (2020) Mitigating N2O and NO emissions from direct-seeded rice with nitrification inhibitor and urea deep placement. Rice Sci 27(5):434–444. https://doi.org/10.1016/j.rsci.2020.03.005
Ghashghavi M, Jetten MSM, Lüke C (2017) Survey of methanotrophic diversity in various ecosystems by degenerate methane monooxygenase gene primers. AMB Express 7:162. https://doi.org/10.1186/s13568-017-0466-2
Gholamalizadeh R, Khodakaramian G, Ebadi AA (2017) Assessment of rice associated bacterial ability to enhance rice seed germination and rice growth promotion. Braz Arch Biol Technol 60:1–13. https://doi.org/10.1590/1678-4324-2017160410
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica. https://doi.org/10.6064/2012/963401
Gogoi N, Baruah KK, Gupta PK (2008) Selection of rice genotypes for lower methane emission. Agron Sustain Dev 28:181–186. https://doi.org/10.1051/agro:2008005
Govorukhina NI, Trotsenko YA (1991) Methylovorus, a new genus of restricted facultatively methylotrophic bacteria. Int J Syst Bacteriol 41(1):158–162. https://doi.org/10.1099/00207713-41-1-158
Grossi CEM, Fantino E, Serral F, Zawoznik MS, Fernandez DPDA, Ulloa RM (2020) Methylobacterium sp. 2A is a plant growth-promoting rhizobacteria that has the potential to improve potato crop yield under adverse conditions. Front Plant Sci 11:71. https://doi.org/10.3389/fpls.2020.00071
Grover M, Ali SZ, Sandhya V, Rasul A, Venkateswarlu B (2011) Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J Microbiol Biotechnol 27(5):1231–1240. https://doi.org/10.1007/s11274-010-0572-7
Guerrero-Cruz S, Stultiens K, van Kessel MAHJ, Versantvoort W, Jetten MSM, Op den Camp HJM, Kartal B (2019) Key Physiology of a Nitrite-Dependent Methane-Oxidizing Enrichment Culture. Appl Environ Microbiol 85(8):e00124–e00119. https://doi.org/10.1128/aem.00124-19
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471. https://doi.org/10.1128/mr.60.2.439-471.1996
Haroon MF, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan Z, Tyson GW (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500(7464):567–570. https://doi.org/10.1038/nature12375
Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, Matsuoka M (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460:1026–1030. https://doi.org/10.1038/nature08258
Hill EA, Chrisler WB, Beliaev AS, Bernstein HC (2017) A flexible microbial co-culture platform for simultaneous utilization of methane and carbon dioxide from gas feedstocks. Bioresour Technol 228:250–256. https://doi.org/10.1016/j.biortech.2016.12.111
Ho A, De Roy K, Thas O, De Neve J, Hoefman S, Vandamme P, Heylen K, Boon N (2014) The more, the merrier: heterotroph richness stimulates methanotrophic activity. ISME J 8:1945–1948. https://doi.org/10.1038/ismej.2014.74
Ho A, El-Hawwary A, Sang YK, Meima-Franke M, Bodelier P (2015) Manure-associated stimulation of soil-borne methanogenic activity in agricultural soils. Biol Fertil Soils 51:1–6. https://doi.org/10.1007/s00374-015-0995-2
Hogendoorn C, Picone N, van Hout F, Vijverberg S, Poghosyan L, van Alen TA, Frank J, Pol A, Gagliano AL, Jetten MS, D’Alessandro W (2021) Draft genome of a novel methanotrophic Methylobacter sp. from the volcanic soils of Pantelleria Island. Antonie Van Leeuwenhoek 114(3):313–324. https://doi.org/10.1007/s10482-021-01525-7
Holzapfel-Pschorn A, Conrad R, Seiler W (1986) Effects of vegetation on the emission of methane from submerged paddy soil. Plant Soil 92:223–233. https://doi.org/10.1007/bf02372636
Hooper AB, Vannelli T, Bergmann DJ, Arciero D (1997) Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie van Leeuwenhoek 71:59–67. https://doi.org/10.1023/A:1000133919203
