Abstract
Dodonaea viscosa, a wild and perennial shrub that can tolerate harsh environmental conditions, was used for the isolation of its endophytic bacteria and their potential was explored for the promotion of Canola growth. The bacteria identified through 16S rRNA gene sequencing, belonged to ten different genera namely Inquilinus, Xanthomonas, Pseudomonas, Rhizobium, Brevundimonas, Microbacterium, Bacillus, Streptomyces, Agrococcus and Stenotrophomonas. All the strains produced small amount of IAA (indole acetic acid) in the absence of tryptophan and comparatively more in the presence of tryptophan. All the bacterial strains were positive for ammonia production, cellulase and pectinase activity, but few of them showed phosphate solubilization, siderophore and hydrogen cyanide production. Only three strains showed ACC (1-aminocyclopropane-1-carboxylate) deaminase activity when tested using in-vitro enzyme assay. Members of genera Bacillus, Pseudomonas and Streptomyces showed positive chitinase, protease and antifungal activity against two phytopathogenic fungi Aspergillus niger and Fusarium oxysoprum, while members of Xanthomonas, Pseudomonas and Bacillus showed significant root elongation of Canola which could be related with their positive plant-growth-promoting (PGP) traits. Among the three plant growth promoting Bacillus strains, B. idriensis is never reported before for its PGP activities. These results showed the potential of Dodonaea viscosa endophytic bacteria as PGPBs, which in future can be further explored for their host range/molecular mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern agriculture is relying on the use of chemical fertilizers, pesticides and growth regulators to enhance yield due to increase in population growth and food demand. This reliance is linked with difficulties, such as environmental contamination, health threats, disruption of natural ecological nutrient cycling and damage to biological communities that otherwise maintain crop production (Enebak et al. 1998). As a result, there has been a revival of awareness in ecofriendly, viable and organic agronomic practices (Esitken et al. 2005). Environment-friendly agricultural practices such as use of bio-fertilizers and bio-pesticides has become an ideal approach for many countries in the world.
Endophytic bacteria has been reported as good candidates for plant growth promoting activity, as they can serve their host effectively and efficiently under a wide range of environmental conditions compared to those that bind exclusively to the plant’s rhizosphere (Compant et al. 2010). Plant growth-promoting endophytic bacteria have the ability to inhabit the plant tissues with no apparent harmful effect to the host plant (Reiter and Sessitsch 2006) and known to provide growth benefits to their host. They may establish a biological alternative to chemical fertilizers and increase crop yield. They possess such distinct qualities that make them best candidates for the plant growth promotion. They can safely be used as fertilizers or against disease causing agents, as they do not exert ecological pressures and are environment friendly (Pavlov et al. 2011). They promote plant growth by producing phytohormones (such as IAA), siderophores, solubilizing phosphate or lowering of ethylene levels in plants etc. (Ahemad and Khan 2011).
A large array of bacteria including species of Pseudomonas, Azospirillum, Azotobacter, Klebsiella, Enterobacter, Alcaligenes, Arthrobacter, Burkholderia, Bacillus and Serratia have been reported to enhance plant growth (Ji et al. 2014). Endophytic bacteria with plant growth promoting characters have been reported from different plants (Fernandes et al. 2013; Zhao et al. 2015). These endophytic bacteria improve the growth by improving the biological fixation of nitrogen, production of growth regulators or by increasing various resistances. Endophytes with diverse properties isolated from unexplored and wild sources will have much effective role to manipulate and improve plant growth (Jasim et al. 2014).
In this study, we selected Dodonaea viscosa L. (Sanatha) as a target plant for the isolation of endophytic bacteria. It is an evergreen, woody, perennial and wild shrub, though it is a native plant of Australia, wide spread throughout the tropics known for its medicinal use (Rajamanickam et al. 2010). Dodonaea is drought tolerant, shade tolerant, wind hardy and has the ability to withstand wildfires (Duvauchelle 2009). The aim of the present study was to isolate endophytic bacteria of wild Dodonaea visocosa for the evaluation of their plant growth promoting activities. Canola (Brassica napus) was selected as the experimental crop for the inoculation of isolated endophytic bacteria with potential for the improvement of plant growth.
