Abstract
The treatment of bacterial biofilms has been progressively troubled due to increasing antibiotic resistance. Biofilms exacerbate the fight against infections as they provide a protective environment for microbial cells, hindering the penetration of antimicrobial agents and favoring the uptake of elements necessary for cell survival like water, oxygen, and nutrients. Indeed, many first-line antimicrobial agents have become ineffective in treating biofilm-related infections, instigating the search for new antimicrobial agents. Natural products, particularly plant-derived compounds known as phytochemicals, have been shown to be effective, with an excellent broad-spectrum antibacterial profile, even against drug-resistant bacteria. This chapter addresses the main concepts of biofilm-associated mechanisms that promote bacterial survival. Moreover, different phytochemicals are described for biofilm prevention and control, correlating the mode of action of phytochemicals to the inhibition/alteration of virulence factors and other biofilm mechanisms. The scientific evidence of a wide variety of phytochemicals being described in this chapter should support future efforts to fast-track in vitro research to clinical applications to fight biofilm-related infectious diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Since their discovery, antibiotics have been essential for the treatment and prevention of diseases, allowing many previously fatal infections to be treated or controlled (Nigam et al. 2014; Allen et al. 2019; Micoli et al. 2021). Nevertheless, the improper use and over-prescription of antibiotics, poor hygiene and sanitation habits, poor infection control, and the absence of new antibiotics led to a rapid emergence and spread of resistant microbial strains (Allen et al. 2019; Liu et al. 2021; Hawkings et al. 2007; Ferri et al. 2017; Barbieri et al. 2017). In fact, antibiotic resistance is one of the greatest threats to public health, limiting the prevention and treatment of a large range of previously treatable infectious diseases (Micoli et al. 2021; Jindal et al. 2015; Monte et al. 2014; Levy and Marshall 2004). Antimicrobial resistance (AMR) is a natural result of the adaptation of infectious pathogens to antibiotics used in a variety of fields. Moreover, the economic burden associated with these multidrug-resistant bacteria is massive (Monte et al. 2014; Prestinaci et al. 2015). Since antibiotic resistance is multifactorial, the WHO recommends a series of coordinated actions, from preventing the spread of the infection to promoting the research and development of new treatment strategies (Barbieri et al. 2017; Neu 1992; Ventola 2015). In addition, microorganisms may live in biofilms, which consist of aggregates of microbial cells attached to biotic or abiotic surfaces and embedded in a self-produced matrix of extracellular polymeric substances that protect cells from environmental stressors. It is well-known that the acquisition of resistance to antibiotics by bacteria is facilitated when they are present in biofilms (de Carvalho 2018; Yin et al. 2019; Kostakioti et al. 2013; Bridier et al. 2011). Biofilms are linked to serious and difficult-to-treat infections and combating them can be unmanageable or requires high doses of antibiotics (Llor and Bjerrum 2014; Frieri et al. 2017). Bacteria in biofilms can be up to 1000 times more tolerant to antibiotics than free-floating bacteria, which severely limits treatment options (Potera 2010; Sharma et al. 2019; Venkatesan et al. 2015).
To find novel antimicrobial agents distinct from antibiotics, plants have been explored as a source of compounds that show promising effectiveness against a variety of organisms, including fungi, yeasts, bacteria, and viruses (Monte et al. 2014; Wintola and Afolayan 2015; Cowan 1999). Phytochemicals are non-nutritive plant secondary metabolites with biological activity (Huang et al. 2016; Jimenez-Garcia et al. 2018; Liu 2013; Arendt and Zannini 2013; Diep et al. 2015). Different parts of plants from several species, such as leaves, roots, fruits, seeds, barks, stem bark, and flowers, are rich in different classes of phytochemicals, which may be a source of effective, inexpensive, and safe antimicrobial agents (Barbieri et al. 2017; Aung et al. 2020; Chouhan et al. 2017). They have been shown to inhibit peptidoglycan synthesis, destroy the structure of microbial membranes, change the hydrophobicity of bacterial membranes, and interfere with quorum sensing (QS) (Monte et al. 2014; Nazzaro et al. 2013). Plant extracts and their phytochemicals have been highlighted as promising antimicrobial agents due to their cost-effectiveness, eco-friendliness, large structural diversity, and the possibility of reducing the resistance to antimicrobial drugs (Sakarikou et al. 2020; Giaouris and Simões 2018).