Hossain M, Sultana F, Islam S (2017) Plant growth-promoting fungi (PGPF): phytostimulation and induced systemic resistance. Plant Microbe Interact Agro-Ecol Perspect. https://doi.org/10.1007/978-981-10-6593-4_6
Iguchi H, Yurimoto H, Sakai Y (2011) Stimulation of methanotrophic growth in cocultures by cobalamin excreted by rhizobia. Appl Environ Microbiol 77:8509–8515. https://doi.org/10.1128/aem.05834-11
Iguchi H, Izuru S, Maiko S, Hiroya Y, Yasuyoshi S (2012) Distribution of methanotrophs in the phyllosphere. Biosci Biotechnol Biochem 76(8):1580–1583. https://doi.org/10.1271/bbb.120281
Islam MR, Siddique IA, Ali MH, Islam MR, Mahmud AA (2019) Rice genotypic variation in methane emission patterns underirrigated culture. Fundam Appl Agric 4(1):693–703
Islam T, Gessesse A, Garcia-Moyano A, Murrell JC, Ovreas L (2020) A Novel Moderately Thermophilic Type Ib Methanotroph Isolated from an Alkaline Thermal Spring in the Ethiopian Rift Valley. Microorganisms 8(2):250. https://doi.org/10.3390/microorganisms8020250
Ivanova EG, Doronina NV, Trotsenko YA (2001) Aerobic methylobacteria are capable of synthesizing auxins. Microbiology 70:392–397
Jain N, Pathak H, Mitra S, Bhatia A (2004) Emission of methane from rice fields—a review. J Sci Ind Res 63:101–115
Jhala YK, Vyas RV, Shelat HN, Patel HK, Patel KT (2014) Isolation and characterization of methane utilizing bacteria from wetland paddy ecosystem. World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-014-1606-3
Ji Y, Mao G, Wang Y, Bartlam M (2013) Structural insights into diversity and n-alkane biodegradation mechanisms of alkane hydroxylases. Front Microbiol 4:58. https://doi.org/10.3389/fmicb.2013.00058
Jia ZJ, Cai ZC, Tsuruta H (2006) Effect of rice cultivar on CH4 production potential of rice soil and CH4 emission in a pot experiment. Soil Sci Plant Nutr 52:341–348. https://doi.org/10.1111/j.1747-0765.2006.00043.x
Jorge GL, Kisiala A, Morrison E, Aoki M, Paula A, Nogueira O, Emery RJN (2019) Endosymbiotic Methylobacterium oryzae mitigates the impact of limited water availability in lentil (Lens culinaris Medik) by increasing plant cytokinin levels. Environ Exp Bot 162:525–540. https://doi.org/10.1016/j.envexpbot.2019.03.028
Karhu K, Mattila T, Bergstrom I, Regina K (2011) Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—results from a short-term pilot field study. Agric Ecosyst Environ 140:309–313. https://doi.org/10.1016/j.agee.2010.12.005
Keltjens JT, Pol A, Reimann J, Op den Camp HJ (2014) PQQ-dependent methanol dehydrogenases: rare-earth elements make a difference. Appl Microbiol Biotechnol 98(14):6163–6183. https://doi.org/10.1007/s00253-014-5766-8
Khmelenina VN, Kalyuzhnaya MG, Starostina NG, Suzina NE, Trotsenko YA (1997) Isolation and characterization of halotolerant alkaliphilic methanotrophic bacteria from Tuva soda lakes. Curr Microbiol 35(5):257–261. https://doi.org/10.1007/s002849900249
Khosa MK, Sidhu BS, Benbi DK (2011) Methane emission from rice fields in relation to management of irrigation water. J Environ Biol 32(2):169–172
Kim SY, Pramanik P, Bodelier PLE, Kim PJ (2014) Cattle manure enhances methanogens diversity and methane emissions compared to swine manure under rice paddy. PLoS ONE 9(12):e113593. https://doi.org/10.1371/journal.pone.0113593
Kim J, Yoo G, Kim D, Ding W, Kang H (2017) Combined application of biochar and slow-release fertilizer reduces methane emission but enhances rice yield by different mechanisms. Appl Soil Ecol 117–118:57–62. https://doi.org/10.1016/j.apsoil.2017.05.006
Kim IT, Ahn KH, Lee YE, Jeong Y, Park JR, Shin DC, Jung J (2021) An Experimental Study on the Biological Fixation and Effective Use of Carbon Using Biogas and Bacterial Community Dominated by Methanotrophs, Methanol-Oxidizing Bacteria, and Ammonia-Oxidizing Bacteria. Catalysts 11(11):1342. https://doi.org/10.3390/catal11111342
