Abstract
Irrigated transplanted flooded rice is a major source of methane (CH4) emission. We carried out experiments for 2 years in irrigated flooded rice to study if interventions like methane-utilizing bacteria, Blue-green algae (BGA), and Azolla could mitigate the emission of CH4 and nitrous oxide (N2O) and lower the yield-scaled global warming potential (GWP). The experiment included nine treatments: T1 (120 kg N ha−1 urea), T2 (90 kg N ha−1 urea + 30 kg N ha−1 fresh Azolla), T3 (90 kg N ha−1 urea + 30 kg N ha−1 Blue-green algae (BGA), T4 (60 kg N ha−1 urea + 30 kg N ha−1 BGA + 30 kg N ha−1 Azolla, T5 (120 kg N ha−1 urea + Hyphomicrobium facile MaAL69), T6 (120 kg N ha−1 by urea + Burkholderia vietnamiensis AAAr40), T7 (120 kg N ha−1 by urea + Methylobacteruim oryzae MNL7), T8 (120 kg N ha−1 urea + combination of Burkholderia AAAr40, Hyphomicrobium facile MaAL69, Methylobacteruim oryzae MNL7), and T9 (no N fertilizer). Maximum decrease in cumulative CH4 emission was observed with the application of Methylobacteruim oryzae MNL7 in T7 (19.9%), followed by Azolla + BGA in T4 (13.2%) as compared to T1 control. N2O emissions were not significantly affected by the application of CH4-oxidizing bacteria. However, significantly lower (P<0.01) cumulative N2O emissions was observed in T4 (40.7%) among the fertilized treatments. Highest yields were observed in Azolla treatment T2 with 25% less urea N application. The reduction in yield-scaled GWP was at par in T4 (Azolla and BGA) and T7 (Methylobacteruim oryzae MNL7) treatments and reduced by 27.4% and 15.2% in T4 and T7, respectively, as compared to the T1 (control). K-means clustering analysis showed that the application of Methylobacteruim oryzae MNL7, Azolla, and Azolla + BGA can be an effective mitigation option to reduce the global warming potential while increasing the yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate change is undoubtedly a result of the enhanced greenhouse effect. IPCC (2014) reported that anthropogenic greenhouse gas (GHG) emission reached 49 Gigatons of CO2 equivalent in 2010 at the global level. Agriculture is a source of anthropogenic emission of two of the major GHGs methane (CH4) and nitrous oxide (N2O) to the atmosphere. Emission from agriculture has been increasing with time due to increased requirement for feeding more than 7.6 billion global population leading to an intensification of farming practices. Anthropogenic emission from agricultural soil occupy over 13% of the total global GHG emission (Zhao et al. 2019) and play a significant role in global warming and climate change. Emission of CH4 and N2O from agriculture are about 47.5% and 72.3%, respectively, of the total emission (Ritchie and Roser 2018). Rice (Oryza sativa L.) is a stable food for more than 50% of the world’s population and rice fields are a major source of CH4 emission from agricultural soils (Shin et al. 2020; Bhatia et al. 2013). Generally, water management and nitrogen fertilizer application govern CH4 and N2O emission from rice. Standing water in lowland and irrigated rice provides suitable anaerobic environment which facilitates the process of methanogenesis by methanogenic bacteria (strictly anaerobic) consuming soil organic matter and liberating CH4 as an end product (Malyan et al. 2016). Higher soil redox (Eh) potential existing in upland rice or aerobic condition during the cropping period favor higher N2O emission (Kumar et al. 2020; Yu et al. 2001). Emission of CH4 and N2O from rice soil are effected by several factors such as soil Eh, pH, temperature, organic matter, fertilizer application, and water management (Bhattacharyya et al. 2019; Yao et al. 2019; Tan et al. 2018; Jain et al. 2016; Hussain et al. 2015).
Mid-season drainage for mitigation of CH4 from submerged rice (Tariq et al. 2017) and N fertilizer management for lowering N2O emission has been widely recommended for rice soils (Aliyu et al. 2021; Malla et al. 2005). This practice though has a limitation, as water management is a difficult task in lowland rice conditions. The farmers growing rice cannot risk draining their flooded fields, as there is no certainty of the next rainfall event and thus this practice is not followed by the farmers. Some chemical and fertilizer interventions such as application of sulfate and nitrate fertilizers for CH4 mitigation have been reported from rice soil (Hussain et al. 2015; Ali et al. 2015), but a few of them may have environmental concerns. The application of nitrate-based fertilizer may be a source of N2O emission through the denitrification pathway and may result in increased leaching of nitrate in frequently irrigated rice. Leaching and runoff of nitrate into ground and surface water, respectively, may impact human health. The application of ammonium sulfate may lead to a potential increase in ammonia volatilization (Choudhaury and Kenneday 2005). Ammonia volatilization is also an indirect potential source of N2O emission (Inubushi et al. 1996) which contributes to global warming.
The application of Azolla-based biofertilizers may have beneficial effects on growth and yield of rice (Kollah et al. 2016; Dubey 2005). Azolla is a floating pteridophyte, occurs in symbiotic association with a nitrogen-fixing cyanobacterium Anabaena azollae (Nostocaceae family), and has been reported to reduce plant nitrogen requirement, but their impact on CH4 and N2O emission has been less reported. Some studies conducted in eastern India (Bharati et al. 2000) and in Southern China (Xu et al. 2017) have shown that the application of Azolla may reduce CH4 emission, whereas opposite results were reported in the studies conducted in Northeastern China (Chen et al. 1997). In addition, Azolla may reduce NH3 volatilization by lowering the pH of floodwater when urea is applied (Liu et al. 2017). Blue green algae (BGA) are photosynthetic nitrogen fixers and are free living. Azolla and BGA cyanobacteria both are oxygen-liberating biofertilizers and can reduce CH4 emission by directly stimulating CH4 oxidation at the soil–water interface and indirectly by promoting CH4 oxidation in flooded paddy soils by increasing soil Eh and thereby inhibiting CH4 production. Their impact on N2O emission has not been reported in literature.
In addition, methane-oxidizing/utilizing bacteria (MOB) prevailing in aerobic zone of paddy ecosystem may utilize CH4 produced by the methanogenic archaea. Methane emission from soil is the net balance of CH4 production by methanogens in anaerobic layer followed by CH4 oxidation by methanotrophs or MOB under aerobic conditions (Conrad 2007). MOB inhabits flooded rice soil due to the presence of CH4 at the soil–water interface and near root hairs as a result of CH4 leakage from root hairs (Aulakh et al. 2000; Dubey and Singh 2001). Methanotrophs have generally been considered to be obligate in nature, i.e., growing only on CH4 as their sole source of carbon and energy. However, facultative methylotrophic organisms also have been found in major clades of microbial life such as gram-negative methylotrophs, belonging to the α, β, and γ subgroups of the proteobacteria, firmicutes, archaea, and yeasts which utilize C1 compounds including CH4 to generate energy (Rani et al. 2021a, b; Iguchi et al. 2015). Methanotrophs and methylotrophs oxidize CH4 to form formaldehyde, which is at the diverging point for further oxidation to CO2 for energy source and assimilation for biosynthesis. The facultative methane- and/or methanol-utilizing bacteria can play a significant role in reducing the net methane flux by utilization of emitted methane at the source level (Rani et al. 2021a, b; Davamani et al. 2020). Previously, we isolated and characterized a large number of facultative methane-utilizing bacteria having plant growth–promoting traits from different rice-growing regions of India (Rani et al. 2021a, b). Among these isolates, the three isolates which showed significant methane utilization potential, i.e., Methylobacterium oryzae MNL7, Hyphomicrobium facile MaAL69, and Burkholderia vietnamiensis AAAr40, were used in the present study as bio-inoculant. Hyphomicrobium facile, an aerobic chemoorganotroph, has been used for denitrification of nitrate in drinking water treatment facilities (Liessens et al. 1993). Fewer reports on utilization of methane-utilizing bacteria as bio-inoculant for reducing methane emission through its oxidation at the source level are available in the literature; however, no significant findings have been reported on the role of different algal and bacterial based interventions in reducing the emissions of CH4 and N2O from the rice ecosystem.
