Abstract
Bacterial biofilms are multicellular aggregates enclosed in a self-created biopolymer matrix. Biofilm-producing bacteria have become a great public health problem worldwide because biofilms enable these microorganisms to evade several clearance mechanisms produced by host and synthetic sources. Over the past years, different flavonoids including quercetin have engrossed considerable interest among researchers owing to their potential anti-biofilm properties. To our knowledge, there is no review regarding effects of quercetin towards bacterial biofilms, prompting us to summarize experimental evidence on its anti-biofilm properties. Quercetin inhibits biofilm development by a diverse array of bacterial pathogens such as Enterococcus faecalis, Staphylococcus aureus, Streptococcus mutans, Escherichia coli, and Pseudomonas aeruginosa. Prevention of bacterial adhesion, suppression of quorum-sensing pathways, disruption or alteration of plasma membrane, inhibition of efflux pumps, and blocking nucleic acid synthesis have been documented as major anti-biofilm mechanisms of quercetin. Overall, anti-biofilm activity of quercetin can open up new horizons in a wide range of biomedical areas, from food industry to medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biofilm is an organized, complex, and sessile microbial community enclosed in a self-created biopolymer matrix, which can be formed on both biotic and abiotic surfaces (Flemming et al. 2016). From the human perspective, biofilm has a tremendous impact in the field of medicine, in particular healthcare-associated infections related to indwelling devices such as catheters, implants, artificial heart valves, and prosthetic joints. Indeed, biofilm formation is an adaptive mechanism of bacterial cells, allowing them to survive and persist in harsh environments (Koo et al. 2017). Due to low-metabolic activity of biofilm-encased bacteria and insufficient penetration of antibiotics into biofilm, bacteria residing within biofilm are able to withstand up to thousand times greater concentrations of antibiotics compared with their planktonic counterparts (Wu et al. 2015; Memariani et al. 2019a).
Inhibition of biofilm development is considered as a major drug target for the treatment of numerous bacterial infections. Heretofore, a plethora of diverse anti-biofilm molecules with unique structures including herbal compounds, chelating agents, anti-microbial peptides, lantibiotics, and synthetic chemicals has been discovered (Sadekuzzaman et al. 2015). These molecules hamper biofilm formation in a number of different ways such as impediment in AHL (N-acyl homo-serine lactones)-mediated quorum-sensing, inhibition of stringent response by bacteria, dispersion of extracellular polysaccharide substance of biofilm, cleavage of peptidoglycan, biofilm disassembly, neutralization of lipopolysaccharides (LPS), alteration of membrane permeabilization, prevention of cell division or survivability, direct interaction with nucleic acid synthesis, interfering with cyclic di-GMP (c-di-GMP) signaling system, and blocking curli biogenesis (Roy et al. 2018).
Utilization of naturally occurring compounds of plant origin has proved to be a promising strategy for prevention and treatment of various diseases since ancient times (Vikram et al. 2010). Plant secondary metabolites have been extensively exploited in pharmaceutical industry as a source of food additives, flavors, drugs, dyes, insecticides, and fragrances (Hussain et al. 2012). In this respect, flavonoids and other phenolic compounds constitute one of the chief classes of secondary metabolites. Flavonoids have two or more aromatic rings, each bearing at least one aromatic hydroxyl and connected with a carbon bridge (Panche et al. 2016). They are categorized into several classes including flavonols, flavones, isoflavonoids, proanthocyanidins, catechins, and anthocyanidins (Nabavi and Silva 2019). Flavonoids can exert multiple health-promoting features including anti-oxidant, anti-inflammatory, anti-neoplastic, and anti-microbial effects. In particular, anti-bacterial and anti-biofilm properties of different flavonoids have engrossed considerable interest among researchers during the last decade (Vipin et al. 2019a).
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a plant-derived flavonol that usually exists in various foods including capers, onions, peppers, cranberries, tomatoes, apples, and grapes. The name quercetin stems from the Latin word “Quercetum” (oak woodland), and has been used since 1857 (Nabavi and Silva 2019). Quercetin is continuing to receive great attention owing to its potential anti-oxidant, anti-allergic, anti-cancer, anti-inflammatory, anti-diabetic, anti-microbial, and cardioprotective activities (Anand David et al. 2016). Reviews on physicochemical properties, biologic activities, and therapeutic values of quercetin have been previously published (Smith et al. 2016; Li et al. 2016). However, there is no review on anti-biofilm properties of quercetin. Thus, the present review sought to summarize findings from multiple studies with regard to anti-biofilm effects of quercetin on a broad spectrum of bacterial pathogens. For the reader’s convenience, we described the importance of biofilm-producing bacteria for which anti-biofilm effects of quercetin have been evaluated. Besides, the details of bacterial targets for anti-biofilm action of quercetin are further discussed in this review.
Anti-biofilm effects on Gram-positive bacteria
Bacillus subtilis
Bacillus subtilis is a rod-shaped, endospore-forming bacterium that long-served as a facile model organism to delineate the molecular mechanisms of biofilm establishment. Information with regard to food-poisoning due to B. subtilis is rare (Earl et al. 2008). Quercetin is able to decrease in vitro biofilm formation by B. subtilis. In this context, 500 μg/mL of quercetin was sufficient for 84% reduction of biofilm development in comparison to untreated control (Bordeleau et al. 2018). A quercetin/multi-walled carbon nano-tube/titanium dioxide nano-composite (Q/MWCNTs/TiO2) has been demonstrated to lessen either adhesion or biofilm formation in B. subtilis compared to quercetin alone, as evidenced by confocal laser-scanning microscopy (CLSM). Unlike MWCNTs/TiO2, Q/MWCNTs/TiO2 was less protective against biofilm formation. This can be attributed to the improved hydrophilicity of the glass surface in the presence of quercetin, thereby decreasing electrostatic repulsion between negatively charged B. subtilis surface and Q/MWCNTs/TiO2 coated surface (Raie et al. 2018).
Enterococcus faecalis
As a gut-dwelling opportunistic pathogen, Enterococcus faecalis has long been recognized to be notoriously associated with nosocomial infections as a result of its capability to form biofilms on stents and artificial devices (Ch’ng et al. 2019). This is further exacerbated by the fact that this organism is intrinsically resistant to numerous classes of antibiotics and has the propensity to obtain antibiotic resistance determinants through horizontal gene transfer (Miller et al. 2015).
In a recent survey, quercetin was found to be effective against E. faecalis MTCC 2729 at sub-minimum inhibitory concentrations (sub-MICs). At 1/2 × MIC (256 μg/mL), quercetin inhibited 95% of biofilm formation, which was further confirmed by scanning electron microscopy (SEM) and CLSM (Qayyum et al. 2019). In another study, Kim et al. (2018) found that a quercetin–pivaloxymethyl conjugate (Q-POM) at 5 μg/mL waned 70% of biofilm establishment by a vancomycin-resistant E. faecium isolate. Two-dimensional gel electrophoresis (2DE) and matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF/MS) analysis divulged that nineteen proteins represented differential intensities in biofilm-inhibited condition after exposure to quercetin, among which ten and nine proteins were over-expressed and suppressed, respectively (Qayyum et al. 2019). Noticeably, quercetin augmented expression of stress marker proteins DnaK and GroES, both of which are involved in protein folding as well as stress management of the cells (Bøhle et al. 2010). Additionally, quercetin is able to suppress several glycolytic enzymes such as 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase (GpmA) and ATP-dependent phosphofructokinase (PfkA). Data relating to interactome networks also exhibited robust connections among translation elongation factors, namely Tuf, Frr, Tsf, and FusA (Qayyum et al. 2019). Hence, quercetin simultaneously affects multiple proteins to cease E. faecalis biofilm formation. This multi-target mode of action can decrease the likelihood of resistance to quercetin.
Enterococcus faecium
Enterococcus faecium is the second-most frequently encountered Enterococcus species linked with diseases in hospitalized patients, particularly urinary tract infections, bacteremia, and endocarditis (Ch’ng et al. 2019; Lee 2017). The species also exhibits natural resistance to a broad range of antibiotics (Miller et al. 2015). Q-POM has been demonstrated to mitigate biofilm development by a vancomycin-susceptible E. faecium isolate in a dose-dependent manner (Kim et al. 2018).
