Abstract
The emergence of antibiotic-resistant bacteria, dubbed superbugs, together with relative stagnation in developing efficient antibiotics has led to enormous health and economic problems, necessitating the need for discovering and developing novel antimicrobial agents. In this respect, animal venoms represent a rich repertoire of pharmacologically active components. As a major component in the venom of European honeybee Apis mellifera, melittin has a great potential in medical applications. In this mini-review, we summarize a multitude of studies on anti-bacterial effects of melittin against planktonic and biofilm-embedded bacteria. Several investigations regarding synergistic effects between melittin and antibiotics were also described. On the whole, the properties of melittin can open up new horizons in a range of biomedical areas, from agriculture to veterinary and clinical microbiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern medicine has conquered many life-threatening illnesses, but threat of antibiotic-resistant bacteria seems to be a never-ending challenge facing humankind. It is anticipated that annual deaths attributable to anti-microbial resistance will surpass those of cancer by 2050, if we do not take action (O'Neill 2016). The greatest concern is imposed by the ESKAPE pathogens (i.e., Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) which have the ability to “escape” the lethal action of conventional anti-microbials and host immune responses (Rice 2008).
Extensive exposure to antibiotics has rapidly led to emergence and nationwide propagation of multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) bacteria, often dubbed superbugs, making the choice of chemotherapeutics for treatment more problematic (Deslouches et al. 2015; Mohamed et al. 2017). Such fear is even further compounded once pathogens establish biofilms on tissues and medical devices. In this respect, biofilm formation empowers bacteria to abscond from various clearance mechanisms produced by host and synthetic sources (Batoni et al. 2016; Overhage et al. 2008).
While admirable efforts are underway to potentiate current anti-microbial arsenal, the problem of antibiotic-resistance in superbugs is of sufficient importance that effective anti-microbial materials ought to be discovered and evaluated (Deslouches et al. 2015). Among the limited numbers of new anti-microbials in the pipeline, natural peptides from animal venoms have been demonstrated to possess promising biological properties, which warrant their development as efficacious agents against recalcitrant pathogens (Almeida et al. 2018; Memariani et al. 2017; Hale and Hancock 2007). This review summarizes empirical evidences on anti-bacterial and anti-biofilm properties of melittin, a major component of honeybee venom.
Animal venoms
Natural products originated from both plants and animals possess a diverse array of as-yet unidentified substances that suggest seemingly limitless possibilities for finding potential leads (Gajski and Garaj-Vrhovac 2013). Animal venoms are poisonous secretions which involved in protection against predators or immobilizing/killing preys (Andreotti et al. 2010). Though the composition of venoms varies from animal to animal, the majority of venoms comprise a myriad of enzymes, peptides, low molecular weight organic molecules, and inorganic salts (Pennington et al. 2018). Animal venoms have been exploited for thousands of years, in many traditional remedies and medicines, to cure a plethora of maladies including atopic dermatitis, arthritis, chronic pain, multiple sclerosis, infectious diseases, cancers, gastrointestinal issues, and cardiovascular diseases (Pennington et al. 2018; Andreotti et al. 2010; Lewis and Garcia 2003).
Recent propitious progresses toward high-throughput screening and characterization of venom components have provided the impetus to search for novel venom-based therapeutics such as those with anti-microbial activity (King 2011). In this context, venom-derived anti-microbial peptides (AMPs) are of particular interest because of their selectivity, broad-spectrum microbicidal activity, and relative safety (Pennington et al. 2018; Memariani et al. 2018a).
Bee venom
Unquestionably, venoms from various animals such as bees, snakes, scorpions, spiders, toads, octopus, and marine cone snails represent a rich source of pharmacologically active components, creating unique avenues for discovering promising biomolecules which can be used per se or as lead compounds in the development of therapeutic drugs (Sabatier 2011; Almeida et al. 2018). Honeybee venom is a complex concoction of biologically active substances, such as melittin, secapin, apamine, hyaluronidase, phospholipase A2, phospholipids, saccharides, noradrenaline, histamine, and dopamine, with enormous chemical and functional variability (Hider 1988).
Bee venom has been exploited since ancient times to treat several ailments. In oriental traditional medicine, bee venom has been used to treat skin maladies, palliate the back pain, and attenuate chronic inflammation conditions caused by both multiple sclerosis and rheumatoid arthritis (Oršolić 2012; Son et al. 2007). Likewise, honeybee venom can exert anti-atopic dermatitis (An et al. 2018), radioprotective (Varanda and Tavares 1998), anti-mutagenic (Varanda et al. 1999), anti-cancer (Oršolić 2012), and anti-microbial (AL-Ani et al. 2015) activities, attesting to the therapeutic potential of honeybee venom and its major constituent melittin as well.
Thus far, numerous peptides with anti-microbial activities have been isolated from bee venoms such as melittin (Fennell et al. 1967), melectin (Cerovský et al. 2008), macropin (Monincová et al. 2014), HYL (Nešuta et al. 2016), and Xac-1 (Kawakami et al. 2017), suggesting their potential use as natural antibiotics. Furthermore, there are several reviews in the literature regarding anti-cancer effects (Rady et al. 2017; Gajski and Garaj-Vrhovac 2013), anti-inflammatory properties (Lee and Bae 2016), and anti-diabetic activities (Hossen et al. 2017) of melittin. As far as we know, however, reviews of the anti-bacterial and anti-biofilm activities of melittin are currently not available.
Physiochemical, structural, and biological properties of melittin
Melittin is a major component in the venom of European honeybee Apis mellifera. It is a small cationic linear peptide (Fig. 1), comprising at least half of the venom dry weight (Tacón 2016). Melittin is composed of 26 amino acid residues (GIGAVLKVLTTGLPALISWIKRKRQQ-CONH2). At physiological pH, melittin has a net charge of + 6 due to the presence of arginine and lysine residues. The N- and C-terminal regions of melittin are mainly hydrophobic and hydrophilic, respectively (Rady et al. 2017). Polar and non-polar amino acid residues distribute asymmetrical in melittin, suggesting its amphipathic nature when it is adopted an α-helical conformation. This feature makes the peptide not only water-soluble but also membrane-active (Terwilliger and Eisenberg 1982).