Based on previous studies, we hypothesize that (1) application of biofertilizers alone and in combination may have a differential impact on CH4 and N2O emission and yield-scaled GWP emission and (2) microbial inoculations of methane-utilizing bacteria may result in significant reduction of CH4 emission in submerged rice and on overall yield-scaled GWP emission. To test our hypothesis, a 2-year field study was conducted for quantifying CH4 and N2O emission, rice yield, and yield-scaled GWP under Azolla, BGA, and methane-utilizing bacteria in rice.
Materials and methods
Experimental site
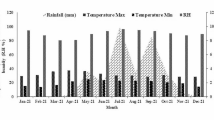
The 2-year study was conducted during Kharif season of 2014 and 2015 at the research farm of Indian Agricultural Research Institute (IARI) (28°40′ N latitude and 77°12′ E longitude), New Delhi, India. The soil of experimental site had 46% sand, 32% silt, and 22% clay and bulk density of 1.39 g cm−3. The initial soil had soil organic carbon of 0.59%, pH (1:2 soil/water) of 8.10, electrical conductivity of 0.43 dS m−1, and CEC of 7.3 C mol (P+) kg−1. The climatic condition of the region is sub-tropical, semi-arid characterized by prolonged hot summer and rainfall occurring during late June to mid-September. Metrological data of the study site for both years are presented in Fig. 1. The average minimum and maximum temperature during the first and second growing season was 21.7 °C; 22.1 °C and 33.8 °C; 34.9 °C, respectively (Fig. 1). The rainfall was higher in the month of July during both years.
Experimental design and treatment details
Twenty-three-day-old seedlings of rice variety Pusa-1509 were transplanted at 20 × 15 cm spacing in the month of July during both cropping years. The experiment consisted of nine treatments in three replications arranged in randomized block design (Table 1). Nitrogen was applied through urea in three split of 50% (basal) and 25% each as two top dressings at tillering and panicle initiation stages. Phosphorus (60 kg P2O5 ha−1) and potassium (40 kg K2O ha−1) was applied as basal dose in all the treatments. The Azolla and commercial formulation blue green algae (BGA) biofertilizer (Anabaena torulosa) for treatments T2, T3, and T4 procured from the Centre for Conservation and Utilization of Blue Green Algae, Indian Agriculture Research Institute, New Delhi, India was applied to the standing water in puddled plots 15 days before transplanting. The methane-utilizing/plant growth–promoting bacterial cultures of Hyphomicrobium facile MaAL69 (NCBI accession no. KY810635), Methylobacterium oryzae MNL7 (NCBI accession no. KY810615), and Burkholderia vietnamiensis AAAr40 (NCBI accession no. KY810624) were obtained from Division of Microbiology, IARI, New Delhi. These cultures were previously isolated from rice rhizosphere and phyllosphere and characterized for methane oxidation potential and plant growth–promoting attributes such as indole acetic acid production, and P, K, and Zn solubilization (Rani et al. 2021a). The liquid culture of the three bacterial isolates was raised individually in ammonium mineral salt medium (Whittenbury et al. 1970) to get a population density of 108 cells/mL. The liquid culture of each of the bacterial isolate was applied alone (T5, T6, and T7) and in combination (T8) (having each culture in 1:1:1 ratio) during nursery preparation through seed treatment, at the time of transplanting by root dip, and spray inoculated at the tillering and panicle initiation stage as per methodology described by Rani et al. (2021b). Then 150 mL of liquid formulation of these cultures was diluted to 1 L with irrigation water for root dip treatment for 2 h before transplanting. In the standing crop, the culture broth of these microbes was mixed with water at 20% and was sprayed two times during the crop period (2 days before second and third split of urea application) for maintaining the population of the CH4-utilizing bacteria. The water level of 6 ± 4 cm was maintained by irrigation during rice growth period. The field was allowed to get dry naturally about 15–20 days before rice harvesting. No pesticide and herbicide was applied to avoid any additional effects. Number of panicles and leaf area index was quantified at flowering stage and the grain yield and test weight (average weight of 1000 grains of rice) were recorded at harvest.
Greenhouse gas sampling and analysis
Air sampling for determination soil CH4 and N2O fluxes was carried out using static-closed chamber technique (Bhatia et al. 2011). The air sampling was performed between 8:30 am and 11:30 am once a week throughout the crop season except after the three events of urea fertilization when air sampling was performed four times a week. Gas samples were collected from the top of the static closed chamber using 50 mL air-tight syringes at 0, 1/2, and 1 h. Temporal increases of the CH4 and N2O concentration in the air within the close chamber represented CH4 and N2O fluxes (Pathak et al. 2002, 2003). Concentration of CH4 and N2O gases in the collected gas samples were measured by using gas chromatograph equipped with a flame ionization detector and electron capture detector, respectively. Nitrogen was used as carrier gas and hydrogen and air were used for igniting the flame for analysis. Emission of CH4 and N2O from soil was calculated from the increase in CH4/N2O concentrations per unit surface area of the chamber within a specific time interval by the following equation:
where F is the CH4/N2O flux (mg CH4 m−2 h−1/μg N2O m−2 h−1), ρ is the gas density, V is the volume of the close chamber (m3), “A” is the surface area of the closed chamber (m2), Δc/Δt is the rate of increase of CH4/N2O gas concentration in the chamber (mg/μg m−3 h−1), and T (absolute temperature) is calculated as 273 + mean temperature in (°C) of the chamber. Total CH4/N2O flux for the entire cultivation period was computed by linear interpolation (Bhatia et al. 2012) using the following equation:
where Ri was the CH4/N2O emission flux (g m−2 day−1) on the ith sampling interval, Di is the number of days in the ith sampling interval, and n is the number of sampling intervals.
Global warming potential (GWP) and yield-scaled GWP
Global warming potential (GWP) is the quantification of warming potential of a mole of trace gas released into the atmosphere relative to a mole of CO2 as a standard gas. GWP of CH4 is 21 and that of N2O oxide is 310 on a 100-years time horizon (Gupta et al. 2016; Bhatia et al. 2005). The GWP and the yield-scaled GWP that is carbon emitted per unit of grain yield of rice was estimated using the following equations:
Soil redox potential and dissolved oxygen
Soil redox potential (Eh) and dissolved oxygen (DO) were measured weekly at regular intervals by a multi-parameter portable ORP meter (CONTECH-Cor-1) during the cropping period.