Materials and methods
Isolation of endophytic bacteria
Endophytic bacteria were isolated from stress resistant, perennial and wild shrub Dodoneae viscosa collected from Quaid-i-Azam University, Islamabad. Young plants were collected in the month of February 2014. Roots were cleaned thoroughly with tap water and cut into small pieces, which were sterilized with chlorox (Commercial bleach) for 5 min and five washes with autoclaved distilled water. Surface sterilized roots were macerated in mortar and pestle, followed by the serial dilution of the extract in 0.03 M MgSO4 up to 10−3 and plated on Tryptic Soya Agar (TSA) medium at 30 °C. Pure and morphologically distinct colonies were selected for further studies and stored on TSA slants at 4 °C and glycerol stocks at −80 °C.
Plant growth promoting activities
Indole-3-acetic-acid (IAA) production
Ability of bacterial endophytes to produce IAA was assessed as described by Rashid et al. (2012). Bacterial cells were grown in tryptophan supplemented medium at 37 °C for 24 h cell harvesting (10,000 rpm for 10 min) was followed by addition of 2 ml of Salkowski’s reagent. Appearance of pink color indicated IAA synthesis after 25 min. OD was monitored at 535 nm using a Spectrophotometer (UV 3000 spectrophotometer). IAA was used as standard.
Phosphate solubilization
Mineral phosphate solubilization activity was determined following Kuklinsky-Sobral et al. (2004). NBRIP medium was supplemented with 5 g l−1 tricalcium phosphate. Bacterial culture was stabbed and incubated as described before. Appearance of halo zone around the colony was taken as positive result.
Siderophore, ammonia and hydrogen cyanide production
Siderophore production of isolates was checked on CAS agar medium according to the modified method of Schwyn and Neilands (1987). Bacterial isolates were streaked on Chrome azurol S agar medium and incubated at 30 °C for 48–72 h. Development of a yellow to orange halo around the bacterial growth showed a positive result for siderophore production.
Bacterial culture were grown in peptone water and incubated for 48 h at 30 °C for determining the ammonia production potential. After the addition of Nesseler’s reagent, development of yellow to brown color indicated positive activity (Marques et al. 2010).
Hydrogen cyanide (HCN) production by the bacterial isolates was analysed by amending the TSA medium with 4.4 g glycine. Isolates were streaked on this modified agar plates. Whatman no.1 filter paper soaked in a 2 % sodium carbonate in 0.5 % picric acid solution was placed on top of streaked plates and incubated at 30 °C for 4 days, after which development of orange to red color indicated HCN production by the isolates (Ahmad et al. 2008).
ACC deaminase enzyme production
ACC deaminase enzyme activity was assayed following the method of Penrose and Glick (2003). The absorbance of the bacterial extracts in the presence of assay reagent, with ACC (the substrate) and without ACC was measured by UV Spectrophotometer at 540 nm. The amount of α-ketobutyrate produced was calculated by preparing its standard curve ranging between 0 and 10 µmol.
Production of cell wall degrading enzymes
Cellulose Congo Red agar was prepared to analyse the cellulose activity of the isolates. Bacterial cultures were inoculated on the cellulose- Congo red agar and incubated for 7 days at 30 °C. Following incubation, the colonies exhibiting zones of clearing were taken to be cellulase positive (Hendricks et al. 1995).
For pectinase activity, isolates were spot-inoculated on a selective agar medium supplemented with polyglacturonic acid (5 g l−1) as a source of substrate. The plates were observed for clear zones around the colonies after flooding with 1 % iodine solution (Kumar and Sharma 2012).
For assessing protease activity of isolates, they were inoculated on LB supplemented with 2 % skimmed milk agar medium (Adinarayana et al. 2003). Chitinase medium was prepared by using ½ strength TSA supplemented with 0.6 % w/v colloidal chitin. Bacteria were spot inoculated on medium and incubated at 30 °C for 5 days. The chitinolytic activity was confirmed by the presence of clear zone around the inoculated bacteria (Bibi et al. 2012).
Antagonistic activities against pathogenic fungi
Bacterial endophytes were tested for their anti-fungal capabilities against Aspergillus niger (accession no: 1109) and Fusarium oxysporum (Accession no: 1114) obtained from Fungal culture bank, University of the Punjab, using a dual culturing technique on screening media containing half strength PDA and half strength TSA. Bacterial isolates were inoculated 2 cm away from fungal disc on media plate and uninoculated plate with fungal disc served as negative control (Kumar et al. 2012). Antifungal activity was evaluated after incubating inoculated plates at 30 °C up to 7 days.