Listeria monocytogenes
Listeriosis is a relatively rare but potentially life-menacing foodborne illness, usually affecting pregnants, the elderly, and immunocompromized individuals. Listeria monocytogenes biofilms have been shown to grow on polystyrene, stainless steel, rubber or glass surfaces in food processing facilities (Rodríguez-López et al. 2018). Recent evidence reveals that teichoic acid is the major polysaccharide in L. monocytogenes biofilm matrix, which ostensibly resembles cell wall teichoic acid (Brauge et al. 2016).
Quercetin, at sub-MIC concentrations, hinders abiotic surface colonization of L. monocytogenes (Vazquez-Armenta et al. 2018). Following administration of quercetin at 0.4 mM for 2 h, the attachment of L. monocytogenes to stainless steel was entirely abrogated, while this concentration significantly decreased viability of bacterial cells (p < 0.05) after 24 h. No viable bacterial cells were recovered from the stainless steel coupons following 24 h of exposure to 0.8 mM of quercetin. Compared to the untreated control, 1 h treatment with quercetin was enough for substantial reduction of bacteria encased in 24 h-old biofilms (p < 0.05). Likewise, 0.2 mM of quercetin considerably diminished bacterial cell density on stainless steel during early and late stages of biofilm development (Table 1). A 41% reduction in total extracellular protein content was also discernible in quercetin-treated biofilms, whereas neither DNA nor polysaccharide content in biofilms was affected by quercetin (Vazquez-Armenta et al. 2018). Some phenolic compounds including gallic acid and ferulic acid have been proven to influence physico-chemical characteristics of bacteria such as free energy of adhesion between the bacterial cells and polystyrene, thereby making the surface attachment unfavorable (Borges et al. 2012). Furthermore, it has been reported that quercetin can repress genes associated with bacterial adhesion (Lee et al. 2013). Overall, these studies showed that quercetin has the potential to be applied as a food additive to minimize adhesion, proliferation, and biofilm growth of L. monocytogenes.
Staphylococcus aureus
Over the past decades, Staphylococcus aureus has caused wreaking havoc in both the community and healthcare settings (Memariani et al. 2018). The bacterium produces a wide spectrum of diseases, ranging from relatively mild skin infections, such as impetigo, furuncles, and folliculitis, to even serious and potentially fatal diseases, including sepsis, endocarditis, and osteomyelitis. Indeed, an extraordinary repertoire of virulence factors contributes to the pathogenicity of S. aureus (Kane et al. 2018; Memariani et al. 2019b). Other than that, the aptitude of S. aureus to form biofilms on indwelling medical devices such as artificial heart valves, prosthetic joints, and catheters impedes successful treatment of infections (Moormeier and Bayles 2017).
A contemporary study demonstrated inhibitory impacts of quercetin (at both MIC and sub-MIC) on biofilm production in both reference and clinical isolates of S. aureus. In this context, quercetin (at concentrations ranging from 250 to 500 μg/mL) was enough to decrease almost half of biofilm formation by methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA) (da Costa Júnior et al. 2018). A study conducted on methicillin-sensitive S. aureus strain (MSSA) ATCC 6538 showed that quercetin significantly (p < 0.05) inhibited biofilm production at both 20 μg/mL and 50 μg/mL after 24 h (Cho et al. 2014). It has been evinced that both number and position of the hydroxyl group in flavonoid structures affect their anti-biofilm activities. For instance, quercetin with five hydroxyl groups displayed the highest inhibitory effects on biofilm establishment (more than 80%) compared to other flavonoids. The authors also demonstrated that red wines markedly enhanced viability of S. aureus-infected Caenorhabditis elegans, probably thanks to anti-virulence and anti-biofilm properties of quercetin in red wines (Cho et al. 2014).
In another investigation, Q-POM crippled biofilm production by six S. aureus isolates (Table 1) in the range of 1–50 μg/mL. In this regard, 5 μg/mL of Q-POM inhibited biofilm formation of S. aureus isolates by 24–83%. As for cytotoxicity, more than 70% of human liver epithelial cells were viable at 50 μg/mL, demonstrating the higher selectivity of Q-POM against S. aureus in comparison to human cells (Kim et al. 2018). Vanaraj et al. (2017) designed hybrid silver nano-particles (AgNPs) combined with quercetin, which gave satisfactory results (92% inhibition of biofilm formation) when applied at a concentration of 100 μg/mL against a clinical isolate of S. aureus. The hybrid also considerably diminished exopolysaccharide (EPS) production by S. aureus (p < 0.001), and dispersed biofilm-enclosed bacterial aggregates. Concerning toxicity, negligible hemolytic activity (< 5% at 120 μg/mL) was reported for the hybrid (Vanaraj et al. 2017). It seems that the hybrid permeabilizes the bacterial membranes, interacts with intracellular components, and induces oxidative stress in bacteria, thereby killing microorganisms. Another survey revealed 28.5% (p ≤ 0.01), 58% (p ≤ 0.01), and 73.7% (p ≤ 0.05) reduction in MRSA biofilm formation after exposure to 10, 20, and 50 μg/mL of quercetin-AgNPs, respectively, in comparison to untreated control (Ahmed et al. 2018). In general, nano-particles enhance stability, water solubility, and bioactivity of quercetin.
A noticeable inhibition of biofilm establishment by S. aureus ATCC 6538 was observed when quercetin was exploited at 20 μg/mL in vitro (Lee et al. 2013). Quercetin at 1 μg/mL was sufficient to abolish > 50% of biofilm production by two MSSA strains as well as one MRSA strain (Table 1). To shed some light on molecular mechanisms behind the inhibition of biofilm production, quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was applied. In this respect, quercetin (10 μg/mL) efficiently repressed expression of adhesion related genes icaA and icaD, both of which are indubitably implicated in biofilm formation (O’Gara 2007). Furthermore, quercetin suppressed expression of the quorum-sensing gene agrA together with virulence-regulatory genes sigB and sarA (Lee et al. 2013). Inhibition of quorum-sensing genes interferes with bacterial cell-to-cell communication and subsequent biofilm formation (Markowska et al. 2013).
Interestingly, quercetin prohibited the hemolysis of human erythrocytes by S. aureus in a concentration-dependent fashion, suggesting its inhibitory effects on S. aureus hemolysin (Lee et al. 2013). This finding is in line with a study conducted by Cho et al. (2014) in which 20 μg/mL of quercetin significantly (p < 0.05) lessened lysis of human erythrocyte by S. aureus after a 16-h of incubation (Cho et al. 2014). Likewise, satisfactory results were reported from a recent survey in which Q-POM dose-dependently repressed the hemolysis by MRSA, MSSA, and VISA strains (Kim et al. 2018). As a general rule, targeting both biofilm and exotoxin production by S. aureus simultaneously kills two birds with one stone.
Staphylococcus saprophyticus
Staphylococcus saprophyticus is the second-most prevalent etiologic agent of cystitis in young women (Ronald 2003). Quercetin was shown to hamper biofilm development by antibiotic-resistant isolates of S. saprophyticus. Recently, da Costa Júnior et al. (2018) demonstrated 39% to 56% inhibition of biofilm production by moderate and strong biofilm producer S. saprophyticus isolates at sub-MICs of quercetin (≤ 500 μg/mL).
Streptococcus mutans
Streptococcus mutans is the main culprit in causing dental caries. In fact, one of the most important virulence properties of the bacterium is its capacity to form biofilms, also known as dental plaques, on tooth surfaces (Dani et al. 2016). Quercetin has been shown to exert anti-biofilm effects on S. mutans (Zeng et al. 2019). In this context, minimum biofilm inhibition concentration (MBIC50) and minimum biofilm reduction concentration (MBRC50) of quercetin were 16 mg/mL and 32 mg/mL, respectively. Quercetin lessened viability of biofilm-encased bacteria cells, biofilm dry-weight, total protein, glucans formation, and acid production. Quercetin was superior to chlorhexidine (0.12%) with respect to reduction in biofilm biomass, interlinked bacteria-extracellular matrix, and viability of biofilm-residing bacteria, as evidenced by SEM and CLSM. Structural analysis also revealed that quercetin wanes the biofilm thickness and renders it porous (Zeng et al. 2019). These findings suggest that quercetin degrades biofilm-extracellular matrix and penetrates deep into biofilm, where it can inhibit metabolically active, slow growing, or even persister cells.