Statistical analysis
Statistical analysis of the experimental data was performed using SPSS (16.0, USA). ANOVA was carried out to check if the variations between the means were statistically significant. When the ANOVA was found significant at 5% level of significance and the error variances were homogeneous, we followed it up with Tukey’s post hoc test to compare which treatment means were significantly different.
We carried out non-hierarchical cluster analysis using the k-means clustering algorithm using the data. For determining the optimal number of clusters, average silhouette method was used which determines how well each object lies within its cluster. A high average silhouette width indicates a good clustering. Average silhouette method computes the average silhouette of observations for different values of k. The optimal number of clusters is the one that maximizes the average silhouette over a range of possible values for k.
Results
Nitrous oxide emission
The daily N2O flux ranged from 136 to 1850 μg m−2 day−1 during the rice growth period (Fig. 2a). Three main peaks of N2O emission were observed during the rice growth period after each split fertilizer application. The peak N2O flux was observed 2 to 3 days after each N fertilizer application. The magnitude of cumulative N2O emission was highest in T1 (120 kg N ha−1) treatment. The presence of plant growth–promoting, methane-utilizing bacteria (T5 to T8) did not have any significant impact on the N2O emission. Lowest cumulative N2O emission was observed in the T4 (Azolla + BGA) treatment. The substitution of 30 kg N ha−1 with Azolla and BGA biofertilizers significantly (P < 0.01) reduced the mean cumulative N2O emission by 8.7% and 12.0%, respectively, over the T1 treatment in the 2 years, respectively. The substitution of 60 kg N ha−1 by Azolla and BGA in T4 treatments resulted in 40.7% less cumulative N2O emission (significant at P < 0.01) as compared to T1 treatment over the 2 years (Table 2). The daily average N2O flux during the whole rice growth period varied from 275 to 868 μg N2O day−1 (Fig. 3b) under the different treatments. The mean cumulative N2O emission varied from 0.245 kg N2O ha−1 to 0.785 kg N2O ha−1 (Table 2).
Methane emission
Methane (CH4) emission from rice soil varied considerably among the treatments and the dynamics of CH4 flux during both cropping years is presented in Fig. 2b. The CH4 flux increased significantly with plant growth in all the treatments. Irrespective of the treatments, the highest CH4 fluxes were observed around 35 days after transplanting (DAT) in both years. The highest peak (79.1 mg CH4 m−2 day−1) was observed in T8 (Hyphomicrobium facile MaAL69, Methylobacterium oryzae MNL7, and Burkholderia vietnamiensis AAAr40) treatments and lowest peak (41.0 mg CH4 m−2 day−1) was recorded in T2 (Azolla) treatments, respectively, in the first year (Fig. 2b). The second highest CH4 peak was recorded around 63 DAT in both years (Fig. 2b). The CH4 flux rates decreased sharply at rice maturity in all the plots.
The daily average CH4 flux during the entire crop growth period (seasonal daily average) varied from 29.0 to 39.98 mg CH4 m−2 day−1 (Fig. 3a) in the different treatments. The highest daily average CH4 flux was recorded in T8 treatment and the lowest in T7 treatment (Fig. 3a). Among the treatments, application of Azolla–BGA and Methylobacterium oryzae MLN7 significantly reduced the rate of CH4 flux during the study period (Fig. 2b).
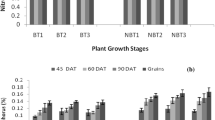
In Pusa Basmati-1509, being a short cycle variety, the total crop cycle varied from 106 to 110 days after transplanting in the two cropping years. We partitioned the CH4 emission in three agronomic phases (Moldenhauer and Slaton 2001): vegetative phase (transplanting to panicle initiation), reproductive phase (panicle initiation to heading), and ripening phase (heading to maturity). Vegetative phase was observed up to 42–46 DAT; subsequently, reproductive phase was observed up to 72–76 DAT and the ripening phase was observed up to harvest. Among the different growth phases of rice, CH4 emission was the highest during the vegetative growth phase and lowest during the ripening phase (Fig. 4). During the vegetative phase, the average CH4 emission in the 2 years ranged from 55% (T2) to 67% (T8) of the total emission (Fig. 4), while in the reproductive phase, CH4 emission ranged from 26% (T9) to 33% (T2) of the total emission and varied from 7% to 13% during the ripening phase in the different treatments.
There was a significant impact of different treatments on CH4 emission in both years and is presented in Table 2. Among the treatments, T7 (Methylobacterium oryzae MNL7), T4 (Azolla + BGA), and T2 (Azolla) significantly (P < 0.05) reduced total seasonal CH4 emission by 19.9%, 13.3%, and 9.7%, respectively (Table 2) as compared to the T1 control averaging over the 2 years. Treatment T3, T5, and T6, reduced CH4 emission by 7.1%, 4.9%, and 4.1%, respectively, as compared to T1 (Table 2) over the 2 years. The cumulative CH4 emission under T8 treatment (Hyphomicrobium facile MaAL69 + Burkholderia vietnamiensis AAAr40 +Methylobacterium oryzae MNL7) was 10.3% higher than T1 (Table 2).
Soil redox potential, dissolved oxygen, and soil carbon and nitrogen
Soil Eh decreased sharply 2 weeks after transplanting in all the treatments (Fig. 2c). Eh declined sharply under flooding condition and lowest Eh was observed at 35 DAT irrespective of the treatments (Fig. 2c). Lowest Eh was observed in T8 (−240 mV) at 35 DAT (Fig. 2c). Eh showed less fluctuation between 35 and 63 DAT, and it sharply rose after 63 DAT (Fig. 2c). The DO values ranged from 3.06 to 0.85 mg L−1 in the first year while it was slightly lower in the second year and ranged from 2.90 to 0.94 mg L−1 during the crop growth period. Average DO levels were observed to be the highest in T2 (1.74 mg L−1) and lowest in T1 (1.65 mg L−1) (Fig. 3a).
We measured the change in soil organic carbon, total N, and pH after rice harvest in both years. There was a slight increase in soil organic carbon and total N in the T2 and T4 treatments; however, the increase was not statistically significant (results not shown).
Growth and yield attributes
The highest leaf area index (LAI) was observed in T2 (4.2) with Azolla substitution and was significantly higher (P < 0.05) than all the other treatments followed by T4 (4.1) treatment. No significant difference was observed in plant height and tillers/hill among the treatments. The test weight did not change significantly, but the number of productive panicles was significantly higher under Azolla treatment (T2) as compared to control. The number of panicles varied under the different treatments from 186 to 240 panicles m−2. Number of tillers was observed to be the highest in T4 with Azolla and BGA application (12.2 per hill) and was the lowest in unfertilized control T9 (10.3 per hill) (result not shown); however, the differences were not significant. Highest grain yield was observed in T2 treatment with substitution of 30 kg N ha−1 with Azolla. The application of plant growth–promoting bacteria with CH4-utilizing ability did not have any significant effect on rice yield (Table 2). The rice yield was higher in T2 (14.8%) followed by T4 (9.9%) as compared to T1. In T9 treatment which was having no fertilizer, the rice yield was significantly (44.8 %) lower than T1 (control).