Kirschke S, Bousquet P, Ciais P, Saunois M, Canadell JG, Dlugokencky EJ, Bergamaschi P, Bergmann D, Blake DR, Bruhwiler L, Cameron-Smith P (2013) Three decades of global methane sources and sinks. Nat Geosci 6:813–823
Kluber HD, Conrad R (1998) Effects of nitrate, nitrite, NO and N2O on methanogenesis and other redox processes in anoxic rice field soil. FEMS Microbiol Ecol 25(3):301–318. https://doi.org/10.1016/s0168-6496(98)00011-7
Kolb S, Horn MA (2012) Microbial CH4 and N2O consumption in acidic wetlands. Front Microbiol 3:78. https://doi.org/10.3389/fmicb.2012.00078
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55(1):485–529. https://doi.org/10.1146/annurev.micro.55.1.485
Kravchenko I, Sukhacheva M (2017) Methane oxidation and diversity of aerobic methanotrophs in forest and agricultural soddy–podzolic soils. Appl Soil Ecol 119:267–274. https://doi.org/10.1016/j.apsoil.2017.06.034
Kruistum H, Bodelier PL, Ho A, Meima-Franke M, Veraart AJ (2018) Resistance and Recovery of Methane-Oxidizing Communities Depends on Stress Regime and History; A Microcosm Study. Front Microbiol 9:1714. https://doi.org/10.3389/fmicb.2018.01714
Kumar S, Vikram S, Raghava GPS (2012) Genome sequence of the nitroaromatic compound- degrading bacterium Burkholderia sp. Strain SJ98. J Bacteriol 194:3286. https://doi.org/10.1128/jb.00497-12
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14 C labelling. Soil Biol Biochem 41:210–219. https://doi.org/10.1016/j.soilbio.2008.10.016
Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol 37:25–50. https://doi.org/10.1016/s1164-5563(01)01067-6
Lee HJ, Jeong SE, Kim PJ, Madsen E, Jeon CO (2015) High resolution depth distribution of Bacteria, Archaea, methanotrophs, and methanogens in the bulk and rhizosphere soils of a flooded rice paddy. Front Microbiol 6:639. https://doi.org/10.3389/fmicb.2015.00639
Lidstrom ME, Chistoserdova L (2002) Plants in the Pink: Cytokinin Production by Methylobacterium. J Bacteriol 184(7):1818. https://doi.org/10.1128/jb.184.7.1818.2002
Liechty Z, Santos-Medellín C, Edwards J, Nguyen B, Mikhail D, Eason S, Phillip G, Sundaresan V (2020) Comparative Analysis of Root Microbiomes of Rice Cultivars with High and Low Methane Emissions Reveals Differences in Abundance of Methanogenic Archaea and Putative Upstream Fermenters. Msystems 5(1):1–20. https://doi.org/10.1128/msystems.00897-19
Lindau CW, Delaune RD, Patrick WH, Bolloch PK (1990) Fertilizer effects on dinitrogen, nitrous oxide, and methane emissions from lowland rice. Soil Sci Soc Am J 54(6):1789–1794. https://doi.org/10.2136/sssaj1990.03615995005400060048x
Linquist BA, Adviento-Borbe MA, Pittelkow CM, van Kessel C, van Groenigen KJ (2012) Fertilizer management practices and greenhouse gas emissions from rice systems: a quantitative review and analysis. Field Crops Res 135:10–21. https://doi.org/10.1016/j.fcr.2012.06.007
Liu LL, Greaver TL (2009) A review of nitrogen enrichment effects on three biogenic GHGs: The CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol Lett 12:1103–1117. https://doi.org/10.1111/j.1461-0248.2009.01351.x
Lu Y, Wassmann R, Neue HU, Huang C, Bueno CS (2000) Methanogenic responses to exogenous substrates in anaerobic rice soils. Soil Biol Biochem 32:1683–1690. https://doi.org/10.1016/s0038-0717(00)00085-7
Ly P, Vu QD, Jensen LS, Pandey A, Neergaard A (2015) Effects of rice straw, biochar and mineral fertiliser on methane (CH4) and nitrous oxide (N2O) emissions from rice (Oryza sativa L.) grown in a rain-fed lowland rice soil of Cambodia: a pot experiment. Paddy Water Environ 13:465–475. https://doi.org/10.1007/s10333-014-0464-9