In another study, quercetin suppressed biofilm production and maturation by S. mutans UA159 (Table 1). It exhibited synergistic activity with plant-based compound Deoxynojirimycin (DNJ) against biofilm formation by S. mutans (Hasan et al. 2014). When administrated alone or combined with DNJ, quercetin noticeably lessened the synthesis of both water soluble and alkali soluble polysaccharides by crude glucosyltransferases from S. mutans. Moreover, quercetin (32 μg/mL) significantly (p < 0.05) diminished cell surface hydrophobicity of S. mutans in comparison to untreated bacteria. At sub-MICs, quercetin in combination with DNJ also repressed glass-dependent adherence of S. mutans, regardless of the presence or absence of 5% sucrose. An in-depth analysis of gene expression disclosed that quercetin down-regulates a plethora of virulence genes including those related to adhesion promotion, surface biogenesis, quorum-sensing, and biofilm formation (Hasan et al. 2014). In this respect, 49.07% and 61.79% suppression was achieved in expression levels of brpA and smu630, respectively, both of which are involved in quorum-sensing regulation of biofilm formation (Brown et al. 2005; Wen et al. 2006). Incorporation of quercetin into a commercial adhesive has been reported to impede growth of S. mutans biofilm (Yang et al. 2017). XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] analysis and CLSM evaluation of S. mutans biofilms revealed that quercetin-doped adhesive groups exhibited lower metabolic activity and higher dead bacteria, respectively, in comparison to the unmodified adhesive group. Quercetin-doped adhesive also retained its bonding properties towards collagenase ageing. Efficient inhibition of matrix metalloproteinase activity and acceptable biocompatibility are the other beneficial properties of quercetin-doped adhesives (Gennaris and Collet 2013). On the whole, considering the detrimental effects of dental plaques on oral health, quercetin can be added to toothpaste and mouthwash formulations.
Streptococcus pneumoniae
More than a century ago, Streptococcus pneumoniae has been branded as the “Captain of the Men of Death” by Sir William Osler owing to its extreme prowess at killing. Today, the bacterium remains one of the major pathogens afflicting children and elderly throughout the world (Chao et al. 2015). Over the past years, studies shed light on the role of pneumococcal biofilm formation during asymptomatic colonization as well as disease states such as otitis media, chronic rhinosinusitis, and, to lesser extent, pneumonia (Chao et al. 2015).
Sialic acid serves as a prominent molecule for both S. pneumoniae colonization and biofilm development (Parker et al. 2009; Trappetti et al. 2009). It is worthwhile to mention that S. pneumoniae neuraminidase (NanA) cleaves sialic acid residues from the airway epithelium, thereby facilitating bacterial biofilm development (Parker et al. 2009). Furthermore, nanA expression is up-regulated once S. pneumoniae is grown under biofilm conditions (Oggioni et al. 2006). NanA also needs transpeptidation by sortase A (SrtA) for appropriate anchoring to the cell wall of S. pneumoniae. These findings together with direct role of SrtA in bacterial adherence to host tissues have instigated researchers to examine the possible effects of quercetin on pneumococcal biofilms. Quercetin dose-dependently mitigated catalytic activity of S. pneumoniae sortase in vitro (p < 0.01). Quercetin exhibited an inhibitory effect on S. pneumoniae biofilm formation, and reduced colony-forming units (CFUs) of biofilm-encased bacteria (Wang et al. 2018a). Based on western blot analysis, quercetin did not influence expression of pneumolysin, which has been previously shown to be involved in early biofilm formation (Shak et al. 2013). Indeed, inhibition of SrtA transpeptidase activity is the main mechanism by which quercetin blocks biofilm development. This inhibition prevents the normal anchoring of NanA. Noticeably, addition of sialic acid waned inhibitory effects of quercetin on biofilm growth, corroborating the findings of a previous study (Trappetti et al. 2009). In general, these findings show that indirect decrement in sialic acid production is a highly possible mechanism for reducing biofilm development.
Molecular modelling also revealed that quercetin occupies the substrate channel, thereby causing steric hindrance between the substrate and S. pneumoniae SrtA. Additionally, mutational analysis confirmed that both Leu113 and Leu118 play a pivotal role in the engagement of quercetin with the channel of SrtA (Wang et al. 2018a). Nevertheless, future studies should explore other factors that interact with quercetin in S. pneumoniae. Collectively, these data suggest that quercetin in aerosol form or gargle solution can be applied for prevention or treatment of S. pneumoniae infections.
Anti-biofilm effects on Gram-negative bacteria
Escherichia coli
Escherichia coli is a highly versatile species encompassing both commensals and pathogenic strains (Memariani et al. 2014). It has long been a major Gram-negative model organism for in vitro analysis of biofilm formation on abiotic surfaces. In many bacterial species, signal autoinducer 2 (AI-2), a potential quorum-sensing signal, has been shown to be associated with biofilm formation (González Barrios et al. 2006). Because of this, some researchers assessed the plausible effects of quercetin on biofilm growth of E. coli strains. For instance, one study demonstrated that quercetin meaningfully (p < 0.01) curtailed biofilm production by E. coli O157:H7 in a concentration-dependent fashion (Vikram et al. 2010).
Samoilova et al. (2014) examined the effects of quercetin on biofilm development by E. coli (Table 2). Contrary to expectations, substantial increase in biofilm production was observed when quercetin administrated at concentrations ranged from 1 to 50 μM. This discrepancy can be attributed to the different concentrations of quercetin which do not influence the bacterial survival. Another possible explanation is the culture media used for biofilm formation (Naves et al. 2008). It should also be borne in mind that biofilm production greatly varies among bacterial strains of the same species (López et al. 2010). Moreover, quercetin has been shown to diminish swimming motility of E. coli, reduce expression of rpoS, induce transition from exponential to stationary growth phases, and eventually influence maturation of E. coli biofilms (Ito et al. 2008). Experiments conducted on E. coli knockout mutants suggest that the excess of RpoS rather than its lack negatively affects biofilm production (Adnan et al. 2017; Samoilova et al. 2014; López et al. 2010).
In a study conducted by Yu and co-workers, significant reductions in biofilm development by E. coli ECDCM1 treated with quercetin (20 μg/mL; p < 0.05) and quercetin-AgNPs (1 μg/mL; p < 0.01) were evident (Yu et al. 2018). Similarly, Ahmed et al. (2018) found that quercetin-AgNPs lessened extracellular polymeric substance formation by an extended-spectrum β-lactamase (ESBL)-producing E. coli isolate. Severe damage to the biofilm integrity of E. coli was palpable in SEM images following quercetin treatment (Yu et al. 2018). Upon the addition of quercetin or quercetin-AgNPs, transcription levels of several biofilm-related genes including bcsA, csgA, fliC, fimA, motA, and wcaF were substantially decreased (p < 0.01) as compared to the control (Yu et al. 2018). Altogether, quercetin, especially when conjugated with nano-particles, is a promising agent for eradication of E. coli biofilms associated with indwelling implants as well as recurrent urinary tract infections.
Pseudomonas aeruginosa
Classified as an opportunistic pathogen, Pseudomonas aeruginosa produces a wide array of deadly infections, particularly in immunocompromized patients (Memariani et al. 2016). Alginate, the principal component of exopolysaccharide matrix in biofilms, plays a crucial role in clinical outcomes of patients with cystic fibrosis (CF) and chronic wounds (Moradali et al. 2017). There is extensive evidence that blocking the quorum-sensing regulatory systems in P. aeruginosa interferes with biofilm development. The most defined quorum-sensing pathways in P. aeruginosa are the las and rhl systems (Waters and Smyth 2015).