In vitro assay for plant growth promotion
Gnotobiotic canola root elongation assay
Canola seeds were obtained from National Agricultural Research Centre (NARC), Islamabad. To evaluate the ability of bacterial isolates to promote root growth in-vitro, method described by Penrose and Glick (2003) of gnotobiotic Canola root elongation was followed. Bacterial inoculated and un-inoculated seeds (control) on filter paper plates were placed in a plant growth chamber under controlled conditions (12 h light/dark cycles), constant temperature (25 °C) and relative humidity (60 %). Root lengths of the resulting plantlets were measured on the fifth day.
Molecular identification of isolated endophytic bacteria
The selected isolates were identified through 16S rRNA Gene Sequencing. Total genomic DNA from each isolate was extracted using alkaline lysis method (Afzal et al. 2015). 16S rRNA gene was amplified by performing colony PCR using universal bacterial primers 27F and 1492R (Chen et al. 2010), which produced a product of size 1465 base pairs (Bibi et al. 2012). The PCR was run for 35 cycles (denaturation at 94 °C and 30 s, annealing at 56 °C and 30 s, extension at 72 °C and 90 s) with one initial denaturation step (94 °C, 5 min) and a final extension step (72 °C, 7 min). PureLink™ Quick PCR Purification Kit (Invitrogen) was used to purify PCR product and were commercially sequenced for the bacterial 16S rRNA gene using 27F through Sanger sequencing technology of ABI from Macrogen (South Korea). Depending on the quality of the sequencing result EzTaxon-e server version 2.1 and National Centre for Biotechnology Information (NCBI) database using Mega BLAST algorithm (http://www.ncbi.nlm.nih.gov/BLAST) were used to determine the closest match (Kim et al. 2012).
Statistical analysis
Data was analyzed statistically by ANOVA using Statistix 8.1 software. Significance was determined at P ≤ 0.05.
Results and discussion
Isolation and identification of isolates by 16S rRNA gene sequencing
A total of 15 morphologically distinct bacterial isolates were selected and evaluated for their PGP and antagonistic traits. The isolates were identified through 16S rRNA gene analysis. The identified bacteria belonged to ten different genera namely Inquilinus, Xanthomonas, Pseudomonas, Rhizobium, Brevundimonas, Microbacterium, Bacillus, Streptomyces, Agrococcus and Stenotrophomonas. The nucleotide sequences obtained in this work were deposited in GenBank under accession numbers KJ591012-KJ591026. Details of 16S rRNA gene based identification of the bacteria isolated from D. viscosa are given in Table 1.
Bacillus as predominant genera in the present study is same as reported by Kim et al. (2011). Bacillus has also been reported as an endophyte of Polygonum cuspidatum (Figueiredo et al. 2009); Tomato (Feng et al. 2013); Aquilaria sp. etc. (Krishnan et al. 2012). Bacillus and Pseudomonas have been extensively reported and studied to colonize the roots (Kumar et al. 2011). Rashid et al. (2012) reported Microbacterium sp. as an endophyte of tomato. Recovery of these bacteria from surface sterilized canola plants, originating from the inoculated seeds, confirmed their endophytic nature (Rashid et al. 2012).
Characterization of endophytic bacteria as PGPB
Bacterial plant growth promotion is a deep-rooted and multifaceted phenomenon that is often proficient via the activities of more than one PGP trait showed by the associated bacteria (Lifshitz et al. 1987). In this study, we found that endophytic bacterial isolates of perennial and wild shrub Dodonaea viscosa possessed multiple PGP traits such as P-solubilization, IAA production, ACC deaminase, cell wall degrading enzymes, ammonia, siderophore, HCN production and antifungal activity against phytopathogens.
IAA production
All the isolates produced IAA in the absence of tryptophan and comparatively more in the presence of tryptophan. Amounts of IAA production by bacteria can produce positive effect on plant growth (Marques et al. 2010). IAA concentrations in 48 h grown liquid cultures supplemented with tryptophan ranged from 0.88 to 23.7 µg/ml and in the absence of tryptophan ranged from 0.36 to 9.02 µg/ml (Table 2). Six isolates produced more than 4 µg IAA ml−1culture. Few isolates Pseudomonas taiwanensis, Pseudomonas geniculata, Bacillus idriensis, Bacillus cereus, Bacillus subtilis and Agrococcus terreus showed significantly increased IAA production.