2 Biofilm-Related Infections
Biofilms are surface-related microbial structures that can be present in a large variety of environments, either abiotic or biotic (Moshynets and Spiers 2016; Balcázar et al. 2015; Lebeaux et al. 2014). One of the most important characteristics of biofilms is their capacity to ensure greater protection of bacterial cells and survive in the presence of external stressors, such as high concentrations of antimicrobials. Biofilms recalcitrance may lead to treatment failure and infection recurrence (Lebeaux et al. 2014; Aggarwal et al. 2015; Giulia and O'Toole 2021). Therefore, even in unfavorable environments, biofilms can promote the survival of pathogenic microorganisms and facilitate their spread and the recolonization of new niches (Muhammad et al. 2020; Del Pozo 2018; Roy et al. 2018; Wu et al. 2015; Kostakioti et al. 2013). Biofilms are also associated with device-related infectious diseases, which can be responsible for the development of malfunctions of devices and even chronic infections (Del Pozo 2018; Nandakumar et al. 2013; Khatoon et al. 2018). In fact, biofilms are responsible for up to 80% of human infections, such as cystic fibrosis (CFs), endocarditis, osteomyelitis, and sepsis. Thus, given the relevance of infections caused by biofilms, it is essential to highlight the pathogens considered most critical, as well as the stages of development of a biofilm, their main specific tolerance mechanisms, and their respective virulence factors.
2.1 Critical Multidrug-Resistant Pathogens
According to the WHO, certain microorganisms are particularly problematic for human health, including Gram-negative bacteria, such as Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa and ESKAPE pathogens, i.e. a group of six highly virulent, pathogenic, and antibiotic-resistant pathogens, including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. These Gram-positive and Gram-negative bacteria can evade or “escape” commonly used antibiotics due to the spread of multidrug resistance, being linked to increasing AMR infections worldwide (Santajit and Indrawattana 2016; Schultz et al. 2020; Mulani et al. 2019; Skariyachan and Garka 2018; Julian and Blumberg 2017; Karlowsky et al. 2017). Table 1 shows the main virulence factors associated with some of the most concerning bacteria, as defined by the WHO. These virulence factors are correlated with their tolerance to antimicrobial agents, as well as the development of infection in the host system.
2.2 Biofilm Formation and Specific Mechanisms
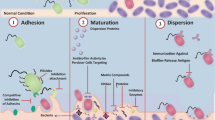
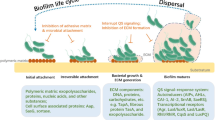
Biofilm formation has been recognized as a multistage process. It is believed that the biofilm development process occurs through several stages, as schematized in Fig. 1.
For the successful development of a biofilm, specific mechanisms and associated virulence factors are crucial. Virulence factors are molecules produced by bacteria, which are needed to enable bacteria to attack eukaryotic cells and cause infections. They are usually encoded on mobile and integrative genetic elements, namely plasmids, bacteriophages, conjugative transposons, integrative and conjugative elements, and pathogenicity islands (Abedon et al. 2009; Sharma et al. 2017; Lami 2019; Boyd 2012). Virulence factors allow the pathogens to evade the host immune response and facilitate the establishment and long-term survival of biofilms in tissues (Zhao and Ma 2015; Phillips and Schultz 2012). Besides virulence factors, biofilms also developed several mechanisms, which are extremely important for their survival and proliferation in host tissues (summary in Table 2).