X-ray crystallographic and nuclear magnetic resonance (NMR) studies revealed that each melittin chain has a structure consisting of two α-helical segments, one α-helix containing residues 1–10 and the longer one formed by residues 13–26. These two α-helices are joined by a “hinge” region between residues 11 and 12 to constitute a bent rod (Anderson et al. 1980; Terwilliger and Eisenberg 1982; Bazzo et al. 1988; Lam et al. 2001). Four conformationally identical monomers of melittin are packed together to form a tetramer which is non-lytic and it is predominant at concentrations found in bee’s abdominal sack (Tacón 2016; Terwilliger and Eisenberg 1982). It has been shown that melittin exists as a monomer at the minimum concentrations necessary for cell lysis. When the venom is released, dissociation of the tetramer occurs which yields the monomer (Hider 1988; Picoli et al. 2017). It is now apparent that melittin attaches to the membrane surface as monomers but acts on the membrane collectively to produce pores (Lee et al. 2013).
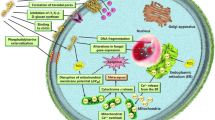
It has been proposed that melittin can form a short-lived pore in the membrane, as evidenced by the release of calcein dye from the liposomes, and the size of pore increases with the peptide-to-lipid molar ratio (P/L) (Matsuzaki et al. 1997). In addition, the peptide induces stable pores in the micromolar concentration range (Lee et al. 2013). Melittin is able to orient either parallel or perpendicular to a lipid bilayer (Smith et al. 1994; Yang et al. 2001). In this context, parallel binding of melittin to the membrane prohibits other melittin molecules from incorporating into lipid bilayer and creating pores, thereby protecting the membrane from leakage (van den Bogaart et al. 2008). As P/L exceeds a certain threshold, an increasing fraction of melittin molecules shifts toward the perpendicular orientation (Yang et al. 2001; van den Bogaart et al. 2008). Perpendicular orientation of melittin to the plane of the membrane is required for the formation of transmembrane pores, while the parallel orientation is inactive (Yang et al. 2001; van den Bogaart et al. 2008). When inserted in the membrane bilayers, the attached peptides aggregate, inducing the lipids to bend. It inevitably gives rise to the formation of toroidal pores and the subsequent leakage of cytoplasmic contents (Park et al. 2006). This level of knowledge with regard to membrane-disrupting mechanism of melittin is necessary for developing novel anti-microbial agents with improved therapeutic indices.
Melittin attacks all lipid membranes including those found in the erythrocyte membrane, resulting in hemolysis. Indeed, the release of hemoglobin is succeeded by the formation of ion-permeable pores (Raghuraman and Chattopadhyay 2007). Melittin has also been shown to exert allergenic activity by increasing serum immunoglobulin E (IgE) in nearly one-third of honeybee venom-sensitive individuals (Paull et al. 1977). However, this adverse feature might result from contamination with other bee venom constituents (Lee and Bae 2016). Furthermore, melittin can incorporate into phospholipid bilayers of the cell membranes and induce morphological changes in a dose- and time-dependent manner, thereby leading to cell lysis. Though cytotoxic to normal cells, the toxic effect of melittin against tumor cells is more pronounced (Gajski and Garaj-Vrhovac 2013; Lee and Bae 2016). Thus, possible adverse effects of melittin should be considered before assessing its potential therapeutic applications.
Anti-bacterial effects
In vitro studies
Historically, anti-bacterial activity of bee venom was first reported by Schmidt-Lange (1941). This finding was extended by Ortel and Markwardt (1955) as well as Benton et al. (1963). In the early 1950s, melittin was discovered after electrophoretically separation of direct hemolysin from the indirect hemolysin phospholipase A (Habermann 1972; Neumann et al. 1952). Melittin was proposed as the anti-bacterial constituent in bee venom by Fennell et al. (1967). They demonstrated that anti-bacterial activity of whole bee venom is of the same order of magnitude as that of melittin in vitro. The authors found that both honeybee venom and its melittin fraction had anti-bacterial effects on a penicillin-resistant strain of S. aureus (strain 80). It has been also shown that melittin had higher anti-bacterial activity against Gram-positive bacteria in comparison to Gram-negative bacteria (Fennell et al. 1967). Numerous attempts were made to ascertain the susceptibilities of various pathogens to melittin from the 1960s onwards, as evidenced in Table 1.
In vitro anti-mycobacterial activities of melittin were first demonstrated in 1971 by Dorman and Markley (1971). Subsequent surveys during the 1980s and 1990s vividly confirmed that melittin has significant anti-bacterial activities against both reference and clinical strains of bacteria (Steiner et al. 1981; Stocker 1984; Boman et al. 1989; Blondelle and Houghten 1991; Wade et al. 1992; Piers et al. 1994; Oren and Shai 1996). It also showed minimum inhibitory concentration (MIC) values of ≤ 16 μg/mL for a large number of Gram-negative bacteria (Piers et al. 1994). However, there had been major discrepancies between the MIC values reported by previous studies (listed in Table 1), which could be convincingly explained by differences in purities of melittin, bacterial strains, and methodologies.
Melittin has been reported to exhibit an immediate inhibitory activity against Borrelia burgdorferi, the etiologic agent of Lyme disease (Lubke and Garon 1997; Socarras et al. 2017). For instance, Lubke and Garon (1997) showed a dramatic decline in the optical density of melittin-treated cultures of B. burgdorferi compared to untreated cultures. In this regard, ultrastructural observation of melittin-treated Borrelia spirochetes by field emission scanning electron microscopy (FE-SEM) divulged tangible alterations in the surface envelope of bacteria including augmented blebbing of surface components and pore formation. Dark-field microscopy also confirmed that melittin at a concentration equivalent to 100 μg/mL is capable of ceasing spirochete motility within seconds after exposure (Lubke and Garon 1997). Another study indicated that daily administration of melittin could significantly diminish the numbers of Borrelia persisters (p value ≤ 0.05) in comparison to the negative control (sterile PBS buffer) using SYBR Green I/propidium iodide (PI) assay (Socarras et al. 2017). Compared to doxycycline, melittin significantly lessens the numbers of spirochetes (p value ≤ 0.01) and B. burgdorferi persister cells (p value ≤ 0.01) at all concentrations, suggesting melittin as an appropriate candidate to extirpate different forms of B. burgdorferi (Socarras et al. 2017).