Global warming potential (GWP) and yield-scaled GWP
In the present study, the GWP in the two rice-growing years was the highest in the combined methane-utilizing bacteria treatment T8 (996 kg CO2 eq. ha−1) and lowest in combined BGA and Azolla treatment T4 (739 kg CO2 ha−1) (Table 2). The mean GWP during the 2 years was significantly higher (P < 0.001) in T8 by 7.5% as compared to the T1 treatment. The share of CH4 in the total GWP ranged from 554 (kg CO2 eq. ha−1) to 764 (kg CO2 eq. ha−1) in the different treatments (Fig. 5). N2O share in total GWP was the highest in T6 (238 kg CO2 eq. ha−1) treatment. The share of CH4 to the total GWP ranged from 74 to 89% among the different treatments (Fig. 5), and for N2O it was the lowest in T1 (11%) and the highest in T7 treatment (31%).
The yield scaled GWP was the least in T4 (0.172 kg CO2 equivalent kg−1 grain yield) and the highest in T9 (0.329 kg CO2 equivalent kg−1 grain yield) (Table 2). In T1, yield-scaled emission was 0.237 kg CO2 eq. kg−1 grain yield and was significantly (P<0.001) reduced by 21.1%, 13.8%, and 27.4%, respectively, with the application of T2 (Azolla), T3 (BGA), and T4 (Azolla + BGA). The application of plant growth–promoting, methane-utilizing bacteria reduced the yield-scaled GWP by 8.6%, 1.8%, and 15.1% in T5, T6, and T7 treatments, respectively, over T1. The application of combination of bacteria (T8), however, did not reduce the yield-scaled emission and were statistically at par with the control (T1).
Non-hierarchical k-means clustering
We carried out non-hierarchical k-means clustering to analyze data and for finding subgroups (clusters) within treatments and for identifying the outliers. The k-means clustering was done on the mean data of the 2-year experiment and the cluster plot obtained is shown in Fig. 6. The clustering segregated the treatments into four subgroups. Out of these, there were two major subgroups and two outliers. The grouping showed that the treatments T2, T4, and T7 having GHG mitigation potential were grouped in one cluster. The control (T1), T3, T5, and T6 treatments were clustered together. The no-fertilizer N treatment T9 was another subgroup. The treatment T8 (combines bacteria) formed a separate subgroup and was an outlier among the treatments.
Discussion
Variation in CH4 and N2O emission during crop growth
In the present study, CH4 flux pattern was similar in both cropping years. The cumulative CH4 fluxes under all treatments during year I was higher as compared to year II (Fig. 2b). This was likely due to higher rainfall (Fig. 1) in the first year as compared to the second year. Rainfall enhances the methanogenic activity by maintaining optimum soil temperature and increases CH4 flux (Hussain et al. 2015). Kim et al. (2016) reported enhanced CH4 emission from paddy soil due to occurrence of rainfall during the crop growth period. Variations in CH4 emission were observed in the different treatments; however, maximum fluxes of CH4 were observed during tillering and reproductive stages in all the treatments in both years (Fig. 2b). It might be due to the combined effect of high root exudation during tillering that provided substrate for methanogenesis (Singh et al. 2009) and direct transport of generated CH4 to the atmosphere by the rice tiller through parenchyma, reducing chances of oxidation near the surface soil (Sass and Cicerone 2002). At the beginning of the crop cycle, when rice plants were little developed, bubble formation and vertical movement in the bulk of the soil was the main transfer mechanism. After tillering, diffusion through the parenchyma becomes the dominant process, and was responsible for more than 90% of the CH4 emission during active tillering and reproductive stages (Tyler et al. 1997). The N2O fluxes were driven by the fertilizer application events. Peaks of N2O flux were obtained after each fertilization event. Malyan et al. (2019) reported that applied urea fertilizer was hydrolyzed to ammonium and further nitrified and denitrified producing high fluxes of N2O.
Impact of methane-utilizing bacteria on CH4 and N2O emission
In the present study, three plant growth–promoting bacteria capable of utilizing CH4 as sole C source were evaluated for their ability to consume CH4 in rice. In our previous work, a commercial liquid formulation of these cultures was developed. They were isolated from the rhizosphere and phyllosphere of different rice-growing regions of India and were evaluated for their plant growth–promoting attributes and CH4 oxidation potential by culturing them in NMS media having different CH4 concentration from (0.5 to 5%) as sole C source (Rani et al. 2021a, b). Facultative methylotrophy in all the three bacterial cultures have been reported earlier by various workers (Van Aken et al. 2004; Mcdonald et al. 2001); however, genetic analysis of the ability to utilize CH4 as sole C source by bacteria belonging to these genera is a topic of further research (Dedysh and Dunfield 2011; Theisen and Murrell 2005). In order to reduce CH4 emission in rice rhizosphere under flooded condition, it is essentially required for methanotroph population to be maintained above the threshold level not only in rhizosphere but also in phyllosphere (Iguchi et al. 2015). In the present study, while carrying out rhizosphere and phyllosphere inoculations, the populations of all the three bacteria, whether inoculated alone or in combination, were maintained above >108 cells mL−1.
Inoculation of Methylobacterium oryzae MNL7 (T7) caused significant reduction in CH4 emission by ~20% as compared to un-inoculated treatment (T1). Previously certain strains of Methylobacterium sp. have been reported to have the ability to utilize CH4 as sole C source of energy. Methylobacterium strain, BJ001T, had been isolated from poplar tissues and has been reported to be able to use CH4 as the sole source of carbon and energy (Van Aken et al. 2004). In contrast, inoculation of Hyphomicrobium facile (T5) and Burkholderia sp. (T6) did not cause any significant reduction in CH4 emission. Difference in the ability of the isolates to act differently under field conditions can be attributed to several factors such as decline in population due to competition with native population, utilization of C sources other than CH4 due to their facultative methylotrophy in nature, survival under anoxic conditions, etc. (Iguchi et al. 2015).
In order to avoid population decline two spray schedules were carried out as stated earlier. Dubey (2005) observed that temperature, CH4 concentration, soil moisture, oxygen availability, nitrogenous compounds, and soil pH play a significant role in CH4 oxidation by bacteria. Results showed that inoculation with Methylobacterium oryzae MNL7 (T7) alone was capable of significantly reducing CH4 emission and could be used for developing commercial-scale technology for use in flooded paddies. From this study, it was observed that the stage of bacterial inoculation was also important in getting the desired level of reduction in CH4 emission. Spraying of cultures to enrich the population in phyllosphere and on water surface at the right stage of the crop may also be important for significant reduction in CH4 emission. The growth and activity of CH4-oxidizing microbes in the rice rhizosphere may also be stimulated by ammonium-based fertilization. Urea has been reported to enhance the activity and population size of methanotrophs in rice rhizosphere (Dong et al. 2011; Xie et al. 2010). No effect of plant growth–promoting bacteria on N2O emission was observed in our study.