3 Antibacterial Strategies Based on Natural Products to Target Biofilms
Bacterial resistance mechanisms are specific strategies of microbial evolution (Simões et al. 2009; Upmanyu et al. 2020; Ray et al. 2017). Thus, it is not surprising that new antibiotic resistance mechanisms will emerge (Barbieri et al. 2017; Diggle and Whiteley 2020; Munita and Arias 2016; Sekyere and Asante 2018). Some phenomena can explain the resistance of microorganisms to antibiotics: (i) cells can present an intrinsic and natural resistance to drugs, being “genetically programmed” to resist antibiotics (Holmes et al. 2016; Beceiro et al. 2013); (ii) microorganisms may suffer some genetic modifications like mutations, which make them insensitive to antibiotics (Fair and Tor 2014; Beceiro et al. 2013; Meredith et al. 2015); (iii) cells may become resistant to antimicrobial agents through the horizontal acquisition of genes that confer resistance to certain antibiotics from other microorganisms, which is a very extensive phenomenon and increases the probability of survival under the selective pressure caused by antibiotics (Beceiro et al. 2013; Meredith et al. 2015; Bello-López et al. 2019). Moreover, bringing a new antibiotic to the market may take up to 10 years and it is very expensive and time consuming (Barbieri et al. 2017; Spellberg 2014; Power 2006). Thus, new strategies aiming to fight bacterial infections, especially those caused by biofilms, are highly necessary (Bi et al. 2021; Srinivasan et al. 2021; Zhang et al. 2020a; Simões et al. 2009). Indeed, natural compounds, especially those obtained from plants, have become promising candidates for these much-needed treatments due to excellent antibacterial activity (Barbieri et al. 2017; Ćirić et al. 2019; Melander et al. 2020; Chassagne et al. 2021; Lahlou 2013).
Medicinal plants are the most abundant biological resources in traditional and modern medicine, nutraceuticals, food supplements, folk medicines, pharmaceutical intermediates, and chemical components of synthetic medicines (Desai et al. 2015; Saranraj et al. 2016; Pan et al. 2013). The intake of phytochemicals in the diet can promote health and protect the human body from diseases, being also able to reduce the risk of some chronic diseases (Jimenez-Garcia et al. 2018; Liu 2013; Yoo et al. 2018; Septembre-Malaterre et al. 2017). Phytochemicals with nutritious and health-care properties in foods are of great significance due to their beneficial effects on human health. They are able to prevent and assist in the combat of a large variety of diseases, such as cancer, coronary heart disease, diabetes, hypertension, inflammation, microbial, viral, and parasitic infections, psychosis, spastic diseases, ulcers, osteoporosis and related diseases, among others (Thakur et al. 2020; Zhang et al. 2015; Forni et al. 2019; Guan et al. 2021; Kibe et al. 2017; Visioli et al. 2000; Howes and Simmonds 2014).
A large variety of well-known phytochemicals have been identified over the years, such as lycopene present in tomatoes, isoflavones in soybeans, and flavonoids found in fruits (Zhang et al. 2015; Ghoshal 2018; Waheed Janabi et al. 2020). An interesting feature of these compounds is their powerful antioxidant potential. In fact, the regular consumption of fruits, vegetables, and whole grains has shown to reduce the risk of various diseases related to oxidative damage, acting as free radical scavengers like hydrogen donors, electron donors, peroxide decomposers, singlet oxygen quenchers, enzyme inhibitors, synergists, and metal chelators (Thatoi et al. 2014; Yu et al. 2021; Chen et al. 2014; Asaduzzaman 2018; Manganaris et al. 2018; Martínez et al. 2014). Phytochemicals that have significant health benefits belong to the categories of phenolic compounds, alkaloids, organosulfur compounds, phytoestrogens, terpenoids, carotenoids, limonoids, and phytosterols. All of these have shown to be highly effective in preventing and combating a wide variety of diseases (Huang et al. 2016; Jimenez-Garcia et al. 2018; Thakur et al. 2020; Singh et al. 2020; Bayir et al. 2019; Andrade et al. 2020; Koche et al. 2018; Patra 2012). Thus, the exciting properties of phytochemicals, combined with the growing need for new antimicrobial agents highlight these molecules as potential antibiofilm strategies of the future.