The first report on anti-bacterial action of melittin against several mollicutes dates back to 1997, when Béven and Wróblewski (1997) investigated effects of ten naturally occurring peptides on viability, morphology, and motility of mollicutes. Mollicutes are distinguished phenotypically from other class of bacteria by the absence of cell wall and their minute size. Melittin was found active against six different genera of mollicutes with MIC values in the range of 0.6–10 μM. Probing mollicute cells through a fluorescent dye (3,3′-dipropylthiodicarbocyanine iodides) revealed that melittin induced depolarization of bacterial membranes (Béven and Wróblewski 1997). Melittin, expressed within plasmid constructs, has been also reported to be effective in intracellular inhibition of urogenital pathogens including Mycoplasma hominis and Chlamydia trachomatis (Lazarev et al. 2002).
An abundance of evidence with regard to broad-spectrum bactericidal activity of melittin has propelled researchers to use melittin as a positive control AMP for comparison of its anti-microbial activity to other novel-discovered/developed AMPs. For instance, Moerman et al. (2002) used melittin as a positive control and found that it was active against reference strains of both Gram-positive and Gram-negative bacteria (Table 1). In particular, they found that melittin is effective in inhibiting reference strains of Listeria monocytogenes and Nocardia asteroides (Moerman et al. 2002). When the authors observed MIC increment in the presence of 5-mM extracellular Mg2+ ions, they suggested that electrostatic interaction occurs between melittin as a cationic peptide and negatively charged lipopolysaccharide (LPS) in Gram-negative bacterial membrane (Moerman et al. 2002). Furthermore, melittin has proven to be effective in inhibiting Vibrio parahaemolyticus KTCT 2471 (MIC of 1.56 μg/mL) and Edwardsiella tarda NUF251 (MIC of 0.78 μg/mL), both of which are able to cause disease in aquatic creatures (Kim et al. 2007). Using confocal microscopy technique, Pandey et al. (2010) pointed out that rhodamine-labeled melittin localized onto E. coli cells and altered their morphology. It has been also found to create holes of varying sizes onto Bacillus megaterium, as revealed by confocal microscopy (Pandey et al. 2010).
Melittin has the ability to inhibit plant-associated bacteria (González-Rodríguez et al. 2005; Shi et al. 2016). In an investigation of the inhibitory effects of melittin on 39 strains belonging to seven genera and 12 different species of plant pathogenic bacteria, the peptide marvelously exhibited 100% growth inhibition in vitro. Except for one strain (Dickeya chrysanthemi), the MIC values of melittin against the tested pathogens varied between 6.5 and 65 μM. In another study, using an agar well diffusion assay, Shi et al. (2016) noticed the anti-bacterial potency of melittin against Xanthomonas oryzae pathovar oryzae, causing agent of rice blight disease (Table 1). SEM revealed that melittin is able to induce surface roughening and shrinking, and pore formation, thereby resulting in rapid bacterial cell death (Shi et al. 2016). Melittin is also capable of binding to bacterial DNA/RNA in vitro, suggesting its probable role in inhibition of intracellular targets (Shi et al. 2016). Thus, these findings open up a range of new applications for melittin in the field of agricultural microbiology.
There are multiple lines of evidence that confirm the anti-bacterial activity of melittin toward antibiotic-resistant bacteria. In a study conducted on 20 MDR nosocomial isolates of A. baumannii, MIC and MBC values of melittin were in the range of 0.50–16 and 0.50–32 μg/mL, respectively (Giacometti et al. 2003). Another investigation revealed that melittin has a strong anti-bacterial effect on 41 clinical bacterial strains comprising 11 methicillin-resistant S. aureus (MRSA), 15 methicillin-susceptible S. aureus (MSSA), and 15 E. faecalis isolates (Table 1), with MIC values from 2 to 8 μg/mL (Dosler and Gerceker 2012). One survey also demonstrated that melittin inhibited 32 isolates of antibiotic-resistant bacteria such as S. aureus, P. aeruginosa, E. coli, and Salmonella typhimurium strains at concentrations equal to or less than 16 μM (Gopal et al. 2013), suggesting its potential for treating intractable infections caused by nosocomial pathogens.
A study conducted by Leandro et al. (2015) demonstrated in vitro anti-bacterial potency of melittin against prominent etiologic agents of tooth decay with MIC values lying in the 4–40 μg/mL range (Table 1). Considering the adverse impacts of tooth decay on people’s health, melittin has potential of inhibiting oral pathogens (Leandro et al. 2015).
In a contemporary study, Khozani et al. (2018) observed that there was a major difference between inhibitory (p value < 0.05) or bactericidal (p value < 0.05) activities of melittin and certain antibiotics including ceftazidime, doripenem, and colistin against 33 P. aeruginosa strains from patients who suffered from third-degree burns. The superior in vitro antibacterial activity of melittin compared to mentioned antibiotics prompted the authors to suggest melittin as a candidate for evaluating its topical anti-microbial activity in a mouse model of burn infection (Khozani et al. 2018).
Synergism with other anti-microbials
Several investigations have addressed possible synergistic effects between melittin and other anti-microbial agents, in particular conventional antibiotics (Moerman et al. 2002; Giacometti et al. 2003; Dosler and Gerceker 2012; AL-Ani et al. 2015; Bardbari et al. 2018). For instance, one study indicated that melittin exhibited synergistic activity with erythromycin against K. pneumoniae. Moreover, melittin has the ability to act synergistically when combined with amoxicillin, cefuroxime, and erythromycin against Listeria monocytogenes (Moerman et al. 2002). Using chequerboard titration method, synergistic effects observed between melittin and β-lactam antibiotics against A. baumannii ATCC 19606 and a clinical isolate of A. baumannii (04–01) (Giacometti et al. 2003). When exploited either alone or in combination with frequently used antibiotics, melittin exhibited concentration-dependent and rapid bactericidal activity against MRSA, MSSA, and E. faecalis isolates according to killing kinetic curves (Dosler and Gerceker 2012). It is noteworthy to mention that fast microbicidal action of AMPs such as melittin not only lessens the duration of treatment but also the possibility of developing anti-microbial resistance among bacterial pathogens (Memariani et al. 2018b). A recent survey assessed conceivable synergistic interactions of melittin in combination with several antibiotics against five clinical isolates of MDR A. baumannii. The authors pointed out that melittin exerted significant synergistic behaviors when combined with colistin and imipenem toward MDR A. baumannii strains (Bardbari et al. 2018), corroborating previous findings reported by Giacometti et al. (2003).