Quercetin (8–64 μg/mL) has been shown to exert fairly strong anti-biofilm effects on P. aeruginosa strain PAO1, as evidenced by CLSM (Ouyang et al. 2016). Similar inhibitory properties of quercetin were reported in a study conducted on clinical isolates (Vipin et al. 2019a). Using colony-counting method, the authors revealed that 16 μg/mL of quercetin is more effective than 32 μg/mL of azithromycin in prevention of PAO1 adhesion to the microtiter plate surface. Of note, quercetin (16 μg/mL) significantly (p < 0.05) repressed expression levels of quorum-sensing associated genes lasI, lasR, rhlI, and rhlR by 34%, 68%, 57%, and 50%, respectively (Ouyang et al. 2016). Interestingly, quercetin can inhibit twitching motility (Vipin et al. 2019a), which has been linked with enhanced surface attachment, cell-to-cell adhesion, and biofilm production (Shreeram et al. 2018). Moreover, quercetin displayed negligible cytotoxicity (3.8–4.8%) against HEK 293T cells, even at concentrations up to 10,000 μg/mL. The agent also neutralized cytotoxic effects of P. aeruginosa isolates on HEK 293T cells, demonstrating cell protective efficacy of quercetin during bacterial infection (Vipin et al. 2019a).
One study showed the beneficial role of quercetin-AgNPs in mitigating either biofilm establishment or extracellular polymeric substance production by an ESBL-producing P. aeruginosa (Table 2). In this respect, quercetin-AgNPs at 10, 20, and 50 μg/mL reduced 39.2% (p ≤ 0.01), 62% (p ≤ 0.01), and 81% (p ≤ 0.05) of biofilm formation, respectively (Ahmed et al. 2018). CLSM analysis also demonstrated that quercetin-AgNPs induced not only active oxygen species generation but also damage to bacterial cell membrane and extracellular DNA (eDNA) release. Combinations of 1/2 × MIC of quercetin (250 μg/mL) with either 1/2 × MIC of amikacin (4 μg/mL) or 1/2 × MIC of tobramycin (1.5 μg/mL) completely extirpated biofilm-embedded bacterial cells following a 8-h of exposure at 37 °C, suggesting its potential to enhance anti-bacterial efficiency of existing antibiotics. CLSM analysis by live/dead assay further confirmed bactericidal synergism of quercetin-antibiotics toward biofilm-embedded P. aeruginosa isolates (Vipin et al. 2019b). Therefore, combinations of quercetin with conventional antibiotics or nano-particles, particularly in an aerosolized form, propound an auspicious strategy for eradication of biofilm-producing P. aeruginosa in cystic fibrosis patients.
Pseudomonas fluorescens
Pseudomonas fluorescens is an uncommon pathogen in humans, and usually occurs in patients with compromized immune systems. The bacterium is also one of the etiologic agents that are involved in fin rot disease in fish (Nishimura et al. 2017). In an attempt to determine the influence of plate material on biofilm inhibitory activity of quercetin, biofilm assays for Pseudomonas fluorescens Pf0-1 were performed in sandblasted polypropylene and polystyrene microtiter plates containing M63 medium supplemented with quercetin (50 and 100 μg/mL). Biofilm inhibition in polypropylene was noticeably higher than that of polystyrene microtiter plates (Bordeleau et al. 2018). This finding can be attributed to the aromatic nature of quercetin, resulting in higher degree of quercetin absorption onto polystyrene in comparison to polypropylene microtiter plates. The absorption causes insufficient concentrations of quercetin in media required for biofilm inhibition.
Proteus mirabilis
Proteus mirabilis is a common cause of urinary tract infections (UTIs) in patients with functional or structural abnormalities or with long-term catheterization (Armbruster et al. 2018). In a study performed by Aygül et al. (2019), quercetin (0.12 to 1.00 mM) was shown to activate biofilm production by P. mirabilis HI4320 compared to the untreated control. Furthermore, it reduced swarming motility in a concentration-dependent fashion after 12 and 24 h of incubation. The authors found that quercetin decreased expression levels of several virulence genes such as those involved in polyamine synthesis, swarming motility, and flagella expression (Aygül et al. 2019). Nevertheless, the possible mechanisms for stimulatory effects of quercetin on biofilm formation by P. mirabilis remained unknown and need to be scrutinized in future studies.
Vibrio harveyi
Vibrio harveyi is a pathogen of marine creatures including both fish and shrimps (Austin and Zhang 2006). Quercetin has been demonstrated to reduce biofilm formation and bacterial cell–cell communication by V. harveyi BB120 in a dose-dependent manner. It seems that quercetin at lower concentrations mostly inhibits quorum-sensing systems, while bacterial growth is affected at higher concentrations (Vikram et al. 2010). In general, quercetin can be used in aquaculture as prophylactic or therapeutic measures.
Bacterial targets for anti-biofilm effects
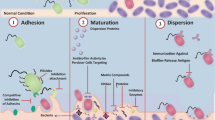
The detailed mechanisms of action underlying the anti-biofilm effects of quercetin will definitely help us to optimize our strategies for combating biofilms. Thus, in this section, we describe bacterial targets for anti-biofilm action of quercetin, as depicted in Fig. 1.
Disruption or alteration of plasma membrane
Bacterial plasma membrane executes a plethora of essential functions including transport, osmoregulation, respiration processes, biosynthesis of peptidoglycan, and synthesis of lipids. For instance, one study showed that both quercetin and quercetin-3-O-rhamnoglucoside (rutin) can decrease bilayer thickness of the cellular membrane, while only rutin is able to disrupt the lipid monolayer structure (Sanver et al. 2016). In a study conducted by Amin et al., potassium release was measured to determine the effects of antibiotic-flavonoids combinations on the plasma membrane of 100 clinical MRSA strains. In this regard, potassium leakage was highest for morin + rutin + quercetin which improved further in combination with imipenem, indicating that cytoplasmic membrane damage in conjunction with cell wall damage can be assumed to be the mechanism of action of these combinations (Amin et al. 2015). Likewise, Wang et al. demonstrated that quercetin is capable of damaging plasma membranes and cell walls of S. aureus and E. coli, as evidenced by leakage of β-galactosidase and alkaline phosphatase from the bacterial cells (Wang et al. 2018b). Tentative evidence suggests that the hydroxyl group at C-3 in flavonoids is the primary determinant for significant membrane interaction (Wu et al. 2013). Therefore, quercetin can reduce viability of both planktonic and biofilm-embedded bacteria by permeabilizing the bacterial membrane.
Quercetin has been shown to diminish LPS production in E. coli O157:H7 (Lee et al. 2010).Given the importance of LPS in the structural integrity of the outer membrane of Gram-negative bacteria and the bacterial–surface interactions (Nakao et al. 2012), targeting LPS by quercetin may be beneficial in reducing viability of bacteria within biofilms and bacterial adhesion to surfaces.
Inhibition of cell envelope synthesis
Peptidoglycan is an essential component of the bacterial cell wall. One study demonstrated that quercetin inhibits d-alanine-d-alanine ligase, which is responsible for the production of the terminal dipeptide of peptidoglycan precursor UDPMurNAc-pentapeptide (Wu et al. 2008). Quercetin binds to the active center of d-alanine-d-alanine ligase (Singh et al. 2013; Wu et al. 2008). Inhibition of d-alanine-d-alanine ligase induces a cell wall deficiency, compromising the ability of S. mutans for surface adhesion and biofilm formation (Qiu et al. 2016). Quercetin also impairs biofilm formation in both S. aureus and S. pneumoniae by inhibiting SrtA activity (Wang et al. 2018a; Kang et al. 2006; Liu et al. 2015). As a cysteine transpeptidase in most of Gram-positive bacteria, SrtA can mediate the anchorage of many surface protein virulence factors to the cell wall layer.