Effect of Azolla and BGA on CH4 and N2O emission
Azolla–BGA biofertilizers in rice are globally used and are known to liberate oxygen in flooded water (Kollah et al. 2016; Bharati et al. 2000). In our study, the application of Azolla biofertilizer in treatment T2 and in combination with BGA in T4 reduced cumulative CH4 and N2O emission from the rice soils. This was due to liberation of photosynthetic oxygen in paddy water by Azolla and BGA (Malyan et al. 2016) which increased the DO concentration in flooded water, and eventually decreased the CH4 emission from paddy soil by enhancing the CH4 oxidation (Ali et al. 2015).
Among the treatments, seasonal cumulative CH4 emission were reduced in T2 and T4 by ~9.7 and ~13.3%, respectively, as compared to T1, due to higher average DO concentration during the crop growth (Fig. 3a) and higher soil redox potential (Fig. 2c) which might have enhanced the activity of CH4-oxidizing bacteria (Kimani et al. 2018). Similar reductions in CH4 emission of 20.4% and 12.3% were observed by Ma et al. (2012) and Xu et al. (2017), respectively, after incorporating Azolla in rice. Bharati et al. (2000), however, observed up to 42.5% reduction in the cumulative CH4 emission under Azolla application. Methanogenesis is a multistep process in which methanogenic bacteria uses organic carbon and produces CH4 as an end product under anaerobic environment (Malyan et al. 2016; Ali et al. 2012). In the current study, the oxygen liberated by Azolla–BGA in standing water of rice increased the soil redox potential resulting in suppression of methanogenesis process leading to lower production of CH4 as compared to the T1 control treatment (Fig. 7). However, in some previous studies, Adhya et al. (2000) and Ying et al. (2000) reported that application of Azolla increased cumulative CH4 emission from rice, probably due to decomposition of dead Azolla. Malyan et al. (2019) reported that Azolla has a potential to mitigate the cumulative CH4 emission. Kimani et al. (2018) observed significant reduction in CH4 emission; however, no-significant effect of Azolla on N2O emission was reported in a pot experiment growing rice.
Wagner (1997) reported that Azolla having high photosynthetic ability could release copious amounts of oxygen in standing water thereby increasing the DO concentrations and improving the soil redox potential. Xu et al. (2017) and Prasanna et al. (2002) reported that application of Azolla with N fertilizers like urea has stronger capacity of CH4 oxidation as compared to Azolla alone.
Biological decomposition of organic matter and N fertilizer application are two important sources of N2O emission from agricultural soils (Bremner 1997). In rice, applied Azolla after completing its life span undergoes rapid decomposition and enhanced the N2O emission from rice (Chen et al. 1997). Availability of oxygen is one of the major factors affecting the formation of N2O in rice soil by denitrification pathway (Bhatia et al. 2012). The dissolved oxygen was higher in T2 and T4 treatments due to photosynthesis by Azolla, thereby leading to lower denitrification N2O flux in these treatments. In our study, the cumulative N2O emission under Azolla and BGA applied plots (T2, T3, and T4) were significantly (P < 0.01) lower than T1 (Fig. 2a). Another reason for lower emission was the reduced amount of fertilizer N application in these treatments (Table 2). N2O emission from soil depends on several factors including the rate of N fertilization, type of N applied, and soil-water content (Ladha et al. 2005; Pathak et al. 2002). The urea N application was lower by 25% in T2 and T3, and by 50% in T4 as compared to T1; however, the N2O emission were reduced by 8.7 to 12% in T2 and T3, and by 41% in T4 as compared to T1 treatment. The N fixed by Azolla and BGA was probably more efficiently used for plant growth as compared to 100% synthetic nitrogen applied in the control (T1) treatment leading to reduced N2O losses in T2, T3, and T4 treatments. Kimani et al. (2018) reported that Azolla cover in northeastern Japan rice cultivation reduced the N2O emission from 2.7 to 2.6 mg N m−2. Malyan et al. (2019) observed that application of Azolla along with reduced dose of N fertilizer lowered the GHG intensity in rice by 16 to 19%. Xu et al. (2017) also observed a reduction in N application by the application of Azolla in double rice cropping system in southern China due to nitrogen-fixing properties of these biofertilizers. They also observed lower yield-scaled CH4 emission on the application of Azolla along with nitrogenous fertilizer.
Effect of urea application on CH4 and N2O emission
There are contradictory reports on the effect of N fertilizers on methanotrophs in the rice soil (Hussain et al. 2015; Dubey 2005; Schimel 2000). Datta et al. (2013) reported that cumulative CH4 emission from rice soil increased with the addition of urea fertilizer, whereas Dong et al. (2011) and Xie et al. (2010) reported the stimulation of methanotrophs with the addition of N fertilizers in rice rhizospheric soil (Dong et al. 2011; Xie et al. 2010) leading to lower CH4 emission. In this study, there was higher emission of CH4 in the urea alone treatment (T1) as compared to the no-fertilizer (T9) application. This may be due to the rapid hydrolysis of applied urea fertilizer to ammonium ion. Ammonium ion being similar in chemical structure to CH4 may compete with CH4 for the binding site of methane monooxygenase enzyme, a key enzyme for CH4 oxidation (Bédard and Knowles 1989) and can also lead to the competition between CH4 and ammonium oxidizers for oxygen resulting in increased CH4 emission. N application in the form of urea resulted in 8% higher cumulative CH4 emission as compared to no N fertilizer application. Application of nitrogen fertilizer in T1 resulted in higher below- and above-ground biomass over treatment T9 (no fertilizer) and may have provided higher substrates in the form of root exudates for the methanogenic bacteria to produce more CH4. Datta et al. (2013) observed 26.9% higher CH4 emission under 110 kg N ha−1 urea application over no N fertilizer application in rice fields of Cuttack, India. The high CH4 flux from the urea applied plots could also be due to isostructural and isoelectric symmetry between CH4 molecule and ammonium ion (Schimel 2000). Hanson and Hanson (1996) reported that, due to the presence of high concentration of ammonium ions in soil (such as in urea application conditions), methanotrophic bacteria bind with ammonium ions as a substitute of CH4 molecule and the methanotrophic activity is reduced resulting in higher CH4 emission.
Across all the treatments, the lowest cumulative N2O emission was observed under the T9 (without N fertilizer) treatment in our study (Table 2). In comparison to T1 (control–120 kg N ha−1), the rice cropping without N fertilizer (T9) decreased the cumulative N2O emission by 67.6% (Table 2). Pathak et al. (2002) also reported that total N2O emission from no N fertilizer treatment were reduced by 56.0% as compared to urea-applied rice soils. Das and Adhya (2014) observed a 78.9% decrease in N2O emission in non–N-fertilized soils as compared to urea-applied rice soils. The higher N2O emission in urea treatment is due to availability of mineral N to soil microorganisms which controls the nitrification and denitrification process.