Aside from antibiotics, plant secondary metabolites combined with melittin were also assessed by chequerboard titration assay to investigate whether their combinations are superior against certain bacteria compared to each agent alone (AL-Ani et al. 2015). In this respect, a predominant synergism was found to occur between melittin and either benzyl isothiocyanate or carvacrol toward both MRSA NCTC 10442 and E. coli ATCC 25922, with fractional inhibitory concentration index (FICI) values ranging from 0.24 to 0.5 (AL-Ani et al. 2015).
Synergy of melittin with other anti-microbials might arise from destabilization of outer membrane in Gram-negative bacteria induced by the peptide, facilitating entrance of these anti-microbials into the bacterial cells. In the case of Gram-positive bacteria, it has been suggested that inhibition of peptidoglycan synthesis by β-lactam antibiotics can allow melittin to pass through the altered peptidoglycan layer more easily (Moerman et al. 2002). It should be borne in mind that synergistic activity of melittin with conventional antibiotics decreases the MIC values of both the peptide and antibiotics against tested strains. As a consequence, melittin can be used at non-toxic levels which results in its safe application without cytotoxicity concerns (Bardbari et al. 2018). Based on aforementioned scientific proofs, novel melittin-antibiotic formulations can be applied to eliminate antibiotic-resistant superbugs.
In vivo studies
In addition to in vitro studies, several evidences exist regarding anti-bacterial effectiveness of melittin in animal models. In one of the early attempts to examine the anti-bacterial efficacy of melittin in vivo, Lazarev et al. (2004) observed that aerosolized administration of a plasmid construct expressing melittin gene led to significant inhibition of Mycoplasma gallisepticum infection in chicken (Table 2). The authors suggested that melittin has prophylactic and therapeutic potential for mycoplasma infections in poultry farms (Lazarev et al. 2004). The other study in which mice intravaginally infected with Chlamydia trachomatis, administration of a plasmid expressing gene for melittin through vaginal route caused 45–80% inhibition of infection, as assessed by direct immunofluorescence with monoclonal antibodies (Lazarev et al. 2007). Furthermore, intradermal injections of living Propionibactierium acnes into the mouse ear and subsequent topical treatment with melittin-vaseline mixtures resulted in significant alleviation of swelling and granulomatous response in comparison to mice injected with only living P. acnes, suggesting protective effects of melittin in a P. acnes-induced in vivo inflammatory model (Lee et al. 2014).
Anti-bacterial effects of melittin on mice skin subcutaneously infected with MRSA USA 300 suspension, containing 106 colony forming units (CFUs)/mL, were investigated in a study conducted by Choi et al. (2015). Briefly, in order to examine in vivo efficiency of melittin, each surface lesion was treated with 100 μg of melittin in 80 μL PBS once a day and calipers were applied to gauge lesion dimensions for up to 10 days. The authors demonstrated that treatment of infected zone by melittin for 4 days led to significant decline in diameters of the abscesses in comparison to the PBS-treated group (Table 2). Furthermore, half of the mice intraperitoneally infected with MRSA USA300 were survived after intraperitoneal injection of 5 mg/kg of melittin in 0.1 mL PBS, whereas intraperitoneal injection of either PBS or 2.5 mg/kg melittin failed to survive infected mice after 24 h, as shown by Kaplan-Meier survival curve (Choi et al. 2015). This evidence was the first to confirm significant protective effects of melittin against MRSA infection in vivo.
A new study demonstrated that topical administration of melittin at concentrations of 16 and 32 μg/mL in mice killed 93.3% and 100% of an XDR A. baumannii on a third-degree burned area, respectively (Pashaei et al. 2019). Blood samples of mice treated with melittin (32 μg/mL) exhibited no hemolysis, indicating that the peptide is not entered to blood circulation. Moreover, melittin showed no dermal toxicity toward both normal and burned groups. Remarkably, all the examined mice were alive even after 1 month. This finding might create incentives for investigators to re-examine neglected toxic AMPs for at least topical treatment of burned areas.
Effects on bacterial biofilms
A biofilm is an organized microbial consortium enclosed in a self-created biopolymer matrix. It adheres irreversibly to biotic or abiotic surfaces (Batoni et al. 2016; Høiby et al. 2010). It has become obvious that biofilm formation is an adaptive mechanism of microbial cells, permitting them to survive harsh growth conditions (George et al. 2005). Owing to the presence of the extracellular matrix barrier and slow growth rate, biofilm-encased bacteria might tolerate up to 1000 times greater concentrations of anti-microbials compared to their planktonic counterparts (Memariani et al. 2016; Macià et al. 2014). Furthermore, detached cells from the biofilm can serve as a steady reservoir of pathogens, giving rise to treatment failure and recurrent infections as well (Haagensen et al. 2015). Thus, there is an imperative need for developing efficient anti-biofilm agents to address concerns about biofilm-related infections.
Over the past few years, there have been several attempts to examine efficacy of melittin on the viability of biofilm-embedded bacteria in vitro, as summarized in Table 3. For example, melittin is effective against clinical isolates of biofilm-producing P. aeruginosa, with a minimum biofilm inhibition concentration (MBIC) range of 4–16 μM, which was far more active compared to certain antibiotics including ampicillin, chloramphenicol, and levofloxacin (Gopal et al. 2013). Moreover, melittin has been reported to inhibit either biofilm formation or bacterial surface attachment in a time-dependent manner (Dosler et al. 2016). The peptide was also capable of inhibiting five strong biofilm-producer strains of MDR A. baumannii and removing their biofilm formations (Table 3), alone or in combination with colistin and imipenem (Bardbari et al. 2018). Noticeably, melittin lessened both biofilm biomass and viability of biofilm-embedded B. burgdorferi strain B31 at different concentrations in comparison to PBS-treated biofilms, which was further confirmed by SYBR Green I/(PI) assay and atomic force microscopy (Socarras et al. 2017). Another study divulged that melittin inhibited biofilm production and destroyed bacterial biofilms (Picoli et al. 2017). A recent survey implied that melittin was able to penetrate into biofilm layers of P. aeruginosa gradually and to kill biofilm-residing bacteria kinetically by disrupting bacterial membrane (Khozani et al. 2018). All in all, these evidences suggest that melittin can diminish biofilm formation, biofilm biomass, and viability of bacteria within biofilms in a time- and concentration-dependent manner.