Ma K, Qiu Q, Lu Y (2009) Microbial mechanism for rice variety control on methane emission from rice field soil. Glob Change Biol 16(11):3085–3095. https://doi.org/10.1111/j.1365-2486.2009.02145.x
Ma K, Conrad R, Lu Y (2013) Dry/Wet Cycles Change the Activity and Population Dynamics of Methanotrophs in Rice Field Soil. Appl Environ Microbiol 79(16):4932–4939. https://doi.org/10.1128/aem.00850-13
Macalady JL, McMillan AMS, Dickens AF, Tyler SC, Scow KM (2002) Population dynamics of type I and II methanotrophic bacteria in rice soils. Environ Microbiol 4(3):148–157. https://doi.org/10.1046/j.1462-2920.2002.00278.x
Madhaiyan M, Poonguzhali S, Kwon ASAT (2009) Methylophilus rhizosphaerae sp. nov., a restricted facultative methylotroph isolated from rice rhizosphere soil. Int J Syst Evol MicroBiol 59:2904–2908. https://doi.org/10.1099/ijs.0.009811-0
Malyan SK, Bhatia A, Kumar A, Gupta DK, Singh R, Kumar SS, Tomer R, Kumar O, Jain N (2016) Methane production, oxidation and mitigation: a mechanistic understanding and comprehensive evaluation of influencing factors. Sci Total Environ 572:874–896. https://doi.org/10.1016/j.scitotenv.2016.07.182
Malyan SK, Bhatia A, Tomer R, Harit RC, Jain N, Bhowmik A, Kaushik R (2021) Mitigation of yield-scaled greenhouse gas emissions from irrigated rice through Azolla, Blue-green algae, and plant growth–promoting bacteria. Environ Sci Pollut Res 28(37):51425–51439. https://doi.org/10.1007/s11356-021-14210-z
Mancinelli RL (1995) The regulation of methane oxidation in soil. Annu Rev Microbiol 49(1):581–605. https://doi.org/10.1146/annurev.mi.49.100195.003053
Manjunath M, Prasanna R, Sharma P, Nain L, Singh R (2011) Developing PGPR consortia using novel genera Providencia and Alcaligenes along with cyanobacteria for wheat. Arch Agron Soil Sci 57(8):873–887. https://doi.org/10.1080/03650340.2010.499902
Miao X, Xing X, Ding Y, Ke J, Liu Z, Tang S, Ding C, Wang S, Li G (2016) Yield and nitrogen uptake of bowl-seedling machine-transplanted rice with slow-release nitrogen fertilizer. Agron J 108:313–320. https://doi.org/10.2134/agronj2015.0101
Mostovaya A, Wind-Hansen M, Rousteau P, Bristow LA, Thamdrup B (2022) Sulfate‐and iron‐dependent anaerobic methane oxidation occurring side‐by‐side in freshwater lake sediment. Limnol Oceanogr 67(1):231–246. https://doi.org/10.1002/lno.11988
Murrell JC, Dalton H (1983) Nitrogen fixation in obligate methanotrophs. J Gen Microbiol 129:3481–3486. https://doi.org/10.1099/00221287-129-11-3481
Naitam MG, Kaushik R (2021) Archaea: An Agro-Ecological Perspective. Curr Microbiol 78(7):2510–2521. https://doi.org/10.1007/s00284-021-02537-2
Nakatsu CH, Hristova K, Hanada S, Meng X, Hanson JR, Scow KM, Kamagata Y (2006) Methylibium petroleiphilum gen. nov., sp. nov., a novel methyl tert-butyl ether-degrading methylotroph of the Betaproteobacteria. Int J Syst Evol MicroBiol 56:983–989. https://doi.org/10.1099/ijs.0.63524-0
Nguyen NL, Yu WJ, Gwak JH, Kim SJ, Park SJ, Herbold CW, Kim JG, Jung MY, Rhee SK (2018) Genomic insights into the acid adaptation of novel methanotrophs enriched from acidic forest soils. Front Microbiol. https://doi.org/10.3389/fmicb.2018.01982
Nielsen AK, Gerdes K, Murrell JC (1997) Copper-dependent reciprocal transcriptional regulation of methane oxidation genes in Methylococcus capsulatus Bath and Methylosinus trichosporium OB3b. Mol Microbiol 25:399–409. https://doi.org/10.1046/j.1365-2958.1997.4801846.x
Nontji M, Amran FD (2019) Potential of Indigenous Methanotrophic Bacteria as a Biological Control Agent Against Xanthomonas oryzae pv. oryzae Causing Diseases on Rice. Makara J Sci 23/2:87–90. https://doi.org/10.7454/mss.v23i2.9053
Nontji M, Patenjengi B, Rasyid B, Pirman P (2016) Analysis of Potential Reduce Methane Gas Emission by Methanotrophs Bacteria from Rice Field in Gowa. Mod Appl Sci 10(7):183–187. https://doi.org/10.5539/mas.v10n7p183