Prevention of bacterial adhesion
Adherence of bacteria to surface is an initial step in biofilm development. Various factors such as chemical structure, surface roughness, and surface free energy have been shown to affect bacterial cell–surface interactions (Malhotra et al. 2019). Quercetin is capable of reducing bacterial attachment to surface and blocking the expression of genes involved in bacterial adhesion (Vipin et al. 2019a; Vazquez-Armenta et al. 2018; Lee et al. 2013; Hasan et al. 2014). In this context, alterations in the surface free energy and cell surface hydrophobicity by quercetin are believed to prevent bacterial adhesion to surfaces. Besides, quercetin has the ability to decrease the expression level of Antigen I/II, a surface anchored protein of S. mutans involving in adhesion, biofilm formation, and collagen-dependent bacterial invasion of dentin (Hasan et al. 2014). Quercetin also inhibits S. aureus biofilm development by suppressing expression of polysaccharide intercellular adhesion genes (Lee et al. 2013).
Interfering with quorum-sensing
Autoinducer-2-mediated cell–cell signaling is an important regulatory factor for the biofilm production in different Gram-negative bacteria. Quercetin can act as an antagonist of cell–cell signaling, resulting in inhibition of biofilm formation in E. coli O157:H7 and V. harveyi (Vikram et al. 2010). It is able to diminish the expression of quorum-sensing genes such as lasI, lasR, rhlI, and rhlR in P. aeruginosa (Ouyang et al. 2016). Interestingly, quercetin up-regulates the expression of several iron siderophore proteins, limiting the amount of Fe3+ that is required for biofilm development in P. aeruginosa (Symeonidis and Marangos 2012).
In S. aureus, agr quorum-sensing system modulates the expression of virulence factors as well as biofilm formation (Boles and Horswill 2008). AgrB is an integral membrane endopeptidase that converts the precursor AIP (autoinducer peptide), AgrD, to mature AIP and exports it. AIP is recognized by the AgrC (membrane-bound receptor histidine kinase), which subsequently phosphorylates AgrA in the cytosol. Upon phosphorylation, AgrA binds to P2 and P3, up-regulating agr transcription of RNAII and RNAIII. RNAIII regulates the expression of many genes encoding exoproteins and cell-wall-associated proteins (Tan et al. 2018). Quercetin has been shown to reduce expression of agrA, which can serve as a potential drug for inhibition of agr quorum-sensing system (Lee et al. 2013). On the other hand, many Streptococcus species use quorum-sensing systems to regulate several physiological properties, including the ability to incorporate foreign DNA, tolerate acid, form biofilm, and become virulent (Jimenez and Federle 2014; Kaur et al. 2015). Interference with quorum-sensing systems in different species of Streptococcus, in particular S. mutans, has been reported in the literature (Abachi et al. 2016; Asfour 2018; Lu et al. 2019).
Inhibition of efflux pumps
Although efflux pumps are widely implicated in bacterial antibiotic resistance, new evidence suggests that the pumps also play crucial roles in bacterial pathogenesis, virulence, and biofilm formation. Many well-characterized efflux systems including AcrAB-TolC of E. coli, AcrD of Salmonella enterica, AdeFGH of Acinetobacter baumannii, and MexAB-OprM of P. aeruginosa are involved in biofilm formation (Shriram et al. 2018; Ohene-Agyei et al. 2014; Alav et al. 2018). In silico interaction studies using molecular docking showed that quercetin can bring down Mmr (in Mycobacterium tuberculosis) and EmrE (in E. coli) efflux pumps, suggesting its potential as a non-antibiotic adjuvant for treatment of bacterial infections (Suriyanarayanan and Sarojini Santhosh 2015). Quercetin has also been identified as a high-affinity substrate for TtgR (the transcriptional repressor of TtgABC efflux pump) of Pseudomonas putida (Alguel et al. 2007). Moreover, quercetin has been reported as a substrate for AcrB as deletion of acrB from E. coli resulted in a more than 8-fold reduction in the MIC of quercetin (Al-Karablieh et al. 2009).
Blocking nucleic acid synthesis
Flavonoids are potent topoisomerase inhibitors. DNA gyrase is a type II topoisomerase that introduces or removes negative supercoils, forms or resolves catenanes, and knots or unknots DNA (Górniak et al. 2019). It is pivotal for replication of DNA and transcription, thus affecting cell division (Khan et al. 2018). DNA gyrase is necessary for not only the survival of bacteria within biofilm but also their further spread to a new area (Roy et al. 2018). One study reported that quercetin inhibits this enzyme in E. coli (Ohemeng et al. 1993). Moreover, in silico analysis suggested that subunit B of DNA gyrase (GyrB) from Mycobacterium smegmatis and Mycobacterium tuberculosis can be targeted by quercetin (Suriyanarayanan et al. 2013). It has also been established that quercetin suppresses DNA gyrase by two different mechanisms. Based on the first mechanism, binding of quercetin to DNA stabilizes DNA–gyrase complex, resulting in DNA cleavage. The second mechanism includes competition of quercetin with ATP for the binding site, leading to inhibition of DNA supercoiling activity (Plaper et al. 2003).
Outlook
Anti-biofilm properties of quercetin in conjunction with its negligible adverse effects on human cells suggest this natural herbal flavonol as a safe and inexpensive compound for treatment of recalcitrant infections caused by biofilm-producing pathogens. Various strategies can be exploited in order to introduce novel bio-medical applications and to augment lucrative features of quercetin. It is worth accentuating that catheter-related biofilm infections are a major culprit behind morbidity and mortality in patients who underwent catheterization (Sajeevan et al. 2018). To address this challenge, impregnation of catheters and artificial joints with quercetin may be beneficial in abolishing bacterial adhesion and biofilm establishment. Since quercetin has anti-bacterial and anti-biofilm effects on S. mutans, it can be applied as an anti-caries agent by different ways known for oral administration such as coating on dental floss or even adding into toothpastes and mouth rinse liquids. Besides, quercetin can be incorporated into gels, lotions, ointments, and dressings, or spray as a solution onto a wound to prevent bacterial biofilm development. Another enticing strategy is aerosol administration of quercetin for patients suffering from cystic fibrosis, an inherited disease in which biofilm formation by P. aeruginosa can contribute to treatment failure and even hasten mortality (Oluyombo et al. 2019). Combination of quercetin with existing antibiotics and/or cationic anti-biofilm peptides is the other plausible approach which can minimize not only effective doses of each agent but also the emergence of drug-resistant superbugs. Last but not least, targeted delivery of quercetin by nano-size carriers is rather the other way for prophylaxis or treatment of biofilm-related infections.
Conclusion
As hinted above, quercetin showed broad-spectrum anti-biofilm properties against diverse bacterial pathogens. Fortunately, biofilm inhibition can be achieved at concentrations even lower than required for killing planktonic bacteria. In addition to negligible cytotoxic effects, quercetin has high potential for down-regulation of virulence genes such those associated with hemolysin production in certain pathogens. In future studies, deployment of microfluidic devices for assessing anti-biofilm effects of quercetin on different pathogens will surely expand our knowledge of biofilm biology. All in all, anti-biofilm properties of quercetin can open up new horizons in a wide range of biomedical areas, from food industry to medicine.