Future prospects
As alluded to above, melittin has strong anti-bacterial and anti-biofilm effects on a broad spectrum of bacterial pathogens, though cytotoxicity of melittin at higher doses may hinder its therapeutic application. Several investigations are ongoing to weaken toxicity of native melittin without influencing its microbicidal activity. Development of drug delivery vehicle by incorporating melittin into nanoparticle represents a safe approach for in vivo application of melittin with favorable pharmacokinetics (Soman et al. 2009). Conjugation of melittin with aptamers is another promising strategy for attenuating hemolytic activity of the peptide (Rajabnejad et al. 2018). Besides, incorporation of AMPs into commercially available hydrogels is rather the other way for an innovative therapy of topical infections, especially those related to burn wounds (Silva et al. 2015; Björn et al. 2015). Development of DNA constructs in which the gene of melittin is under the control of an inducible promoter may hold the potential as future prophylactic and therapeutic approaches. Noticeably, combination of natural melittin and current antibiotics and/or AMPs is another solution to minimize doses of melittin which can lessen both cytotoxicity concern of melittin and the likelihood of developing antibiotic-resistant mutant bacteria. These combinatorial therapies can be useful for future treatment of hard-to-treat MDR, XDR, and PDR pathogens.
Conclusions
Over the past half-century, empirical evidences have expanded our knowledge regarding biological effects of melittin. In this respect, published data suggest that melittin is effective against both planktonic and biofilm-embedded bacteria. Furthermore, the synergism between melittin and antibiotics can be a hopeful solution for treatment of antibiotic-resistant superbugs. The double-edged nature of melittin, as a microbicidal and hemolytic constituent of honeybee venom, should not dissuade scientists to scrutinize its conceivable therapeutic applications. Eventually, anti-infective features of melittin will open up new horizons in a range of biomedical areas, particularly from agriculture to veterinary and clinical microbiology.
References
Al-Ani I, Zimmermann S, Reichling J, Wink M (2015) Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 22(2):245–255. https://doi.org/10.1016/j.phymed.2014.11.019
Almeida JR, Palacios ALV, Patiño RSP, Mendes B, Teixeira CAS, Gomes P, da Silva SL (2018) Harnessing snake venom phospholipases A2 to novel approaches for overcoming antibiotic resistance. Drug Dev Res. https://doi.org/10.1002/ddr.21456
An HJ, Kim JY, Kim WH, Gwon MG, Gu HM, Jeon MJ, Han SM, Pak SC, Lee CK, Park IS, Park KK (2018) Therapeutic effects of bee venom and its major component, melittin, on atopic dermatitis in vivo and in vitro. Br J Pharmacol 175(23):4310–4324. https://doi.org/10.1111/bph.14487
Anderson D, Terwilliger TC, Wickner W, Eisenberg D (1980) Melittin forms crystals which are suitable for high resolution X-ray structural analysis and which reveal a molecular 2-fold axis of symmetry. J Biol Chem 255(6):2578–2582
Andreotti N, Jouirou B, Mouhat S, Mouhat L, Sabatier J (2010) Therapeutic value of peptides from animal venoms. In: Mandler L, Liu HW (eds) Comprehensive natural products II. Elsevier, Oxford, pp 287–303
Bardbari AM, Arabestani MR, Karami M, Keramat F, Aghazadeh H, Alikhani MY, Bagheri KP (2018) Highly synergistic activity of melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis 37(3):443–454. https://doi.org/10.1007/s10096-018-3189-7
Batoni G, Maisetta G, Esin S (2016) Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim Biophys Acta 1858(5):1044–1060. https://doi.org/10.1016/j.bbamem.2015.10.013
Bazzo R, Tappin MJ, Pastore A, Harvey TS, Carver JA, Campbell ID (1988) The structure of melittin. A 1H-NMR study in methanol. Eur J Biochem 173(1):139–146. https://doi.org/10.1111/j.1432-1033.1988.tb13977.x
Benton AW, Morse RA, Kosikowski FV (1963) Bioassay and standardization of venom of the honey bee. Nature 198:295–296. https://doi.org/10.1038/198295b0
Béven L, Wróblewski H (1997) Effect of natural amphipathic peptides on viability, membrane potential, cell shape and motility of mollicutes. Res Microbiol 148(2):163–175. https://doi.org/10.1016/S0923-2508(97)87647-4
Björn C, Noppa L, Näslund Salomonsson E, Johansson AL, Nilsson E, Mahlapuu M, Håkansson J (2015) Efficacy and safety profile of the novel antimicrobial peptide PXL150 in a mouse model of infected burn wounds. Int J Antimicrob Agents 45(5):519–524. https://doi.org/10.1016/j.ijantimicag.2014.12.015
Blondelle SE, Houghten RA (1991) Hemolytic and antimicrobial activities of the twenty-four individual omission analogues of melittin. Biochemistry 30(19):4671–4678. https://doi.org/10.1021/bi00233a006
Boman HG, Wade D, Boman IA, Wåhlin B, Merrifield RB (1989) Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett 259(1):103–106. https://doi.org/10.1016/0014-5793(89)81505-4
Bucki R, Pastore JJ, Randhawa P, Vegners R, Weiner DJ, Janmey PA (2004) Antibacterial activities of rhodamine B-conjugated gelsolin-derived peptides compared to those of the antimicrobial peptides cathelicidin LL37, magainin II, and melittin. Antimicrob Agents Chemother 48(5):1526–1533. https://doi.org/10.1128/AAC.48.5.1526-1533.2004
Cerovský V, Hovorka O, Cvacka J, Voburka Z, Bednárová L, Borovicková L, Slaninová J, Fucík V (2008) Melectin: a novel antimicrobial peptide from the venom of the cleptoparasitic bee Melecta albifrons. Chembiochem 9(17):2815–2821. https://doi.org/10.1002/cbic.200800476