Oakley CJ, Murrell JC (1988) nifH genes in the obligate methane oxidizing bacteria. FEMS Micriobiol Lett 49:53–57. https://doi.org/10.1111/j.1574-6968.1988.tb02681.x
Oda M, Nguyen HC (2019) Methane emissions in triple rice cropping: patterns and a method for reduction. F1000Research. https://doi.org/10.12688/f1000research.20046.2
Ojha SK, Benjamin JC, Singh AK (2018) Role on biofertilizer (Blue green algae) in paddy crop. J Pharmacogn Phytochem 7(4):830–832
Olanrewaju OS, Ayangbenro AS, Glick BR, Babalola OO (2019) Plant health: feedback effect of root exudates-rhizobiome interactions. Appl Microbiol Biotechnol 103(3):1155–1166. https://doi.org/10.1007/s00253-018-9556-6
Pennock D, Yates T, Bedard-Haughn A, Phipps K, Farrell R, McDougal R (2010) Landscape controls on N2O and CH4 emissions from freshwater mineral soil wetlands of the Canadian Prairie Pothole region. Geoderma 155:308–319. https://doi.org/10.1016/j.geoderma.2009.12.015
Pingak GMF, Sutanto H, Akhdiya A, Rusmana I (2014) Effectivity of Methanotrophic Bacteria and Ochrobactrum Anthropi as Biofertilizer and Emission Reducer of CH4 and N2O in Inorganic Paddy Fields. J Med Bioeng 3(3):217–221. https://doi.org/10.12720/jomb.3.3.217-221
Prasanna R, Kumar V, Kumar S, Yadav AK, Tripathi U, Singh AK, Jain MC, Gupta P, Singh PK, Sethunathan N (2002) Methane production in rice soil is inhibited by cyanobacteria. Microbiol Res 157(1):1–6. https://doi.org/10.1078/0944-5013-00124
Qin X, Li Y, Wang H, Li J, Wan Y, Gao Q, Liao Y, Fan M (2015) Effect of rice cultivars on yield-scaled methane emissions in a double rice field in South China. J Integr Environ Sci 12(1):47–66. https://doi.org/10.1080/1943815x.2015.1118388
Rahalkar MC, Khatri K, Pandit P, Bahulikar R, Mohite J (2021) Cultivation of important methanotrophs from Indian rice fields. Front Microbiol 3:2492. https://doi.org/10.3389/fmicb.2021.669244
Rani V, Bhatia A, Nain L, Kaushik R (2020) Flooded Paddy Ecosystem Harbors Methanol Oxidizing-Plant Growth Promoting Bacteria Belonging to Order Enterobacterales. Int J Curr Microbiol Appl Sci 9(7):685–696. https://doi.org/10.20546/ijcmas.2020.907.079
Rani V, Bhatia A, Kaushik R (2021a) Inoculation of plant growth promoting-methane utilizing bacteria in different N-fertilizer regime influences methane emission and crop growth of flooded paddy. Sci Total Environ 775:145826. https://doi.org/10.1016/j.scitotenv.2021.145826
Rani V, Bhatia A, Kaushik R (2021b) Methane oxidizing-plant growth-promoting yeast isolated from Indian rice fields. Indian J Agric Sci 91(3):369–373
Rani V, Bhatia A, Nain L, Tomar GS, Kaushik R (2021c) Methane utilizing plant growth-promoting microbial diversity analysis of flooded paddy ecosystem of India. World J Microbiol Biotechnol 37(4):1–22. https://doi.org/10.1007/s11274-021-03018-1
Rani V, Kaushik R, Majumder S, Rani AT, Devi AA, Divekar P, Khati P, Pandey KK, Sing J (2021d) Synergistic Interaction of Methanotrophs and Methylotrophs in Regulating Methane Emission. Microbial Technology for Sustainable Environment. Springer, Singapore, pp 419–437. https://doi.org/10.1007/978-981-16-3840-4_22
Reddy KR, Rai RK, Green SJ, Chetri JK (2020) Effect of pH on methane oxidation and community composition in landfill cover soil. J Environ Eng 146(6):04020037. https://doi.org/10.1061/(asce)ee.1943-7870.0001712
Ross MO, Rosenzweig AC (2017) A tale of two methane monooxygenases. J Biol Inorg Chem 22(2):307–319. https://doi.org/10.1007/s00775-016-1419-y
Ruiz-Ruiz P, Gomez-Borraz TL, Revah S, Morales M (2020) Methanotroph-microalgae co-culture for greenhouse gas mitigation: Effect of initial biomass ratio and methane concentration. Chemosphere 259:127418. https://doi.org/10.1016/j.chemosphere.2020.127418