Effects of different biological interventions on CH4 emission during rice phases
During both years, CH4 emitted during the vegetative stage was higher as compared to the other two stages (Fig. 4). The least amount of CH4 was emitted during ripening stage of rice (Fig. 4). The higher CH4 emission from vegetative stage may be due to higher methanogenic activity (Ali et al. 2015) and higher labile organic carbon present in rice during this stage due to growing plant biomass and more root exudation activity. The low CH4 emission during ripening stage was due to higher soil Eh and lower soil temperature which may have suppressed methanogenesis activity. In our study, we found that there was slightly higher CH4 emission from T2 and T4 treatment as compared to other treatments during ripening stage, which may be due to the degradation of Azolla in the last few weeks of the cropping period.
Impact of different interventions on growth and yield attributes
The application of plant growth–promoting methanotrophs did not lead to any significant impact on growth and yield attributes; however, the application of Azolla led to significant increase in growth and yield attributes of rice (Table 3). Under the Azolla treatments T2 and T4, higher plant height, tillers/hill, and LAI were observed as compared to urea (T1) alone, and this led to an increase in grain yield by 15.2% in the T2 treatment over the control. The increase of growth attributes in Azolla and Azolla + BGA treatments may be due to nitrogen fixation and release of some growth-promoting metabolites (5-aminolevulinic acid and exopolymeric substances) in rice soil (Kantachote et al. 2016) that may have led to an increase in yield. Similar findings were also observed by Ali et al. (2015) and Bharati et al. (2000), and they reported that Azolla plus BGA application increased yield in rice significantly due to its biofertilizer property (Bharati et al. 2000).
Effect of different treatments on GWP and yield-scaled GWP
In our study, maximum reduction in average GWP was observed in T4 treatment (Azolla + BGA, 20.2%) as compared to T1 (fertilized control) (Table 2 and Fig. 5). Higher average DO and higher soil Eh was observed in this treatment in both years. The application of Methylobacterium oryzae MNL7 in T7 lowered the GWP by 13.8% due to reduction in CH4 emission. The lowest yield-scaled GWP was observed in T4 (Azolla + BGA) followed by T2 (Azolla) and T7 (Methylobacterium oryzae MNL7) treatment, and it was 27.4%, 21.1%, and 15.1% lower than control, respectively (Table 2). Lower CH4 and N2O emission in T4 treatment decreased the GWP leading to lower yield-scaled GWP. Lower CH4 and N2O emission was observed due to higher DO concentrations in this treatment whereas lower urea N application (50% N at 60 kg N ha−1) led to 41% less N2O emission in T4. A 25% reduction in application of N fertilizer by 30 kg N ha−1 in T2 and T3 treatment decreased the N2O emission by 9 and 12% as compared to T1.
The k-means cluster analysis grouped the treatments into subgroup having common features of reducing GHG emission and increasing the rice yield (Fig. 6). From the result of the k-means clustering, it was evident that the three treatments T2, T4, and T7 formed one cluster and were the most effective in reducing the GWP and the yield-scaled GWP. The next cluster was of T3, T5, and T6 indicating that these treatments were similar to the control (T1) and had no impact on the GWP. The T8 and T9 treatments were the outliers having very different treatment effects. T8 significantly increased the CH4 emission and T9 was the no-fertilizer treatment which had reduced rice yield and lower N2O emission.
Azolla, BGA, and methane-utilizing bacteria can be used for reducing the GWP of transplanted puddled rice cultivation in the Indo-Gangetic Plains region having around 10.5 Mha of land under rice cultivation. Earlier mitigation options like intermittent irrigation and direct seeded rice have been suggested for reducing the CH4 emissions in this region. The rice yield penalty and weed growth are the major reasons for these options not being successfully implemented and taken up by the farmers of the region. However, the application of Azolla, blue green algae, and plant growth–promoting bacteria can be promoted among the farmers as they not only reduce the yield-scaled GWP but also lead to saving in N (Azolla and BGA substitution). The use of biofertilizers for promoting the growth of different crops is already popular among farmers. Farmers will only use microbial inoculants capable of reducing methane and nitrous oxide emission if they also promote the growth of crops. Hence, it is essential to integrate microbial cultures having dual ability of plant growth promotion and methane utilization with existing package and practices of biofertilizers. A suitable delivery mechanism of such biofertilizers needs to be worked out as it is essential to maintain the population of methane-utilizing microbes in the rhizosphere as well as phyllosphere at the critical stages of crop growth. Further research can be undertaken focusing only to develop suitable delivery mechanisms by integrating popularly used algal and cyanobacteria-based paddy biofertilizers with methane-utilizing bacteria.
Conclusion
Plant growth–promoting bacteria Methylobacterium oryzae and biofertilizers Azolla and Blue-green algae can be effective interventions for reducing the global warming potential and yield-scaled GWP in flooded rice ecosystems by reducing the emission of both CH4 and N2O. Compared with control, Azolla, Azolla + BGA, and Methylobacterium oryzae decreased the yield-scaled GWP by 21.1%, 27.4%, and 15.2% from the rice fields, respectively. Thus, in irrigated flooded rice, the application of Methylobacteruim oryzae MNL7, Azolla alone, or along with BGA could be an effective option for mitigation of yield-scaled GWP, saving inorganic fertilizer and increasing rice yields for achieving the goal of sustainable agriculture. Suitable commercial formulations of the methane-utilizing plant growth–promoting bacteria have to be prepared so that their optimum populations can be maintained during the rice growth period, thus enabling its use by the farmers of the region.
Data availability
All relevant data are within the manuscript and available from the corresponding author on request.