3.1 Targeting Bacterial Biofilms with Phytochemicals
Phytochemicals can present various mechanisms of action that have the capacity to harm the establishment and development of biofilms (Simões et al. 2009). These mechanisms include the inhibition of peptidoglycan synthesis (Monte et al. 2014; Nazzaro et al. 2013), cytoplasmatic membranes and cell wall damage and disruption (Suarez et al. 2005; Oussalah et al. 2006; Haraguchi et al. 1996), modification of hydrophobicity and permeabilization of the bacterial membranes (Cox et al. 2000; Carson et al. 2002; Trombetta et al. 2005; Melzig et al. 2001; Ultee et al. 1999), efflux pump inhibition (Aeschlimann et al. 1999; Schmitz et al. 1998; Khan et al. 2006; Micol et al. 2001), inhibition of RNA or DNA synthesis (Mori et al. 1987; Cushnie and Lamb 2005; Feldberg et al. 1988; Sundar and Chang 1992), inhibition/interference of enzyme activity and of the electron transport chain (Lin et al. 2005; Mirzoeva et al. 1997; Sinha Babu et al. 1997; Mandal et al. 2005). Despite the wide variety of phytochemical molecules that have been described as bioactive and of potential interest as food supplement or drug, only few were approved by the US Food and Drug Administration (FDA) (Kongkham et al. 2020).
Phytochemicals with antibiofilm properties include, for example, quercetin, which can inhibit the production of alginate, leading to a decrease in the adhesion during the biofilm development (Górniak et al. 2019; Memariani et al. 2019; Lee et al. 2013). Another example is (+)-usnic acid, which has been used to modify polyurethane surfaces to evaluate its influence on the development of S. aureus and P. aeruginosa biofilms (Francolini et al. 2004). The results showed that the formation of S. aureus biofilms for a period of up to 6 days was inhibited on polyurethane surfaces with (+)-usnic acid. On the contrary, P. aeruginosa biofilms were able to develop on surfaces of (+)-usnic acid-treated polymer. In fact, the (+)-usnic acid has higher antimicrobial activity against S. aureus than against P. aeruginosa, as translated by the minimum inhibitory concentration (MIC), which was 32 μg/mL for S. aureus and 256 μg/mL for P. aeruginosa. (+)-usnic acid was also able to affect the morphology of P. aeruginosa biofilms, since the biofilm was thinner and flat in the control polymer and in the (+)-usnic acid-treated polymer the biofilm was substantially thicker and rougher (Francolini et al. 2004). Another phytochemical that exhibited very interesting results is emodin, which inhibited the development of biofilms by P. aeruginosa, E. coli, and S. aureus through the decrease of expression of key genes involved in biofilm formation. Moreover, several studies also evaluated the effect of plant extracts on biofilm control, instead of pure and isolated phytochemicals. The use of extracts containing different molecules may result in synergistic interactions, which may explain the positive results obtained with low doses of active compounds in herbal products. This highlights the hypothesis that the use of plant extracts may be advantageous in comparison with the use of single phytochemicals (Borges et al. 2016).
The effectiveness of phytochemicals and plant extracts in combating infections may be justified by alterations caused in specific biofilm mechanisms, as described in the following sections.
3.1.1 Effects of Phytochemicals in QS Mechanisms
QS is a crucial regulatory mechanism involved in biofilm formation and differentiation (Borges et al. 2014a). It allows bacteria to communicate through the delivery and sensing of small signal molecules, ensuring relevant advantages for bacteria in regard to colonization, biofilm development, defense, adaptation, virulence, and pathogenicity (Rutherford and Bassler 2012; Pena et al. 2019; Li and Tian 2012; Grainha et al. 2020). Therefore, studying natural molecules with the ability to interfere with QS and understanding their effects may be an important way to fight bacterial tolerance and biofilms (Rutherford and Bassler 2012; Borges et al. 2014a; Lade et al. 2014). In fact, several studies have shown the action of specific phytochemicals on QS and consequently the possibility to retard or avoid biofilm development. For instance, the synthetic halogenated furanone, a secondary metabolite compound derived from furanone present in the Australian macroalgae Delisea pulchra, has shown great potential in affecting the bacterial signaling QS, as well as the motility of cells. Based on the structural similarity between D. pulchra furanone and Acyl-homoserine lactone (AHL) molecules, it is hypothesized that this furanone may be responsible for interfering with the interaction of the putative regulatory protein and AHL molecule through its competitive binding to the receptor. Moreover, furanones can also inhibit surface aggregation traits, when in an ecologically significant concentration (Roy et al. 2018). Hentzer et al. suggested that rhl system, which is a QS mechanism in P. aeruginosa, is a target to furanone (Hentzer et al. 2002). This compound can penetrate the biofilm matrix of P. aeruginosa, interfering with genes related to QS biofilm maturity expression. Hence, this compound is capable of changing the biofilm structure, which promotes the detachment of bacteria and leads to the loss of bacterial biomass in the matrix, also mediating the displacement of AHL molecules from LuxR receptor sites (Roy et al. 2018; Hentzer et al. 2002; Hentzer and Givskov 2003; Asfour 2018; Alasil et al. 2015).