References
Abachi S, Lee S, Rupasinghe HP (2016) Molecular mechanisms of inhibition of Streptococcus species by phytochemicals. Molecules 21(2):215. https://doi.org/10.3390/molecules21020215
Adnan M, Sousa AM, Machado I, Pereira MO, Khan S, Morton G, Hadi S (2017) Role of bolA and rpoS genes in biofilm formation and adherence pattern by Escherichia coli K-12 MG1655 on polypropylene, stainless steel, and silicone surfaces. Acta Microbiol Immunol Hung 64(2):179–189. https://doi.org/10.1556/030.63.2016.018
Ahmed B, Hashmi A, Khan MS, Musarrat J (2018) ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv Powder Technol 29(7):1601–1616. https://doi.org/10.1016/j.apt.2018.03.025
Alav I, Sutton JM, Rahman KM (2018) Role of bacterial efflux pumps in biofilm formation. J Antimicrob Chemother 73(8):2003–2020. https://doi.org/10.1093/jac/dky042
Alguel Y, Meng C, Terán W, Krell T, Ramos JL, Gallegos MT, Zhang X (2007) Crystal structures of multidrug binding protein TtgR in complex with antibiotics and plant antimicrobials. J Mol Biol 369(3):829–840. https://doi.org/10.1016/j.jmb.2007.03.062
Al-Karablieh N, Weingart H, Ullrich MS (2009) Genetic exchange of multidrug efflux pumps among two enterobacterial species with distinctive ecological Niches. Int J Mol Sci 10(2):629–645. https://doi.org/10.3390/ijms10020629
Amin MU, Khurram M, Khattak B, Khan J (2015) Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement Altern Med 15:59. https://doi.org/10.1186/s12906-015-0580-0
Anand David AV, Arulmoli R, Parasuraman S (2016) Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 10(20):84–89. https://doi.org/10.4103/0973-7847.194044
Armbruster CE, Mobley HLT, Pearson MM (2018) Pathogenesis of Proteus mirabilis infection. EcoSal Plus. https://doi.org/10.1128/ecosalplus.ESP-0009-2017
Asfour HZ (2018) Anti-quorum sensing natural compounds. J Microsc Ultrastruct 6(1):1–10. https://doi.org/10.4103/JMAU.JMAU_10_18
Austin B, Zhang XH (2006) Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol 43(2):119–124. https://doi.org/10.1111/j.1472-765X.2006.01989.x
Aygül A, Öztürk İ, Çilli FF, Ermertcan Ş (2019) Quercetin inhibits swarming motility and activates biofilm production of Proteus mirabilis possibly by interacting with central regulators, metabolic status or active pump proteins. Phytomedicine 57:65–71. https://doi.org/10.1016/j.phymed.2018.12.014
Bøhle LA, Færgestad EM, Veiseth-Kent E, Steinmoen H, Nes IF, Eijsink VGH, Mathiesen G (2010) Identification of proteins related to the stress response in Enterococcus faecalis V583 caused by bovine bile. Proteome Sci 8:37. https://doi.org/10.1186/1477-5956-8-37
Boles BR, Horswill AR (2008) agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4(4):e1000052. https://doi.org/10.1371/journal.ppat.1000052
Bordeleau E, Mazinani SA, Nguyen D, Betancourt F, Yan H (2018) Abrasive treatment of microtiter plates improves the reproducibility of bacterial biofilm assays. RSC Adv 8:32434–32439. https://doi.org/10.1039/c8ra06352d
Borges A, Saavedra MJ, Simões M (2012) The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 28:755–767. https://doi.org/10.1080/08927014.2012.706751
Brauge T, Sadovskaya I, Faille C, Benezech T, Maes E, Guerardel Y, Midelet-Bourdin G (2016) Teichoic acid is the major polysaccharide present in the Listeria monocytogenes biofilm matrix. FEMS Microbiol Lett 363(2):fnv229. https://doi.org/10.1093/femsle/fnv229
Brown TA Jr, Ahn SJ, Frank RN, Chen YY, Lemos JA, Burne RA (2005) A hypothetical protein of Streptococcus mutans is critical for biofilm formation. Infect Immun 73(5):3147–3151. https://doi.org/10.1128/IAI.73.5.3147-3151.2005
Chao Y, Marks LR, Pettigrew MM, Hakansson AP (2015) Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front Cell Infect Microbiol 4:194. https://doi.org/10.3389/fcimb.2014.00194
Ch’ng JH, Chong KKL, Lam LN, Wong JJ, Kline KA (2019) Biofilm-associated infection by enterococci. Nat Rev Microbiol 17(2):82–94. https://doi.org/10.1038/s41579-018-0107-z
Cho HS, Lee JH, Cho MH, Lee J (2014) Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 31(1):1–11. https://doi.org/10.1080/08927014.2014.991319
da Costa Júnior SD, de Oliveira Santos JV, de Almeida Campos LA, Pereira MA, Santos Magalhães NS, Ferro Cavalcanti IS (2018) Antibacterial and antibiofilm activities of quercetin against clinical isolates of Staphyloccocus aureus and Staphylococcus saprophyticus with resistance profile. Int J Env Agr Biotech 3:1948–1958. https://doi.org/10.22161/ijeab/3.5.50
Dani S, Prabhu A, Chaitra KR, Desai NC, Patil SR, Rajeev R (2016) Assessment of Streptococcus mutans in healthy versus gingivitis and chronic periodontitis: a clinico-microbiological study. Contemp Clin Dent 7:529–534. https://doi.org/10.4103/0976-237X.194114
Earl AM, Losick R, Kolter R (2008) Ecology and genomics of Bacillus subtilis. Trends Microbiol 16(6):269–275. https://doi.org/10.1016/j.tim.2008.03.004
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575. https://doi.org/10.1038/nrmicro.2016.94
Gennaris A, Collet JF (2013) The ‘captain of the men of death’, Streptococcus pneumoniae, fights oxidative stress outside the ‘city wall’. EMBO Mol Med 5(12):1798–1800. https://doi.org/10.1002/emmm.201303482
González Barrios AF, Zuo R, Hashimoto Y, Yang L, Bentley WE, Wood TK (2006) Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J Bacteriol 188(1):305–316. https://doi.org/10.1128/JB.188.1.305-316.2006
Górniak I, Bartoszewski R, Króliczewski J (2019) Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 18(1):241–272. https://doi.org/10.1007/s11101-018-9591-z
Hasan S, Singh K, Danisuddin M, Verma PK, Khan AU (2014) Inhibition of major virulence pathways of Streptococcus mutans by quercitrin and deoxynojirimycin: a synergistic approach of infection control. PLoS ONE 9(3):e91736. https://doi.org/10.1371/journal.pone.0091736
Hussain MS, Fareed S, Ansari S, Rahman MA, Ahmad IZ, Saeed M (2012) Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci 4(1):10–20. https://doi.org/10.4103/0975-7406.92725
Ito A, May T, Kawata K, Okabe S (2008) Significance of rpoS during maturation of Escherichia coli biofilms. Biotechnol Bioeng 99(6):1462–1471. https://doi.org/10.1002/bit.21695
Jimenez JC, Federle MJ (2014) Quorum sensing in group A Streptococcus. Front Cell Infect Microbiol 4:127. https://doi.org/10.3389/fcimb.2014.00127
Kane TL, Carothers KE, Lee SW (2018) Virulence factor targeting of the bacterial pathogen Staphylococcus aureus for vaccine and therapeutics. Curr Drug Targets 19(2):111–127. https://doi.org/10.2174/1389450117666161128123536
Kang SS, Kim JG, Lee TH, Oh KB (2006) Flavonols inhibit sortases and sortase-mediated Staphylococcus aureus clumping to fibrinogen. Biol Pharm Bull 29(8):1751–1752. https://doi.org/10.1248/bpb.29.1751
Kaur G, Rajesh S, Princy SA (2015) Plausible drug targets in the Streptococcus mutans quorum sensing pathways to combat dental biofilms and associated risks. Indian J Microbiol 55(4):349–356. https://doi.org/10.1007/s12088-015-0534-8
Khan T, Sankhe K, Suvarna V, Sherje A, Patel K, Dravyakar B (2018) DNA gyrase inhibitors: progress and synthesis of potent compounds as antibacterial agents. Biomed Pharmacother 103:923–938. https://doi.org/10.1016/j.biopha.2018.04.021