Choi JH, Jang AY, Lin S, Lim S, Kim D, Park K, Han SM, Yeo JH, Seo HS (2015) Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol Med Rep 12(5):6483–6490. https://doi.org/10.3892/mmr.2015.4275
Deslouches B, Steckbeck JD, Craigo JK, Doi Y, Burns JL, Montelaro RC (2015) Engineered cationic antimicrobial peptides to overcome multidrug resistance by ESKAPE pathogens. Antimicrob Agents Chemother 59(2):1329–1333. https://doi.org/10.1128/AAC.03937-14
Dorman LC, Markley LD (1971) Solid phase synthesis and antibacterial activity of N-terminal sequences of melittin. J Med Chem 14(1):5–9. https://doi.org/10.1021/jm00283a003
Dosler S, Gerceker AA (2012) In vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram-positive bacteria. J Chemother 24(3):137–143. https://doi.org/10.1179/1973947812Y.0000000007
Dosler S, Karaaslan E, Alev Gerceker A (2016) Antibacterial and anti-biofilm activities of melittin and colistin, alone and in combination with antibiotics against gram-negative bacteria. J Chemother 28(2):95–103. https://doi.org/10.1179/1973947815Y.0000000004
Ebbensgaard A, Mordhorst H, Overgaard MT, Nielsen CG, Aarestrup FM, Hansen EB (2015) Comparative evaluation of the antimicrobial activity of different antimicrobial peptides against a range of pathogenic bacteria. PLoS One 10(12):e0144611. https://doi.org/10.1371/journal.pone.0144611
Fennell JF, Shipman WH, Cole LJ (1967) Antibacterial action of a bee venom fraction (melittin) against a penicillin-resistant Staphylococcus and other microorganisms. USNRDL-TR-67-101. Res Dev Tech Rep 5:1–13
Gajski G, Garaj-Vrhovac V (2013) Melittin: a lytic peptide with anticancer properties. Environ Toxicol Pharmacol 36(2):697–705. https://doi.org/10.1016/j.etap.2013.06.009
George S, Kishen A, Song KP (2005) The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J Endod 31(12):867–872. https://doi.org/10.1097/01.don.0000164855.98346.fc
Giacometti A, Cirioni O, Kamysz W, D'Amato G, Silvestri C, Del Prete MS, Lukasiak J, Scalise G (2003) Comparative activities of cecropin A, melittin, and cecropin A-melittin peptide CA(1-7)M(2-9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 24(9):1315–1318. https://doi.org/10.1016/j.peptides.2003.08.003
González-Rodríguez MÁ, Silva-Rojas HV, Mascorro-Gallardo JO (2005) In vitro assay of the antimicrobial peptide melittin against different phytopathogenic bacteria (article in Spanish). Rev Mex Fitopatol 23(2):176–182
Gopal R, Lee JH, Kim YG, Kim MS, Seo CH, Park Y (2013) Anti-microbial, anti-biofilm activities and cell selectivity of the NRC-16 peptide derived from witch flounder, Glyptocephalus cynoglossus. Mar Drugs 11(6):1836–1852. https://doi.org/10.3390/md11061836
Haagensen JA, Verotta D, Huang L, Spormann A, Yang K (2015) New in vitro model to study the effect of human simulated antibiotic concentrations on bacterial biofilms. Antimicrob Agents Chemother 59(7):4074–4081. https://doi.org/10.1128/AAC.05037-14
Habermann E (1972) Bee and wasp venoms. Science 177(4046):314–322. https://doi.org/10.1126/science.177.4046.314
Hale JD, Hancock RE (2007) Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti-Infect Ther 5(6):951–959. https://doi.org/10.1586/14787210.5.6.951
Han S, Yeo J, Baek H, Lin SM, Meyer S, Molan P (2009) Postantibiotic effect of purified melittin from honeybee (Apis mellifera) venom against Escherichia coli and Staphylococcus aureus. J Asian Nat Prod Res 11(9):796–804. https://doi.org/10.1080/10286020903164277
Hider RC (1988) Honeybee venom: a rich source of pharmacologically active peptides. Endeavour 12(2):60–65. https://doi.org/10.1016/0160-9327(88)90082-8
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35(4):322–332. https://doi.org/10.1016/j.ijantimicag.2009.12.011
Hossen S, Gan SH, Khalil I (2017) Melittin, a potential natural toxin of crude bee venom: probable future arsenal in the treatment of diabetes mellitus. J Chem 2017:1–7. https://doi.org/10.1155/2017/4035626
Kawakami H, Goto SG, Murata K, Matsuda H, Shigeri Y, Imura T, Inagaki H, Shinada T (2017) Isolation of biologically active peptides from the venom of Japanese carpenter bee, Xylocopa appendiculata. J Venom Anim Toxins Incl Trop Dis 23:29. https://doi.org/10.1186/s40409-017-0119-6
Khozani RS, Shahbazzadeh D, Harzandi N, Feizabadi MM, Bagheri KP (2018) Kinetics study of antimicrobial peptide, melittin, in simultaneous biofilm degradation and eradication of potent biofilm producing MDR Pseudomonas aeruginosa isolates. Int J Pept Res Ther 25:329–338. https://doi.org/10.1007/s10989-018-9675-z
Kim IH, Lee DG, Le SH, Ha JM, Ha BJ, Kim SK, Lee JH (2007) Antibacterial activity of Ulva lactuta against methicillin-resistant Staphylococcus aureus (MRSA). Biotechnol Bioproc Engg 12:579–582. https://doi.org/10.1007/BF02931358
King GF (2011) Venoms as a platform for human drugs: translating toxins into therapeutics. Expert Opin Biol Ther 11(11):1469–1484. https://doi.org/10.1517/14712598.2011.621940
Lam YH, Wassall SR, Morton CJ, Smith R, Separovic F (2001) Solid-state NMR structure determination of melittin in a lipid environment. Biophys J 81(5):2752–2761. https://doi.org/10.1016/S0006-3495(01)75918-8
Lazarev VN, Parfenova TM, Gularyan SK, Misyurina OY, Akopian TA, Govorun VM (2002) Induced expression of melittin, an antimicrobial peptide, inhibits infection by Chlamydia trachomatis and Mycoplasma hominis in a HeLa cell line. Int J Antimicrob Agents 19(2):133–137. https://doi.org/10.1016/S0924-8579(01)00479-4
Lazarev VN, Stipkovits L, Biro J, Miklodi D, Shkarupeta MM, Titova GA, Akopian TA, Govorun VM (2004) Induced expression of the antimicrobial peptide melittin inhibits experimental infection by Mycoplasma gallisepticum in chickens. Microbes Infect 6(6):536–541. https://doi.org/10.1016/j.micinf.2004.02.006