Sadasivam BY, Reddy KR (2014) Landfill methane oxidation in soil and bio-based cover systems: a review. Rev Environ Sci Biotechnol 13:79–107. https://doi.org/10.1007/s11157-013-9325-z
Sahoo KK, Goswami G, Das D (2021) Biotransformation of methane and carbon dioxide into high-value products by methanotrophs: current state of art and future prospects. Front Microbiol 12:520
Schnell S, King GM (1994) Mechanistic analysis of ammonium inhibition of atmospheric methane consumption in forest soil. Appl Environ Microbiol 60:3514–3521. https://doi.org/10.1128/aem.60.10.3514-3521.1994
Segers R (1998) Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry 41:23–51
Sepehri A, Sarrafzadeh MH (2019) Activity enhancement of ammonia-oxidizing bacteria and nitrite-oxidizing bacteria in activated sludge process: metabolite reduction and CO2 mitigation intensification process. Appl Water Sci 9:131. https://doi.org/10.1007/s13201-019-1017-6
Shiau YJ, Lin CW, Cai Y, Jia Z, Lin YT, Chiu CY (2020) bNiche differentiation of active methane-oxidizing bacteria in estuarine mangrove forest soils in Taiwan. Microorganisms 8(8):1248. https://doi.org/10.3390/microorganisms8081248
Siddikee MA, Hamayun M, Han G, Sa T (2010) Optimization of gibberellic acid production by Methylobacterium oryzae CBMB20. Korean J Soil Sci Fertil 43(4):522–527
Singh R, Ryu J, Kim SW (2019) Microbial consortia including methanotrophs: some benefits of living together. J Microbiol 57:939–952. https://doi.org/10.1007/s12275-019-9328-8
Sohngen NL (1906) Ueber Bakterien, welche Methan als Kohlenstoffnahrung und Energiequelle gebrauchen. Centralbl Bakteriol Parasitenk Infektionskr Hyg Abt II 15:513–517
Steffens B, Geske T, Sauter M (2010) Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol 190(2):369–378. https://doi.org/10.1111/j.1469-8137.2010.03496.x
Stock M, Hoefman S, Kerckhof FM, Boon N, De Vos P, De Baets B, Heylen K, Waegeman W (2013) Exploration and prediction of interactions between methanotrophs and heterotrophs. Res Microbiol 164(10):1045–1054. https://doi.org/10.1016/j.resmic.2013.08.006
Su J, Hu C, Yan X, Jin Y, Chen Z, Guan Q, Wang Y, Zhong D, Jansson C, Wang F, Schnürer A (2015) Expression of barley SUSIBA2 transcription factor yields high-starch low-methane rice. Nature 523:602–606. https://doi.org/10.1038/nature14673
Sukmawati D, Rusmana I, Mubarik NR (2016) The effectiveness of methanotrophic bacteria and Ochrobactrum anthropi to reduce CH4 and N2O emissions and to promote paddy growth in lowland paddy fields. Malays J Microbiol 12(1):50–55. https://doi.org/10.21161/mjm.75215
Sutanto H, Rusmana I, Mubarik NR (2014) Community succession of methanotrophic bacteria based on pmoA gene in rice fields. Adv Environ Biology 8(14):50–56
Szafranek-Nakonieczna A, Wolińska A, Zielenkiewicz U, Kowalczyk A, Stępniewska Z Błaszczyk M (2019) Activity and identification of methanotrophic bacteria in arable and no-tillage soils from Lublin region (Poland). Microb Ecol 77(3):701–712. https://doi.org/10.1007/s00248-018-1248-3
Taopan RA, Rusmana I, Santosa DA (2018) The effect of methanotrophic bacteria application on paddy growth and methane emission in rainfed rice of Kupang Regency, East Nusa Tenggara, Indonesia. Int J Environ Agric Biotechnol 3(5):1759–1764. https://doi.org/10.22161/ijeab/3.5.25
Theisen AR, Ali MH, Radajewski S, Dumont MG, Dunfield PF, McDonald IR, Dedysh SN, Miguez CB, Murrell JC (2005) Regulation of methane oxidation in the facultative methanotroph Methylocella silvestris BL2. Mol Microbiol 58:682–692. https://doi.org/10.1111/j.1365-2958.2005.04861.x
Thirumurugan D, Asha S (2010) Spore forming bacterial biofertilizer for phosphate solubilization and bio-control agent. Sci Acta Xaver 1(2):51–58