Phosphate solubilization
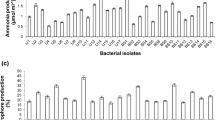
Endophytic bacteria are also known to increase the accessibility of nutrients like insoluble and fixed forms of phosphorus for the host plant (Young et al. 2013). A number of isolates were also found to solubilize inorganic phosphate. Bacillus, Pseudomonas, Serratia and Enterobacter are reported to solubilize the insoluble phosphate compounds and assist in plant growth (Frey-Klett et al. 2005; Hameeda et al. 2008). These genera are well documented for their phosphate solubilizing abilities (Sharma et al. 2013). Among 15 isolates, 12 isolates exhibited the phosphate solubilizing activity by forming clear zones. Isolates having qualitative phosphate solubilizing activity belonged to genera Bacillus, Pseudomonas, Xanthomonas, Rhizobium, Streptomyces, Brevundimonas and Inquilinus (Table 2; Fig. 1a). It is a fact that improved phosphorous nutrition influences overall plant growth and root development (Jones and Darrah 1994). Isolates belonging to Pseudomonas and Bacillus genera were recently reported to induce the plant growth in green gram plants (Saravanakumar et al. 2011).
ACC deaminase activity
The decrease of the level of the plant hormone ethylene by the activity of bacterial enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase is an important mechanism of plant growth promotion (Govindasamy et al. 2008). In this study, only three of 15 isolates gave the ACC deaminase activity. Bacillus idriensis (0.672775 µM), Pseudomonas taiwanensis (4.009774 µM), and Pseudomonas geniculata (0.727172 µM) showed ACC deaminase activity (Table 2). Some bacteria without ACC deaminase are also known to promote plant growth to similar extent compared to ACC deaminase producing bacteria (Sheng et al. 2008; Ma et al. 2011).
Production of cell wall degrading enzymes
All the bacterial strains were screened in vitro for having different cell wall degrading enzymes activity such as cellulase, protease, pectinase and chitinase. Majority of the bacterial strains showed good activity for protease, cellulase and pectinase enzymes.
Plant cell-wall degrading enzymes
Enzymes such as cellulases and pectinases are important for the intracellular root colonization of plant growth promoting bacteria as these are hydrolytic enzymes with the ability to degrade cellulose/pectin material of the plant cell wall (Verma et al. 2001; Reinhold-Hurek and Hurek 2011) and to protect host plant (Hallmann and Kloepper 1996). All strains showed positive cellulase and pectinase activity. All the bacterial isolates showed positive pectinolytic activity and the zone of clearance formed ranged from 10 to 20 mm (Table 2; Fig. 1b).
Fungal cell-wall degrading enzyme
Fungal cell wall degrading enzymes such as chitinases and proteases are also produced by some of the isolated endophytic bacteria that can be employed for the control of plant fungal diseases. Largest zone size for protease activity was observed to be more than 20 mm (Fig. 1c). Streptomyces (alboniger, caeruleatus) Bacillus (idriensis, cereus and subtilis), Pseudomonas taiwanensis and Pseudomonas geniculata showed chitinase activity. Gupta et al. (2006) related the degradation of fungal cell wall to chitinsae. Proteases and chitinases are involved in combating the phytopathogenic fungi via biological control agents (Kim and Chung 2004). Plant growth promoting bacteria function by synthesizing hydrogen cyanide and enzymes such as chitinase for fungal cell wall degradation (Hayat et al. 2010).
Siderophore, ammonia and HCN production
Majority of the strains exhibited siderophore production (Fig. 1d) while all could potentially able to produce ammonia and HCN (except Inquilinus limosus and Rhizobium huautlense) (Table 2). Siderophore production by the isolate assumes significance for acquisition of nutrients such as iron availability to the plant (Glick 2003) and increasing competence for the nutrients with the pathogens in their ecological niche indirectly suppressing disease causing organisms (Pieterse et al. 2001; Wahyudi et al. 2011). Ahmad et al. (2008) reported that bacterial strains belonging to genera Pseudomonas and Bacillus may protect plants from phytopathogenic fungi due to HCN and ammonia production.