References
Adhya TK, Bharati K, Mohanty SR, Ramakrishnan B, Rao VR, Sethunthan N, Wassmann R (2000) Methane emission from rice fields at Cuttack, India. Nutr.Cycl.Agroecosys. 58:95–105
Ali MA, Farouque MG, Haque M, Kabir AU (2012) Influence of soil amendments on mitigating methane emissions and sustaining rice productivity in paddy soil ecosystems of Bangladesh. J Environ Sci Nat Resour 5:179–185
Ali MA, Kim PJ, Inubushi K (2015) Mitigating yield-scaled greenhouse gas emissions through combined application of soil amendments: a comparative study between temperate and subtropical rice paddy soils. Sci Total Environ 529:140–148
Aliyu G, Jiafa LUO, Deyan LIU, Junji YUAN, Zengming CHEN, Tiehu HE, Weixin DING (2021) Yield-scaled nitrous oxide emissions from nitrogen-fertilized croplands in China: a meta-analysis of contrasting mitigation scenarios. Pedosphere 31(2 (2021)):231–242
Aulakh MS, Bodenbender J, Wassmann R, Rennenberg H (2000) Methane trasport capacity of rice plants. II. In variations among different rice cultivars and relationship with morphological characteristics. Nutr Cycl Agroecosyst 58:367–375
Bédard C, Knowles R (1989) Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev 53:68–84. https://doi.org/10.1128/mmbr.53.1.68-84.1989
Bharati K, Mohanty SR, Singh DP, Rao VR, Adhya TK (2000) Influence of incorporation or dual cropping of Azolla on methane emission from a flooded alluvial soil planted to rice in eastern India. Agric Ecosyst Environ 79:73–83
Bhatia A, Pathak H, Jain N, Singh PK, Singh AK (2005) Global warming potential of manure amended soils under rice–wheat system in the Indo-Gangetic plains. Atmos Environ 39:6976–6984
Bhatia A, Ghosh A, Kumar V, Tomer R, Singh SD, Pathak H (2011) Effect of elevated tropospheric ozone on methane and nitrous oxide emission from rice soil in north India. Agric Ecosyst Environ 144:21–28
Bhatia A, Pathak H, Jain N, Singh PK, Tomer R (2012) Greenhouse gas mitigation in rice–wheat system with leaf color chart-based urea application. Environ Monit Assess 184(5):3095–3107
Bhatia A, Kumar A, Das TK, Singh J, Jain N, Pathak H (2013) Methane and nitrous oxide emissions from soils under direct seeded rice. Int J Agric Stat Sci 9(2):729–736
Bhattacharyya P, Dash PK, Swain CK, Padhy SR, Roy KS, Neogi S, Berliner J, Adak T, Pokhare SS, Baig MJ, Mohapatra T (2019) Mechanism of plant mediated methane emission in tropical lowland rice. Sci Total Environ 651:84–92
Bremner JM (1997) Sources of nitrous oxide in soils. Nutr Cycl Agroecosyst 49(7):16
Chen GX, Huang GH, Huang B, Yu KW, Wu J, Xu H (1997) Nitrous oxide and methane emissions from soil–plant systems. Nutr Cycl Agroecosyst 49:41–45
Choudhaury ATMA, Kennedy IR (2005) Nitrogen fertilizer losses from rice soils and control of environmental pollution problems. Commun Soil Sci Plant Anal 36:1625–1639
Conrad R (2007) Microbial ecology of methanogens and methanotrophs. Adv Agron 96:1–63
Das S, Adhya TK (2014) Effect of combine application of organic manure and inorganic fertilizer on methane and nitrous oxide emissions from a tropical flooded soil planted to rice. Geoderma. 213:185–192
Datta A, Yeluripati JB, Nayak DR, Mahata KR, Santra SC, Adhya TK (2013) Seasonal variation of methane flux from coastal saline rice field with the application of different organic manures. Atmos Environ 66:114–122
Davamani V, Parameswari E, Arulmani S (2020) Mitigation of methane gas emissions in flooded paddy soil through the utilization of methanotrophs. Sci Total Environ 726:138570. https://doi.org/10.1016/j.scitotenv.2020.138570
Dedysh SN, Dunfield PF (2011) Facultative and obligate methanotrophs: how to identify and differentiate them. Methods Enzymol 495:31–44
Dong H, Yao Z, Zheng X, Mei B, Xie B, Wang R, Deng J, Cui F, Zhu J (2011) Effect of ammonium-based, non-sulfate fertilizers on CH4 emissions from a paddy field with a typical Chinese water management regime. Atmos Environ 45:1095–1101
Dubey SK (2005) Microbial ecology of methane emission in rice agroecosystem: a review. Appl Ecol Environ Res 3:1–27
Dubey SK, Singh JS (2001) Plant-induced spatial variations in the size of methanotrophic population in dryland and flooded rice agroecosystems. Nutr Cycl Agroecosyst 59:161–167
Gupta DK, Bhatia A, Kumar A, Das TK, Jain N, Tomer R, Malyan SK, Fagodiya RK, Dubey R, Pathak H (2016) Mitigation of greenhouse gas emission from rice–wheat system of the Indo-Gangetic plains: through tillage, irrigation and fertilizer management. Agric Ecosyst Environ 230:1–9
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
Hussain S, Peng S, Fahad S, Khaliq A, Huang J, Cui K, Nie L (2015) Rice management interventions to mitigate greenhouse gas emissions: a review. Environ Sci Pollut Res 22:3342–3360
Iguchi H, Yurimoto H, Sakai Y (2015) Interactions of methylotrophs with plants and other heterotrophic bacteria. Microorganisms. 3:137–151
Inubushi K, Naganuma H, Kitahara S (1996) Contribution of denitrification and autotrophic and heterotrophic nitrification to nitrous oxide production in andosols. Biol Fertil Soils 23:292–298
IPCC (2014) Climate Change 2014: Impacts, Adaptation, and Vulnerability Working Group II Contribution to the Fifth Assessment Report. Cambridge University Press, Cambridge, UK and New York, NY USA
Jain N, Arora P, Tomer R, Vind S, Bhatia A, Pathak H, Chakraborty D, Kumar V, Dubey DS, Harit RC, Singh JP (2016) Greenhouse gases emission from soils under major crops in Northwest India. Sci Total Environ 542:551–561
Kantachote D, Nunkaew T, Kantha T, Chaiprapat S (2016) Biofertilizers from Rhodopseudomonas palustris strains to enhance rice yields and reduce methane emissions. Appl Soil Ecol 100:154–161
Kim Y, Talucder MSA, Kang M, Shim K-M, Kang N, Kim J (2016) Interannual variations in methane emission from an irrigated rice paddy caused by rainfalls during the aeration period. Agric Ecosyst Environ 223:67–75
Kimani SM, Cheng W, Kanno T, Nguyen-Sy T, Abe R, Oo AZ, Tawaraya K, Sudo S (2018) Azolla cover significantly decreased CH4 but not N2O emissions from flooding rice paddy to atmosphere. J Soil Sci Plant Nutr 64:68–76
Kollah B, Patra AK, Mohanty SR (2016) Aquatic microphylla Azolla: a perspective paradigm for sustainable agriculture, environment and global climate change. Environ Sci Pollut Res 23:4358–4369
Kumar A, Medhi K, Fagodiya KR, Subrahmanyam G, Mondal R, Raja P, Malyan SK, Gupta DK, Gupta CK, Pathak H (2020) Molecular and ecological perspectives of nitrous oxide producing microbial communities in agro-ecosystems. Rev Environ Sci Biotechnol 19(4):717–750
Ladha JK, Pathak H, Krupnik TJ, Six J, Kessel C (2005) Efficiency of fertilizer nitrogen in cereal production: retrospects and prospects. Adv Agron 87:85–156
Liessens J, Germonpre R, Kersters I, Beernaert S, Verstraete W (1993) Removing nitrate with a methylotrophic fluidized bed: microbiological water quality. J Am Water Works Assoc 85:155–161
Liu J, Xu H, Jiang Y, Zhang K, Hu Y, Zeng Z (2017) Methane emissions and microbial communities as influenced by dual cropping of Azolla along with early rice. Sci Rep 7:40635