Lazarev VN, Shkarupeta MM, Kostryukova ES, Levitskii SA, Titova GA, Akopian TA, Govorun VM (2007) Recombinant plasmid constructs expressing gene for antimicrobial peptide melittin for the therapy of Mycoplasma and Chlamydia infections. Bull Exp Biol Med 144(3):452–456. https://doi.org/10.1007/s10517-007-0350-1
Leandro LF, Mendes CA, Casemiro LA, Vinholis AH, Cunha WR, de Almeida R, Martins CH (2015) Antimicrobial activity of apitoxin, melittin and phospholipase A2 of honey bee (Apis mellifera) venom against oral pathogens. An Acad Bras Cienc 87(1):147–155. https://doi.org/10.1590/0001-3765201520130511
Lee G, Bae H (2016) Anti-inflammatory applications of melittin, a major component of bee venom: detailed mechanism of action and adverse effects. Molecules 21(5):616. https://doi.org/10.3390/molecules21050616
Lee MT, Sun TL, Hung WC, Huang HW (2013) Process of inducing pores in membranes by melittin. Proc Natl Acad Sci U S A 110(35):14243–14248. https://doi.org/10.1073/pnas.1307010110
Lee WR, Kim KH, An HJ, Kim JY, Chang YC, Chung H, Park YY, Lee ML, Park KK (2014) The protective effects of melittin on Propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J Invest Dermatol 134(7):1922–1930. https://doi.org/10.1038/jid.2014.75
Lewis RJ, Garcia ML (2003) Therapeutic potential of venom peptides. Nat Rev Drug Discov 2(10):790–802. https://doi.org/10.1038/nrd1197
Lubke LL, Garon CF (1997) The antimicrobial agent melittin exhibits powerful in vitro inhibitory effects on the Lyme disease spirochete. Clin Infect Dis 25(Suppl 1):S48–S51. https://doi.org/10.1086/516165
Macià MD, Rojo-Molinero E, Oliver A (2014) Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect 20(10):981–990. https://doi.org/10.1111/1469-0691.12651
Matsuzaki K, Yoneyama S, Miyajima K (1997) Pore formation and translocation of melittin. Biophys J 73(2):831–838. https://doi.org/10.1016/S0006-3495(97)78115-3
Memariani H, Shahbazzadeh D, Sabatier JM, Memariani M, Karbalaeimahdi A, Bagheri KP (2016) Mechanism of action and in vitro activity of short hybrid antimicrobial peptide PV3 against Pseudomonas aeruginosa. Biochem Biophys Res Commun 479(1):103–108. https://doi.org/10.1016/j.bbrc.2016.09.045
Memariani H, Shahbazzadeh D, Ranjbar R, Behdani M, Memariani M, Pooshang Bagheri K (2017) Design and characterization of short hybrid antimicrobial peptides from pEM-2, mastoparan-VT1, and mastoparan-B. Chem Biol Drug Des 89(3):327–338. https://doi.org/10.1111/cbdd.12864
Memariani H, Shahbazzadeh D, Sabatier JM, Pooshang Bagheri K (2018a) Membrane-active peptide PV3 efficiently eradicates multidrug-resistant Pseudomonas aeruginosa in a mouse model of burn infection. APMIS 126(2):114–122. https://doi.org/10.1111/apm.12791
Memariani H, Memariani M, Pourmand MR (2018b) Venom-derived peptide Mastoparan-1 eradicates planktonic and biofilm-embedded methicillin-resistant Staphylococcus aureus isolates. Microb Pathog 119:72–80. https://doi.org/10.1016/j.micpath.2018.04.008
Moerman L, Bosteels S, Noppe W, Willems J, Clynen E, Schoofs L, Thevissen K, Tytgat J, Van Eldere J, Van Der Walt J, Verdonck F (2002) Antibacterial and antifungal properties of alpha-helical, cationic peptides in the venom of scorpions from southern Africa. Eur J Biochem 269(19):4799–4810. https://doi.org/10.1046/j.1432-1033.2002.03177.x
Mohamed MF, Brezden A, Mohammad H, Chmielewski J, Seleem MN (2017) Targeting biofilms and persisters of ESKAPE pathogens with P14KanS, a kanamycin peptide conjugate. Biochim Biophys Acta Gen Subj 1861(4):848–859. https://doi.org/10.1016/j.bbagen.2017.01.029
Monincová L, Veverka V, Slaninová J, Buděšínský M, Fučík V, Bednárová L, Straka J, Ceřovský V (2014) Structure-activity study of macropin, a novel antimicrobial peptide from the venom of solitary bee Macropis fulvipes (Hymenoptera: Melittidae). J Pept Sci 20(6):375–384. https://doi.org/10.1002/psc.2625
Nešuta O, Hexnerová R, Buděšínský M, Slaninová J, Bednárová L, Hadravová R, Straka J, Veverka V, Čeřovský V (2016) Antimicrobial peptide from the wild bee Hylaeus signatus venom and its analogues: structure-activity study and synergistic effect with antibiotics. J Nat Prod 79(4):1073–1083. https://doi.org/10.1021/acs.jnatprod.5b01129
Neumann W, Habermann E, Amend G (1952) Zur papierelektrophoretischen fraktionierung tierischer gifte. Naturwissenschaften 39(12):286–287. https://doi.org/10.1007/BF00591257
O'Neill J (2016) Tackling drug-resistant infections globally: Final report and recommendations. Wellcome Trust and HM Govt. http://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf. Accessed 10 Oct 2018
Oren Z, Shai Y (1996) A class of highly potent antibacterial peptides derived from pardaxin, a pore-forming peptide isolated from Moses sole fish Pardachirus marmoratus. Eur J Biochem 237(1):303–310. https://doi.org/10.1111/j.1432-1033.1996.0303n.x
Oršolić N (2012) Bee venom in cancer therapy. Cancer Metastasis Rev 31(1–2):173–194. https://doi.org/10.1007/s10555-011-9339-3
Ortel S, Markwardt F (1955) Studies on the antibacterial properties of bee venom. [article in German]. Pharmazie 10(12):743–746
Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE (2008) Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 76(9):4176–4182. https://doi.org/10.1128/IAI.00318-08
Pandey BK, Ahmad A, Asthana N, Azmi S, Srivastava RM, Srivastava S, Verma R, Vishwakarma AL, Ghosh JK (2010) Cell-selective lysis by novel analogues of melittin against human red blood cells and Escherichia coli. Biochemistry 49(36):7920–7929. https://doi.org/10.1021/bi100729m