Thomas L, Singh I (2019) Microbial biofertilizers: types and applications. Biofertilizers for sustainable agriculture and environment. Springer, Cham
Traore B, Samake F, Babana A, Hang M (2017) Effects of different fertilizers on methane emission from paddy field of Zhejiang, China. Afr J Environ Sci Technol 11(1):89–93. https://doi.org/10.5897/ajest2016.2189
Tyagi L, Kumari B, Singh SN (2009) Water management—a tool for methane mitigation from irrigated paddy fields. Sci Total Environ 408(5):1085–1090. https://doi.org/10.1016/j.scitotenv.2009.09.010
Valentine DL, Reeburg WS (2000) New perspectives on anaerobic methane oxidation. Environ Microbiol 2(5):477–484. https://doi.org/10.1046/j.1462-2920.2000.00135.x
Van Aken B, Peres CM, Doty SL, Yoon JM, Schnoor JL (2004) Methylobacterium populi sp. nov., a novel aerobic, pink-pigmented, facultatively methylotrophic, methane-utilizing bacterium isolated from poplar trees (Populus deltoides x nigra DN34). Int J Syst Evol MicroBiol 54(Pt 4):1191–1196. https://doi.org/10.1099/ijs.0.02796-0
van Teeseling MC, Pol A, Harhangi HR, van der Zwart S, Jetten MS, Op den Camp HJ, van Niftrik L (2014) Expanding the verrucomicrobial methanotrophic world: description of three novel species of Methylacidimicrobium gen. nov. Appl Environ Microbiol 80(21):6782–6791. https://doi.org/10.1128/aem.01838-14
Veraart AJ, Garbeva P, Van Beersum F, Ho A, Hordijk CA, Meima-Franke M, Zweers AJ, Bodelier PLE (2018) Living apart together-bacterial volatiles influence methanotrophic growth and activity. ISME J 12:1163–1166. https://doi.org/10.1038/s41396-018-0055-7
Verbeke TJ, Dedysh SN, Dunfield PF, McGenity T (2019) Methanotrophy in acidic soils, including northern peatlands. Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology. Springer, Berlin. https://doi.org/10.1007/978-3-030-14785-3_6Nature:133 – 56
Versantvoort W, Guerrero-Cruz S, Speth DR, Frank J, Gambelli L, Cremers G, van Alen T, Jetten MSM, Kartal B, Reimann J (2018) Comparative genomics of Candidatus Methylomirabilis species and description of Ca. Methylomirabilis lanthanidiphila. Front Microbiol 9:1672. https://doi.org/10.3389/fmicb.2018.01672
Wagner D, Pfeiffer EM, Bock E (1999) Methane production in aerated marshland and model soils: effects of microflora and soil texture. Soil Biol Biochem 31:999–1006. https://doi.org/10.1016/s0038-0717(99)00011-5
Wang B, Adachi K (2000) Differences among rice cultivars in root exudation, methane oxidation, and populations of methanogenic and methanotrophic bacteria in relation to methane emission. Nutr Cycl Agrosyst 58:349–356. https://doi.org/10.1007/978-94-010-0898-3_30
Wang B, Neue HU, Samonte HP (1997) Effect of cultivar difference (‘IR72’, ‘IR65598’ and ‘Dular’) on methane emission. Agric Ecosyst Environ 62:31–40. https://doi.org/10.1016/s0167-8809(96)01115-2
Wang B, Li Y, Wan Y, Qin X, Gao Q, Liu S, Li J (2016) Modifying nitrogen fertilizer practices can reduce greenhouse gas emissions from a Chinese double rice cropping system. Agric Ecosyst Environ 215:100–109. https://doi.org/10.1016/j.agee.2015.09.008
Wellner S, Lodders N, Kampfer P (2011) Diversity and biogeography of selected phyllosphere bacteria with special emphasis on Methylobacterium spp. Syst Appl Microbiol 34:621–630. https://doi.org/10.1016/j.syapm.2011.08.005
Whalen SC (2000) Influence of N and non-N salts on atmospheric methane oxidation by upland boreal forest and tundra soils. Biol Fertil Soils 31:279–287. https://doi.org/10.1007/s003740050657
Whittenbury R, Dalton H (1981) The methylotrophic bacteria. In: Starr MP, Stolph H, Tru¨per HG, Balows A, Schlegel HG (eds) The prokaryotes. Springer-Verlag KG, Berlin, pp 894–902. https://doi.org/10.1007/978-3-662-13187-9_71