Ma YY, Tong G, Wang WQ, Zeng CS (2012) Effect of Azolla on CH4 and N2O emissions in Fuzhou Plain paddy fields.pdf. Chin J Eco-Agric 20:723–727
Malla G, Bhatia A, Pathak H, Prasad S, Jain N, Singh J, Kumar V (2005) Mitigating nitrous oxide and methane emissions from soil under rice–wheat system with nitrification inhibitors. Chemosphere 58:141–147. https://doi.org/10.1016/j.chemosphere.2004.09.003
Malyan SK, Bhatia A, Kumar A, Gupta DK, Singh R, Kumar SS, Tomer R, Kumar O, Jain N (2016) Methane production, oxidation and mitigation: a mechanistic understanding and comprehensive evaluation of influencing factors. Sci Total Environ 572:874–896
Malyan SK, Bhatia A, Kumar SS, Fagodiya RK, Pugazhendhi A, Duc PA (2019) Mitigation of greenhouse gas intensity by supplementing with Azolla and moderating the dose of nitrogen fertilizer. Biocatal Agric Biotechnol 20:101266
McDonald IR, Doronina NV, Trotsenko YA, McAnulla C, Murrell JC (2002) Hyphomicrobium chloromethanicum sp. nov. and Methylobacterium chloromethanicum sp. nov., chloromethane-utilizing bacteria isolated from a polluted environment. Int J Syst Evol Microbiol 51(Pt 1):119–122. https://doi.org/10.1099/00207713-51-1-119
Moldenhauer KEWC, Slaton N (2001) Rice growth and development. Rice production handbook, pp 7–14
Pathak H, Bhatia A, Prasad S, Kumar S, Jain MC, Kumar U (2002) Emission of nitrous oxide from rice–wheat systems of Indo-Gangetic Plains of India. Environ Monit Assess 77:163–178
Pathak H, Prasad S, Bhatia A, Singh S, Kumar S, Singh J, Jain MC (2003) Methane emission from rice–wheat cropping system in the Indo-Gangetic plain in relation to irrigation, farmyard manure and dicyandiamide application. Agric Ecosyst Environ 97(1-3):309–316
Prasanna R, Kumar V, Kumar S, Yadav AK, Tripathi U, Singh AK, Jain MC, Gupta P, Singh PK, Sethunathan N (2002) Methane production in rice soil is inhibited by cyanobacteria. Microbiol Res 157:1–6
Rani V, Bhatia A, Kaushik R (2021a) Inoculation of plant growth promoting-methane utilizing bacteria in different N-fertilizer regime influences methane emission and crop growth of flooded paddy. Sci Total Environ 145826. https://doi.org/10.1099/00221287-61-2-205
Rani V, Bhatia A, Nain L, Tomar GS, Kaushik R (2021b) Methane utilizing plant growth-promoting microbial diversity analysis of flooded paddy ecosystem of India. World J Microbiol Biotechnol 37(4):1–22
Ritchie H, Roser M (2018) CO2 and other greenhouse gas emissions. Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions. Accessed Dec 2019
Sass RL, Cicerone RJ (2002) Photosynthate allocations in rice plants: food production or atmospheric methane? Proc Natl Acad Sci U S A 99(19):11993–11995
Schimel J (2000) Rice, microbes and methane. Nature. 403:375–377
Shin D, Lee S, Kim TH, Lee JH, Park J, Lee J, Nam HG (2020) Natural variations at the Stay-Green gene promoter control lifespan and yield in rice cultivars. Nat Commun 11(1):1–11
Singh SK, Bharadwaj V, Thakur TC, Pachauri PC, Singh PP, Mishra AK (2009) Influence of crop establishment methods on methane emission from rice fields. Curr Sci 97:84–89
Tan W, Yu H, Huang C, Li D, Zhang H, Jia Y, Wang G, Xi B (2018) Discrepant responses of methane emissions to additions with different organic compound classes of rice straw in paddy soil. Sci Total Environ 630:141–145
Tariq A, Vu QD, Jensen SL, Tourdonnet S, Sander OB, Wassmann R, Mai TV, Neergaard A (2017) Mitigating CH4 and N2O emissions from intensive rice production systems in northern Vietnam: efficiency of drainage patterns in combination with rice residue incorporation. Agric Ecosyst Environ 249:101–111
Theisen AR, Murrell JC (2005) GUEST COMMENTARY Facultative Methanotrophs Revisited. Society 187:4303–4305
Tyler SC, Bilek RS, Sass RL, Fisher FM (1997) Methane oxidation and pathways of production in a Texas paddy field deduced from measurements of flux, delta-C-13, and delta-D of CH4. Glob Biogeochem Cycles 11:323–348
Van Aken B, Peres CM, Doty SL, Yoon JM, Schnoor JL (2004) Methylobacterium populi sp. nov., a novel aerobic, pink-pigmented, facultatively methylotrophic, methane-utilizing bacterium isolated from poplar trees (Populus deltoides x nigra DN34). Int J Syst Evol Microbiol 54:1191–1196
Wagner GM (1997) Azolla: a review of its biology and utilization. Bot Rev 63:1–26
Whittenbury R, Davies SL, Wilkinson JF (1970) Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61:205–218. https://doi.org/10.1099/00221287-61-2-205
Xie B, Zheng X, Zhou Z, Gu J, Zhu B, Chen X (2010) Effects of nitrogen fertilizer on CH4 emission from rice fields: multi-site field observations. Plant Soil 326:393–401
Xu H, Zhu B, Liu J, Li D, Yang Y, Zhang K, Jiang Y, Hu Y, Zeng Z (2017) Azolla planting reduces methane emission and nitrogen fertilizer application in double rice cropping system in southern China. Agron Sustain Dev 37. https://doi.org/10.1007/s13593-017-0440-z
Yao Z, Zheng X, Wang R, Liu C, Lin S, Butterbach-Bahl K (2019) Benefits of integrated nutrient management on N2O and NO mitigations in water-saving ground cover rice production systems. Sci Total Environ 646:1155–1163
Ying Z, Boeckx P, Chen GX, Cleemput OV (2000) Influence of Azolla on CH4 emission from rice fields. Nutr.Cycl.Agroecosyst. 58:321–326
Yu, K., Wang, Z., Vermoesen, A., Patrick Jr, W. and Van Cleemput, O., 2001. Nitrous oxide and methane emissions from different soil suspensions: effects of soil redox status.
Zhao X, Pu C, Ma ST, Liu SL, Xue JF, Wang X, Wang YQ, Li SS, Lal R, Chen F, Zhang HL (2019) Management-induced greenhouse gases emission mitigation in global rice production. Sci Total Environ 649:1299–1306
Acknowledgments
We thank the Director, Dean, and PG School of Indian Agricultural Research Institute, New Delhi for providing all facilities required in this study. We are also thankful to Head, Dr. G. Abraham and Dr. Sunil Pabbi, Division of Microbiology, IARI, for providing Azolla and BGA for experimentation. Financial support to S.K.M. during Ph.D. as UGC-JRF/SRF provided by University Grant Commission (UGC) is gratefully acknowledged. This study has been supported by the grant of National Innovations in Climate Resilient Agriculture (NICRA, IARI/112-15) project.
Author information
Authors and Affiliations
Contributions
S.K.M.: investigation, writing—original draft. A.B.: conceptualization, supervision, writing—review and editing. R.T.: sample analysis. R.C.H.: field management. A.B.: statistical analysis. N.J.: writing—initial draft. R.K.: conceptualization, supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent for participate
The authors have agreed with the content and all have given consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malyan, S.K., Bhatia, A., Tomer, R. et al. Mitigation of yield-scaled greenhouse gas emissions from irrigated rice through Azolla, Blue-green algae, and plant growth–promoting bacteria. Environ Sci Pollut Res 28, 51425–51439 (2021). https://doi.org/10.1007/s11356-021-14210-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14210-z