Kim MK, Lee TG, Jung M, Park KH, Chong Y (2018) In vitro synergism and anti-biofilm activity of quercetin-pivaloxymethyl conjugate against Staphylococcus aureus and Enterococcus species. Chem Pharm Bull (Tokyo) 66:1019–1022. https://doi.org/10.1248/cpb.c18-00380
Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L (2017) Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15(12):740–755. https://doi.org/10.1038/nrmicro.2017.99
Lee Y (2017) Biofilm formation and antimicrobial resistance in Enterococcus. Infect Chemother 49(3):236–237. https://doi.org/10.3947/ic.2017.49.3.236
Lee KA, Moon SH, Kim KT, Mendonca AF, Paik HD (2010) Antimicrobial effects of various flavonoids on Escherichia coli O157:H7 cell growth and lipopolysaccharide production. Food Sci Biotechnol 19(1):257–261. https://doi.org/10.1007/s10068-010-0037-7
Lee JH, Park JH, Cho HS, Joo SW, Cho MH, Lee J (2013) Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 29:491–499. https://doi.org/10.1080/08927014.2013.788692
Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y (2016) Quercetin, Inflammation and Immunity. Nutrients 8(3):167. https://doi.org/10.3390/nu8030167
Liu B, Chen F, Bi C, Wang L, Zhong X, Cai H, Deng X, Niu X, Wang D (2015) Quercitrin, an inhibitor of Sortase A, interferes with the adhesion of Staphylococcal aureus. Molecules 20(4):6533–6543. https://doi.org/10.3390/molecules20046533
López D, Vlamakis H, Kolter R (2010) Biofilms. Cold Spring Harb Perspect Biol 2(7):a000398. https://doi.org/10.1101/cshperspect.a000398
Lu L, Hu W, Tian Z, Yuan D, Yi G, Zhou Y, Cheng Q, Zhu J, Li M (2019) Developing natural products as potential anti-biofilm agents. Chin Med 14:11. https://doi.org/10.1186/s13020-019-0232-2
Malhotra R, Dhawan B, Garg B, Shankar V, Nag TC (2019) A comparison of bacterial adhesion and biofilm formation on commonly used orthopaedic metal implant materials: an in vitro study. Indian J Orthop 53(1):148–153. https://doi.org/10.4103/ortho.IJOrtho_66_18
Markowska K, Grudniak AM, Wolska KI (2013) Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol 60(4):523–530
Memariani M, Peerayeh SN, Mostafavi SKS, Salehi TZ (2014) Detection of class 1 and 2 integrons among enteropathogenic Escherichia coli isolates. Arch of Pediatr Infect Dis 2(4):e16372. https://doi.org/10.5812/pedinfect.16372
Memariani H, Shahbazzadeh D, Sabatier JM, Memariani M, Karbalaeimahdi A, Bagheri KP (2016) Mechanism of action and in vitro activity of short hybrid antimicrobial peptide PV3 against Pseudomonas aeruginosa. Biochem Biophys Res Commun 479(1):103–108. https://doi.org/10.1016/j.bbrc.2016.09.045
Memariani H, Memariani M, Pourmand MR (2018) Venom-derived peptide Mastoparan-1 eradicates planktonic and biofilm-embedded methicillin-resistant Staphylococcus aureus isolates. Microb Pathog 119:72–80. https://doi.org/10.1016/j.micpath.2018.04.008
Memariani H, Memariani M, Shahidi-Dadras M, Nasiri S, Akhavan MM, Moravvej H (2019a) Melittin: from honeybees to superbugs. Appl Microbiol Biotechnol 103(8):3265–3276. https://doi.org/10.1007/s00253-019-09698-y
Memariani M, Memariani H, Shahidi-Dadras M, Tehranchinia Z, Ghalamkarpour F, Moravvej H (2019b) Contemporary systematic review and meta-analysis of exfoliative toxin-producing Staphylococcus aureus strains isolated from patients in Iran. Rev Med Microbiol. https://doi.org/10.1097/MRM.0000000000000177
Miller WR, Munita JM, Arias CA (2015) Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther 12(10):1221–1236. https://doi.org/10.1586/14787210.2014.956092
Moormeier DE, Bayles KW (2017) Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol 104(3):365–376. https://doi.org/10.1111/mmi.13634
Moradali MF, Ghods S, Rehm BH (2017) Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39. https://doi.org/10.3389/fcimb.2017.00039
Nabavi SM, Silva AS (2019) Nonvitamin and nonmineral nutritional supplements, 1st edn. Academic Press, Cambridge. https://doi.org/10.1016/C2016-0-03546-5
Nakao R, Ramstedt M, Wai SN, Uhlin BE (2012) Enhanced biofilm formation by Escherichia coli LPS mutants defective in Hep biosynthesis. PLoS ONE 7(12):e51241. https://doi.org/10.1371/journal.pone.0051241
Naves P, del Prado G, Huelves L, Gracia M, Ruiz V, Blanco J, Rodríguez-Cerrato V, Ponte MC, Soriano F (2008) Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. J Appl Microbiol 105(2):585–590. https://doi.org/10.1111/j.1365-2672.2008.03791.x
Nishimura T, Hattori K, Inoue A, Ishii T, Yumoto T, Tsukahara K, Nakao A, Ishihara S, Nakayama S (2017) Bacteremia or pseudobacteremia? Review of pseudomonas fluorescens infections. World J Emerg Med 8(2):151–154. https://doi.org/10.5847/wjem.j.1920-8642.2017.02.013
O’Gara JP (2007) ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett 270:179–188. https://doi.org/10.1111/j.1574-6968.2007.00688.x
Oggioni MR, Trappetti C, Kadioglu A, Cassone M, Iannelli F, Ricci S, Andrew PW, Pozzi G (2006) Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol Microbiol 61:1196–1210. https://doi.org/10.1111/j.1365-2958.2006.05310.x
Ohemeng KA, Schwender CF, Barrett JF (1993) DNA gyrase inhibitory and antibacterial activity of some flavones(1). Bioorg Med Chem Lett 3(2):225–230. https://doi.org/10.1016/S0960-894X(01)80881-7
Ohene-Agyei T, Mowla R, Rahman T, Venter H (2014) Phytochemicals increase the antibacterial activity of antibiotics by acting on a drug efflux pump. Microbiologyopen 3(6):885–896. https://doi.org/10.1002/mbo3.212
Oluyombo O, Penfold CN, Diggle SP (2019) Competition in biofilms between cystic fibrosis isolates of Pseudomonas aeruginosa is shaped by R-Pyocins. MBio 10(1):e01828-18. https://doi.org/10.1128/mBio.01828-18
Ouyang J, Sun F, Feng W, Sun Y, Qiu X, Xiong L, Liu Y, Chen Y (2016) Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J Appl Microbiol 120(4):966–974. https://doi.org/10.1111/jam.13073
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47. https://doi.org/10.1017/jns.2016.41
Parker D, Soong G, Planet P, Brower J, Ratner AJ, Prince A (2009) The NanA neuraminidase of Streptococcus pneumoniae is involved in biofilm formation. Infect Immun 77(9):3722–3730. https://doi.org/10.1128/IAI.00228-09
Plaper A, Golob M, Hafner I, Oblak M, Solmajer T, Jerala R (2003) Characterization of quercetin binding site on DNA gyrase. Biochem Biophys Res Commun 306(2):530–536. https://doi.org/10.1016/s0006-291x(03)01006-4
Qayyum S, Sharma D, Bisht D, Khan AU (2019) Identification of factors involved in Enterococcus faecalis biofilm under quercetin stress. Microb Pathog 126:205–211. https://doi.org/10.1016/j.micpath.2018.11.013
Qiu W, Zheng X, Wei Y, Zhou X, Zhang K, Wang S, Cheng L, Li Y, Ren B, Xu X, Li Y, Li M (2016) d-Alanine metabolism is essential for growth and biofilm formation of Streptococcus mutans. Mol Oral Microbiol 31(5):435–444. https://doi.org/10.1111/omi.12146
Raie DS, Mhatre E, El-Desouki DS, Labena A, El-Ghannam G, Farahat LA, Youssef T, Fritzsche W, Kovács ÁT (2018) Effect of novel quercetin titanium dioxide-decorated multi-walled carbon nanotubes nanocomposite on Bacillus subtilis biofilm development. Materials (Basel) 11:157. https://doi.org/10.3390/ma11010157
Rodríguez-López P, Rodríguez-Herrera JJ, Vázquez-Sánchez D, López Cabo M (2018) Current knowledge on Listeria monocytogenes biofilms in food-related environments: incidence, resistance to biocides, ecology and biocontrol. Foods 7(6):E85. https://doi.org/10.3390/foods7060085