Whittenbury R, Phillips KC, Wilkinson JG (1970) Enrichment, isolation and some properties of methane utilizing bacteria. J Gen Microbiol 61:205–218. https://doi.org/10.1099/00221287-61-2-205
Xu ZJ, Zheng XH, Wang YS, Han SH, Huang Y, Zhu JG (2004) Effects of elevated CO2 and N fertilization on CH4 emissions from paddy rice fields. Glob Biogeochem Cycles 18(3):GB3009. https://doi.org/10.1029/2004gb002233
Xu H, Zhu B, Liu J, Li D, Yang Y, Zhang K, Jiang Y, Hu Y, Zeng Z (2017) Azolla planting reduces methane emission and nitrogen fertilizer application in double rice cropping system in southern China. Agron Sustain Dev. https://doi.org/10.1007/s13593-017-0440-z
Yan XY, Yagi K, Akiyama H, Akimoto H (2005) Statistical analysis of the major variables controlling methane emission from rice fields. Glob Change Biol 11:1131–1141. https://doi.org/10.1111/j.1365-2486.2005.00976.x
Yang X, Shang Q, Wu P, Liu J, Shen Q, Guo S, Xiong Z (2010) Methane emissions from double rice agriculture under long-term fertilizing systems in Hunan, China. Agric Ecosyst Environ 137:308–316. https://doi.org/10.1016/j.agee.2010.03.001
Yang HL, Liao YY, Zhang J, Wang XL (2019) Comparative transcriptome analysis of salt tolerance mechanism of Meyerozyma guilliermondii W2 under NaCl stress. 3 Biotech 9(7):286. https://doi.org/10.1007/s13205-019-1817-2
Yim W, Seshadri S, Kim K, Lee G, Sa T (2013) Ethylene emission and PR protein synthesis in ACC deaminase producing Methylobacterium spp. inoculated tomato plants (Lycopersicon esculentum Mill.) challenged with Ralstonia solanacearum under greenhouse conditions. Plant Physiol Biochem 67:95–104. https://doi.org/10.1016/j.plaphy.2013.03.002
Zani S, Mellon MT, Collier JL, Zehr JP (2000) Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected by reverse transcriptase PCR. Appl Environ Microbiol 66:3119–3124. https://doi.org/10.1128/aem.66.7.3119-3124.2000
Zhang W, Sheng R, Zhang M, Xiong G, Hou H, Li S, Wei W (2018) Effects of continuous manure application on methanogenic and methanotrophic communities and methane production potentials in rice paddy soil. Agric Ecosyst Environ 258:121–128. https://doi.org/10.1016/j.agee.2018.02.018
Zheng Y, Huang R, Wang BZ, Bodelier PL, Jia ZJ (2014) Competitive interactions between methane-and ammonia-oxidizing bacteria modulate carbon and nitrogen cycling in paddy soil. Biogeosciences 11(12):3353–3368. https://doi.org/10.5194/bg-11-3353-2014
Acknowledgements
We acknowledge the Indian Council of Agricultural Research (ICAR) funded project National Innovations on Climate Resilient Agriculture (NICRA) for sponsoring the research related to the research findings reported in the review.
Funding
We acknowledge the Indian Council of Agricultural Research (ICAR) funded project on “National Innovations on Climate Resilient Agriculture” for sponsoring this research.We also acknowledge the University Grant Commission, India for providing Senior Research Fellowship to the research scholar.
Author information
Authors and Affiliations
Contributions
VR collected the literature, assisted with the writing, and drew the figures for the manuscript. RP edited the manuscript and helped write the section of the review that highlights the importance of co-inoculating cyanobacteria and methane-utilizing bacteria. RK conceptualized the idea of writing the review and wrote and edited the manuscript and figures.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this review paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rani, V., Prasanna, R. & Kaushik, R. Prospecting the significance of methane-utilizing bacteria in agriculture. World J Microbiol Biotechnol 38, 176 (2022). https://doi.org/10.1007/s11274-022-03331-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03331-3