Antagonistic activities against pathogenic fungi
B. idriensis, B. cereus, B. subtilis, P. taiwanensis, P. geniculata, S alboniger and S. caeruleatus showed antifungal activity against both pathogens F. oxysporum and (A) niger (Table 3; Fig. 2,b). B subtilis and B. cereus are potentially useful as biocontrol agents (Nagórska et al. 2007; Kumar et al. 2012). Azotobacter sp., Pseudomonas sp., Streptomyces sp. and Bacillus sp. are reported for antifungal activity (Ahmad et al. 2008).
Effect of endophytic bacteria as PGPB on growth of Brassica napus (Canola)
Canola seeds inoculated with endophytic bacteria showed that X. sacchari, S. alboniger, B. idriensis, X. translucens, M. trichothecenolyticum, P. taiwanensis, B. cereus, B. subtilis, P. geniculata and A. terreus significantly enhanced root length of Canola seedlings as compared to uninoculated control (Fig. 3). Differences in percent increases possibly lies in the plant-bacterial relationship which varies due to differences in the genetic makeup. Previous reports showed the role of Bacillus sp. and Pseudomonas sp. in the growth promotion of grape wine, tomato, maize, rice and sugar beet through various mechanisms (Chauhan et al. 2013). Patel et al. (2012) explored that members of Pseudomonas enhanced root length. Root and shoot improvement by the members of Bacillus and Pseudomonas are reported by many researchers (Vivas et al. 2003; Patel et al. 2012). In the present study, Bacillus idriensis (MOSEL-RD 7) has been first ever reported as endophytic bacteria and shown to be positive for all plant growth promoting activities.
Root elongation (cm) of Brassica napus (Canola). Results are expressed as mean of replicates. One way ANOVA was performed for each bacterial isolate. Means were compared using the least significant difference (LSD) method; P ≤ 0.05 was considered significant. The F-values of ANOVA for root length is 19.4 (P < 0.05). Dark bars belong to isolates significantly increasing root length compared to control
Bacteria producing IAA in the range of 2–10 µg/ml enhanced root elongation of the host plant under gnotobiotic conditions. IAA promotes root growth by directly stimulating plant cell elongation or cell division (Burd et al. 1998). Ammonia and hydrogen cyanide production by the isolates is positively associated to accumulation of nitrogen and elongation of the roots so, it seemed to influence the plant characters and biomass production of plant (Marques et al. 2010).
The plant growth promoting phenomenon can be endorsed to the ability of the isolates to give positive results for different PGP attributes like IAA, siderophore production, phosphate solubilization, ACC deaminase and cell wall degrading enzymes activity. IAA positively influences root growth and development, thereby enhancing nutrient uptake (Khalid et al. 2004). However, among all PGP traits of the bacteria, the frequency of IAA-producers was found much higher than other PGP traits. IAA increases root size and spreading, resulting in greater nutrient absorption from the soil (Li et al. 2008). Many studies have been embarked on to understand the potential of these unique microbes, which harbor PGP traits.
Conclusion
Dodonaea viscosa can be the potential source of unique endophytic bacteria and for the development of an integrated strategy to be used as bio-inoculants for agronomic crops like canola to enhance their growth. Results revealed that plant growth promoting attributes like IAA production, ammonia, hydrogen cyanide, ACC deaminase and siderophore production may be positively associated with the elongation of the Canola roots in present study. Bacillus idriensis reported in the present study has the potential to promote seedling root growth and is first ever reported as plant growth promoting bacteria. Thus these isolates have potential to be used as plant biofertilizer for plant growth improvement. Experiments related to exploration of more bacterial traits related to growth promotion and field studies are required to further strengthen the findings.