Ronald A (2003) The etiology of urinary tract infection: traditional and emerging pathogens. Dis Mon 49(2):71–82. https://doi.org/10.1067/mda.2003.8
Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9(1):522–554. https://doi.org/10.1080/21505594.2017.1313372
Sadekuzzaman M, Yang S, Mizan MFR, Ha SD (2015) Current and recent advanced strategies for combating biofilms. Compr Rev Food Sci Food Saf 14:491–509. https://doi.org/10.1111/1541-4337.12144
Sajeevan SE, Chatterjee M, Paul V, Baranwal G, Kumar VA, Bose C, Banerji A, Nair BG, Prasanth BP, Biswas R (2018) Impregnation of catheters with anacardic acid from cashew nut shell prevents Staphylococcus aureus biofilm development. J Appl Microbiol 125(5):1286–1295. https://doi.org/10.1111/jam.14040
Samoilova Z, Muzyka N, Lepekhina E, Oktyabrsky O, Smirnova G (2014) Medicinal plant extracts can variously modify biofilm formation in Escherichia coli. Antonie Van Leeuwenhoek 105(4):709–722. https://doi.org/10.1007/s10482-014-0126-3
Sanver D, Murray BS, Sadeghpour A, Rappolt M, Nelson AL (2016) Experimental modeling of flavonoid-biomembrane interactions. Langmuir 32(49):13234–13243. https://doi.org/10.1021/acs.langmuir.6b02219
Shak JR, Ludewick HP, Howery KE, Sakai F, Yi H, Harvey RM, Paton JC, Klugman KP, Vidal JE (2013) Novel role for the Streptococcus pneumoniae toxin pneumolysin in the assembly of biofilms. MBio 4(5):e00655-13. https://doi.org/10.1128/mBio.00655-13
Shreeram DD, Panmanee W, McDaniel CT, Daniel S, Schaefer DW, Hassett DJ (2018) Effect of impaired twitching motility and biofilm dispersion on performance of Pseudomonas aeruginosa–powered microbial fuel cells. J Ind Microbiol Biotechnol 45(2):103–109. https://doi.org/10.1007/s10295-017-1995-z
Shriram V, Khare T, Bhagwat R, Shukla R, Kumar V (2018) Inhibiting bacterial drug efflux pumps via phyto-therapeutics to combat threatening antimicrobial resistance. Front Microbiol 9:2990. https://doi.org/10.3389/fmicb.2018.02990
Singh SP, Konwarh R, Konwar BK, Karak N (2013) Molecular docking studies on analogues of quercetin with d-alanine:d-alanine ligase of Helicobacter pylori. Med Chem Res 22(5):2139–2150. https://doi.org/10.1007/s00044-012-0207-7
Smith AJ, Oertle J, Warren D, Prato D (2016) Quercetin: a promising flavonoid with a dynamic ability to treat various diseases, infections, and cancers. J Can Ther 7:83–95. https://doi.org/10.4236/jct.2016.72010
Suriyanarayanan B, Sarojini Santhosh R (2015) Docking analysis insights quercetin can be a non-antibiotic adjuvant by inhibiting Mmr drug efflux pump in Mycobacterium sp. and its homologue EmrE in Escherichia coli. J Biomol Struct Dyn 33(8):1819–1834. https://doi.org/10.1080/07391102.2014.974211
Suriyanarayanan B, Shanmugam K, Santhosh RS (2013) Synthetic quercetin inhibits mycobacterial growth possibly by interacting with DNA gyrase. Rom Biotechnol Lett 18(5):8587–8593
Symeonidis A, Marangos M (2012) Iron and microbial growth. In: Priti DR (ed) Insight and control of infectious disease in global scenario. InTechOpen, London
Tan L, Li SR, Jiang B, Hu XM, Li S (2018) Therapeutic targeting of the Staphylococcus aureus accessory gene regulator (agr) system. Front Microbiol 9:55. https://doi.org/10.3389/fmicb.2018.00055
Trappetti C, Kadioglu A, Carter M, Hayre J, Iannelli F, Pozzi G, Andrew PW, Oggioni MR (2009) Sialic acid: a preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J Infect Dis 199:1497–1505. https://doi.org/10.1086/598483
Vanaraj S, Keerthana BB, Preethi K (2017) Biosynthesis, characterization of silver nanoparticles using quercetin from Clitoria ternatea L to enhance toxicity against bacterial biofilm. J Inorg Organomet Polym Mater 27(5):1412–1422. https://doi.org/10.1007/s10904-017-0595-8
Vazquez-Armenta FJ, Bernal-Mercado AT, Tapia-Rodriguez MR, Gonzalez-Aguilar GA, Lopez-Zavala AA, Martinez-Tellez MA, Hernandez-Onate MA, Ayala-Zavala JF (2018) Quercetin reduces adhesion and inhibits biofilm development by Listeria monocytogenes by reducing the amount of extracellular proteins. Food Control 90:266–273. https://doi.org/10.1016/j.foodcont.2018.02.041
Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS (2010) Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J Appl Microbiol 109(2):515–527. https://doi.org/10.1111/j.1365-2672.2010.04677.x
Vipin C, Mujeeburahiman M, Ashwini P, Arun AB, Rekha PD (2019a) Anti-biofilm and cytoprotective activities of quercetin against Pseudomonas aeruginosa isolates. Lett Appl Microbiol 68(5):464–471. https://doi.org/10.1111/lam.13129
Vipin C, Mujeeburahiman M, Saptami K, Arun AB, Rekha PD (2019b) Synergistic interactions of quercetin with antibiotics against biofilm associated clinical isolates of Pseudomonas aeruginosa in vitro. bioRxiv. https://doi.org/10.1101/601336
Wang J, Song M, Pan J, Shen X, Liu W, Zhang X, Li H, Deng X (2018a) Quercetin impairs Streptococcus pneumoniae biofilm formation by inhibiting sortase A activity. J Cell Mol Med 22(12):6228–6237. https://doi.org/10.1111/jcmm.1391010.1111/jcmm.13910
Wang S, Yao J, Zhou B, Yang J, Chaudry MT, Wang M, Xiao F, Li Y, Yin W (2018b) Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J Food Prot 81(1):68–78. https://doi.org/10.4315/0362-028X.JFP-17-214
Waters V, Smyth A (2015) Cystic fibrosis microbiology: advances in antimicrobial therapy. J Cyst Fibros 14(5):551–560. https://doi.org/10.1016/j.jcf.2015.02.005
Wen ZT, Baker HV, Burne RA (2006) Influence of BrpA on critical virulence attributes of Streptococcus mutans. J Bacteriol 188(8):2983–2992. https://doi.org/10.1128/JB.188.8.2983-2992.2006
Wu D, Kong Y, Han C, Chen J, Hu L, Jiang H, Shen X (2008) D-Alanine:D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int J Antimicrob Agents 32(5):421–426. https://doi.org/10.1016/j.ijantimicag.2008.06.010
Wu T, He M, Zang X, Zhou Y, Qiu T, Pan S, Xu X (2013) A structure–activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim Biophys Acta 1828(11):2751–2756. https://doi.org/10.1016/j.bbamem.2013.07.029
Wu H, Moser C, Wang HZ, Høiby N, Song Z (2015) Strategies for combating bacterial biofilm infections. Int J Oral Sci 7(1):1–7. https://doi.org/10.1038/ijos.2014.65
Yang H, Li K, Yan H, Liu S, Wang Y, Huang C (2017) High-performance therapeutic quercetin-doped adhesive for adhesive–dentin interfaces. Sci Rep 7:8189. https://doi.org/10.1038/s41598-017-08633-3
Yu L, Shang F, Chen X, Ni J, Yu L, Zhang M, Sun D, Xue T (2018) The anti-biofilm effect of silver-nanoparticle-decorated quercetin nanoparticles on a multi-drug resistant Escherichia coli strain isolated from a dairy cow with mastitis. PeerJ 6:e5711. https://doi.org/10.7717/peerj.5711
Zeng Y, Nikitkova A, Abdelsalam H, Li J, Xiao J (2019) Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch Oral Biol 98:9–16. https://doi.org/10.1016/j.archoralbio.2018.11.005
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
HM and MM wrote the manuscript. AG and MM performed data collection and management. MM and HM contributed to revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Memariani, H., Memariani, M. & Ghasemian, A. An overview on anti-biofilm properties of quercetin against bacterial pathogens. World J Microbiol Biotechnol 35, 143 (2019). https://doi.org/10.1007/s11274-019-2719-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2719-5