Park SC, Kim JY, Shin SO, Jeong CY, Kim MH, Shin SY, Cheong GW, Park Y, Hahm KS (2006) Investigation of toroidal pore and oligomerization by melittin using transmission electron microscopy. Biochem Biophys Res Commun 343(1):222–228. https://doi.org/10.1016/j.bbrc.2006.02.090
Pashaei F, Bevalian P, Akbari R, Pooshang Bagheri K (2019) Single dose eradication of extensively drug resistant Acinetobacter spp. in a mouse model of burn infection by melittin antimicrobial peptide. Microb Pathog 127:60–69. https://doi.org/10.1016/j.micpath.2018.11.055
Paull BR, Yunginger JW, Gleich GJ (1977) Melittin: an allergen of honeybee venom. J Allergy Clin Immunol 59(4):334–338
Pennington MW, Czerwinski A, Norton RS (2018) Peptide therapeutics from venom: current status and potential. Bioorg Med Chem 26(10):2738–2758. https://doi.org/10.1016/j.bmc.2017.09.029
Picoli T, Peter CM, Zani JL, Waller SB, Lopes MG, Boesche KN, Vargas GDÁ, Hübner SO, Fischer G (2017) Melittin and its potential in the destruction and inhibition of the biofilm formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa isolated from bovine milk. Microb Pathog 112:57–62. https://doi.org/10.1016/j.micpath.2017.09.046
Piers KL, Brown MH, Hancock RE (1994) Improvement of outer membrane-permeabilizing and lipopolysaccharide-binding activities of an antimicrobial cationic peptide by C-terminal modification. Antimicrob Agents Chemother 38(10):2311–2316. https://doi.org/10.1128/AAC.38.10.2311
Rady I, Siddiqui IA, Rady M, Mukhtar H (2017) Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett 402:16–31. https://doi.org/10.1016/j.canlet.2017.05.010
Raghuraman H, Chattopadhyay A (2007) Melittin: a membrane-active peptide with diverse functions. Biosci Rep 27(4–5):189–223. https://doi.org/10.1007/s10540-006-9030-z
Rajabnejad SH, Mokhtarzadeh A, Abnous K, Taghdisi SM, Ramezani M, Razavi BM (2018) Targeted delivery of melittin to cancer cells by AS1411 anti-nucleolin aptamer. Drug Dev Ind Pharm 44(6):982–987. https://doi.org/10.1172/JCI38842
Rice LB (2008) Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197(8):1079–1081. https://doi.org/10.1086/533452
Sabatier JM (2011) Animal venoms: from deadly arsenals (toxins) to therapeutic drug candidates. Inflamm Allergy Drug Targets 10(5):312. https://doi.org/10.2174/187152811797200632
Schmidt-Lange W (1941) The germicidal effect of bee venom. Muench Med Wochenschr 83:935
Shi W, Li C, Li M, Zong X, Han D, Chen Y (2016) Antimicrobial peptide melittin against Xanthomonas oryzae pv. oryzae, the bacterial leaf blight pathogen in rice. Appl Microbiol Biotechnol 100(11):5059–5067. https://doi.org/10.1007/s00253-016-7400-4
Silva JP, Dhall S, Garcia M, Chan A, Costa C, Gama M, Martins-Green M (2015) Improved burn wound healing by the antimicrobial peptide LLKKK18 released from conjugates with dextrin embedded in a carbopol gel. Acta Biomater 26:249–262. https://doi.org/10.1016/j.actbio.2015.07.043
Smith R, Separovic F, Milne TJ, Whittaker A, Bennett FM, Cornell BA, Makriyannis A (1994) Structure and orientation of the pore-forming peptide, melittin, in lipid bilayers. J Mol Biol 241(3):456–466. https://doi.org/10.1006/jmbi.1994.1520
Socarras KM, Theophilus PAS, Torres JP, Gupta K, Sapi E (2017) Antimicrobial activity of bee venom and melittin against Borrelia burgdorferi. Antibiotics (Basel) 6(4). https://doi.org/10.3390/antibiotics6040031
Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, Heuser JE, Arbeit JM, Wickline SA, Schlesinger PH (2009) Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest 119(9):2830–2842. https://doi.org/10.1172/JCI38842
Son DJ, Lee JW, Lee YH, Song HS, Lee CK, Hong JT (2007) Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther 115(2):246–270. https://doi.org/10.1016/j.pharmthera.2007.04.004
Steiner H, Hultmark D, Engström A, Bennich H, Boman HG (1981) Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292(5820):246–248. https://doi.org/10.1038/292246a0
Stocker JF (1984) Studies of the action of venom and venom constituents on Escherichia coli. Dissertation, Loughborough University. https://dspace.lboro.ac.uk/2134/25899 (Accessed on December 05, 2018)
Tacón A (2016) Melittin and cancer. J Apither 1(2):51–54. https://doi.org/10.5455/ja.20161027091813
Terwilliger TC, Eisenberg D (1982) The structure of melittin. II. Interpretation of the structure. J Biol Chem 257(11):6016–6022
van den Bogaart G, Guzmán JV, Mika JT, Poolman B (2008) On the mechanism of pore formation by melittin. J Biol Chem 283(49):33854–33857. https://doi.org/10.1074/jbc.M805171200
Varanda EA, Tavares DC (1998) Radioprotection: mechanism and radioprotective agents including honey bee venom. Venom Anim Toxins 4(1):5–21. https://doi.org/10.1590/S0104-79301998000100002
Varanda EA, Monti R, Tavares DC (1999) Inhibitory effect of propolis and bee venom on the mutagenicity of some direct- and indirect-acting mutagens. Teratog Carcinog Mutagen 19(6):403–413. https://doi.org/10.1002/(SICI)1520-6866(1999)19:6<403::AID-TCM4>3.0.CO;2-2
Wade D, Andreu D, Mitchell SA, Silveira AM, Boman A, Boman HG, Merrifield RB (1992) Antibacterial peptides designed as analogs or hybrids of cecropins and melittin. Int J Pept Protein Res 40(5):429–436. https://doi.org/10.1111/j.1399-3011.1992.tb00321.x
Yang L, Harroun TA, Weiss TM, Ding L, Huang HW (2001) Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J 81(3):1475–1485. https://doi.org/10.1016/S0006-3495(01)75802-X
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Memariani, H., Memariani, M., Shahidi-Dadras, M. et al. Melittin: from honeybees to superbugs. Appl Microbiol Biotechnol 103, 3265–3276 (2019). https://doi.org/10.1007/s00253-019-09698-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09698-y