References
Adinarayana K, Ellaiah P, Prasad DS (2003) Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS Pharmscitech 4:440–448
Afzal I, Shinwari ZK, Iqrar I (2015) Selective isolation and characterization of agriculturally beneficial endophytic bacteria from wild hemp using canola. Pak J Bot 47(5). In press
Ahemad M, Khan MS (2011) Functional aspects of plant growth promoting rhizobacteria: recent advancements. Insight Microbiol 1(3):39–54
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163(2):173–181
Bibi F, Yasir M, Song GC, Lee SY, Chung YR (2012) Diversity and characterization of endophytic bacteria associated with tidal flat plants and their antagonistic effects on oomycetous plant pathogens. Plant Pathol J 28(1):20–31
Burd GI, Dixon DG, Glick BR (1998) A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol 64:3663–3668
Chauhan H, Bagyaraj DJ, Sharma A (2013) Plant growth-promoting bacterial endophytes from sugarcane and their potential in promoting growth of the host under field conditions. Expl Agric 49 (1):43–52 doi:10.1017/S0014479712001019
Chen L, Luo S, Xiao X, Guo H, Chen J, Wan Y, Li B, Xu T, Xi Q, Rao C, Liu C, Zeng G (2010) Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl Soil Ecol 46:383–389
Compant S, Clement C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Duvauchelle D (2009). Plant fact sheet for hopbush (Dodonaea viscosa (L) J acq). USDA-National Resources Conservation Service Hawaii Plant Materials Center, Hoolehua 96729
Enebak S, Wei G, Kloepper J (1998) Effects of plant growth-promoting rhizobacteria on loblolly and slash pine seedlings. For Sci 44(1):139–144
Esitken A, Ercisli S, Karlidag H, Sahin F, Libek A, Kaufmane E, Sasnauskas A (2005) Potential use of plant growth promoting rhizobacteria (PGPR) in organic apricot production. In: Proceedings of the international scientific conference: environmentally friendly fruit growing, Polli, Estonia, 7–9 September Tartu University Press, Tartu, pp 90–97
Feng H, Li Y, Liu Q (2013) Endophytic bacterial communities in tomato plants with differential resistance to Ralstonia solanacearum. Afr J Microbiol Res 7(15):1311–1318
Fernandes T P, Nietsche S, Costa M R, Xavier A A, Pereira D F G S, Pereira M C T (2013) Potential use of endophytic bacteria to promote the plant growth of micropropagated banana cultivar Prata Anã. Afr J Biotechnol 12:4915–4919
Figueiredo JE, Gomes EA, Guimaraes CT, Lana UGP, Teixeira MA, Lima GVC, Bressan W (2009) Molecular analysis of endophytic bacteria from the genus Bacillus isolated from the genus Bacillus isolated from tropical maize (Zea mays L.) Braz J Microbiol 40:522–534
Frey-Klett P, Chavatte M, Clausse ML, Courrier S, Roux CL, Raaijmakers J (2005) Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytolist 165:317–28
Glick BR (2003) Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21:383–393
Gupta CP, Kumar B, Dubey RC, Maheshwari DK (2006) Chitinase mediated destructive antagonistic potential of Pseudomonas aeroginosa GRC1 against Sclerotinia sclerotiorum causing charcoal rot of peanut. Biocontrol 51:821–835
Hallmann A, Kloepper JW (1996) Immunological detection and localization of the cotton endophytes Enterobacter asburiae JM22 in different plant species. Can J Microbiol 42(11):1144–1154
Hameeda B, Harini G, Rupela OP, Wani SP, Reddy G (2008) Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol Res 163:234–242
Hayat R, Ali S, Ammara U, Khalid R, Ahmad I (2010) Soil beneficial bacteria and their role in plant growth promotion. Ann Microbiol 60:579–598
Hendricks CW, Doyle JD, Hugley B (1995) A new solid medium for enumerating cellulose-utilizing bacteria in soil. Appl Environ Microbiol 61(5):2016–2019
Jasim B, Aswathy AJ, Jimtha CJ, Jyothis M, Radhakrishnan EK (2014) Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiber officinale. 3. Biotech 4:197–204. DOI:10.1007/s13205-013-0143-3
Ji SH, Gururani MA, Chun SC (2014) Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol Res 169(1):83–98
Jones DL, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Khalid A, Arshad M, Zahir ZA (2004) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96:473–480
Kim PI, Chung KC (2004) Production of antifungal protein for control Colletotrichum lagenarium by Bacillus amyloliquefaciens MET0908. FEMS Microbiol Lett 234:177–183
Kim W, Cho WK, Kim SN, Chu H, Ryu KY, Yun JC, Park CS (2011) Genetic diversity of cultivable plant growth-promoting rhizobacteria in Korea. J Microbiol Biotechnol 21(8):777–790
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA Gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
Krishnan P, Bhat R, Kush A, Ravikumar P (2012) Isolation and functional characterization of bacterial endophytes from Carica papaya fruits. J Appl Microbiol 113:308–317
Kuklinsky-Sobral J, Araujo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA, Azevedo JL (2004) Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ Microbiol 6(12):1244–1251
Kumar A, Sharma R (2012) Production of alkaline pectinase by bacteria (Cocci sps.) isolated from decomposing fruit materials. J Phytol 4(1):01–05
Kumar A, Prakash A, Johri BN (2011) Bacillus as PGPR in crop ecosystem. In Maheshwari DK. (ed) Bacteria in agrobiology: crop ecosystem. Springer, Heidalberg, pp 37–59
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167:493–499
Li JH, Wang ET, Chen WF, Chen WX (2008) Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biol Biochem 40(1):238–246
Lifshitz R, Kloepper JW, Kozlowski M, Simonson C, Carlson J, Tipping EN, Zaleska I (1987) Growth promotion of canola (rapeseed) seedlings by a strain of Pseudomonas putida under gnotobiotic conditions. Can J Microbiol 8:102–106
Ma Y, Rajkumar M, Luo Y, Freitas H (2011) Inoculation of endophytic bacteria on host and non-host plants-effects on plant growth and Ni uptake. J Hazard Mater 195:230–237
Marques APGC, Pires C, Moreira H, Rangel AOSS, Castro PML (2010) Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol Biochem 42:1229–1235
Nagórska K, Bikowski M, Obuchowski M (2007) Multicellular behaviour and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol agent. Acta Biochim Pol 54(3):495–508
Patel D, Jha CK, Tank N, Saraf M (2012) Growth enhancement of Chickpea in saline soils using plant growth promoting rhizobacteria. J Plant Growth Regul 31:222–228
Pavlov A, Leonid O, Iryna Z, Natalia K, Maria PA (2011) Endophytic bacteria enhancing growth and disease resistance of potato. Biolcontrol 56:43–49
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizo-bacteria. Physiol Plant 118:10–15
Pieterse CMJ, van Pelt JA, van Wees SCM, Ton J, Leon-Kloosterziel KM, Keurentjes JJB, Verhagen BMW (2001) Rhizobacteria mediated induced systemic resistance: triggering, signaling and expression. Eur J Plant Pathol 107:51–61
Rajamanickam V, Rajasekaran A, Anandarajagopal K, Sridharan D, Selvakumar K, Rathinaraj BS (2010) Anti-diarrheal activity of Dodonaea viscosa root extracts Int J. Pharm Biol 1(4):182–185
Rashid S, Charles TC, Glick BR (2012) Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl Soil Ecol 61:217–224
Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14:435–443
Reiter B, Sessitsch A (2006) Bacterial endophytes of the wildflower Crocus albiflorus analyzed by characterization of isolates and by a cultivation-independent approach. Can J Microbiol 52:140–149
Saravanakumar D, Kavino M, Raguchander T, Subbian P, Samiyappan R (2011) Plant growth promoting bacteria enhances water stress resistance in green gram plants. ACTA Pyhsiol Plant 33:203–209
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:587
Sheng XF, Xia JJ, Jiang CY, He LY, Qian M (2008) Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut 156:1164–1170
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endo- phytic diazotrophs from deep water rice. J Biotechnol 91:127–141
Vivas A, Marialanda JM, Lozano R, Barea JM (2003) Influence of Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi ad plant responses to PEG- induced drought stress. Mycorrhiza 13:249–256
Wahyudi AT, Astuti RP, Widyawati A, Meryandini AA, Nawangsih AA (2011) Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting rhizobacteria. J Microbiol Antimicrob 3(2):34–40
Young LS, Hameed A, Peng SY, Shan YH, Wu SP (2013) Endophytic establishment of the soil isolate Burkholderia sp. CC-Al74 enhances growth and P-utilization rate in maize (Zea mays L.) Appl Soil Ecol 66:40–47
Zhao L, Xu Y, Lai XH, Shan C, Deng Z, Ji Y (2015) Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz J Microbiol 46(4):977–989
Acknowledgments
The present work was funded by Pakistan Academy of Sciences. Imran Afzal was a recipient of a fellowship from Higher Education Commission of Pakistan. Funding was provided by National Natural Science Foundation of China (Grant No. ID0E5VAI1874) and Henan Agricultural Science and Technology Innovation Fund (CN) (Grant No. ID0ENDBI1892).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afzal, I., Iqrar, I., Shinwari, Z.K. et al. Plant growth-promoting potential of endophytic bacteria isolated from roots of wild Dodonaea viscosa L.. Plant Growth Regul 81, 399–408 (2017). https://doi.org/10.1007/s10725-016-0216-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0216-5