Certain polyphenol compounds, such as epigallocatechin gallate (EGCG), tannic acid, ellagic acid, gallic acid, and ferulic acid are also promising molecules for inhibiting biofilm development. Some studies linked the antibiofilm activity of these polyphenolic molecules to the interference in QS. For instance, Huber et al. demonstrated that polyphenolic compounds, such as gallic acid, (−)-epigallocatechin gallate, (+)-catechin and tannic acid were capable of interfering with QS mechanisms of E. coli and P. putida through the blockade of the AHL system (Quave et al. 2012; Huber et al. 2003). Quercetin, as well as some others flavonoids, have also shown to be efficient in affecting the QS mechanisms of several microorganisms, such as P. aeruginosa, S. aureus, Escherichia coli, Enterococcus faecalis, and Streptococcus mutans.
Curcumin, from the rhizome of Curcuma longa, showed strong and effective biofilm inhibitory activity related to the regulation of the expression of genes involved in QS and associated with the production of alginate and exopolysaccharides. Curcumin has also shown effects in inhibiting the swimming and swarming motilities and increasing biofilm susceptibility to antibiotics (Borges et al. 2016; Packiavathy et al. 2013; Packiavathy et al. 2014). A study performed by Kali et al. demonstrated that curcumin in combination with ciprofloxacin was effective against biofilms of Gram-positive bacteria. Moreover, this compound was also effective against Gram-negative biofilms when combined with the antibiotics amikacin, gentamicin (GEN), and cefepime. These results revealed the potential of using curcumin in a combination therapy (Kali et al. 2016). In addition, emodin induced the proteolysis of E. coli QS signal receptor TraR and enhanced the activity of ampicillin against P. aeruginosa. This compound has also been associated with the downregulation of the cidA gene, which is involved in cell lysis and eDNA release (Borges et al. 2016; Yan et al. 2017; Harapanahalli et al. 2015; Zhang et al. 2020b).
Regarding the use of plant extracts, Zhang et al. evaluated the antibiofilm and QS inhibition activity of an extract from Rosa rugosa tea, whose main components are polyphenols and flavonoids (Zhang et al. 2014). This extract inhibited the production of violacein, controlled by QS in C. violaceum. Furthermore, this extract also inhibited the biofilm formation by E. coli and P. aeruginosa, which may be related not only to its quorum quenching activity, but also to the inhibition of bacteria swarming motility. Glycosyl flavonoids, such as chlorogenic acid, isoorientin, orientin, isovitexin, vitexin, and rutin were also capable of inhibiting the QS in C. violaceum and E. coli. Moreover, there are more plant components with QS inhibition properties, such as extracts from liverwort Lepidozia chordulifera, where it is possible to find sesquiterpenoid viridiflorol, triterpenoids, ursolic and betulinic acids (Zhang et al. 2014). Other relevant and efficient phytochemicals with antiquorum sensing effects include N-(heptylsulfanylacetyl)-L-homoserine lactone, extracted from garlic (able to interrupt QS signaling by competitively inhibiting transcriptional regulators LuxR and LasR), limonoids, and hordenine (Klančnik et al. 2021). Furthermore, baicalin hydrate, cinnamaldehyde, and hamamelitannin have been shown to significantly improve the susceptibility of P. aeruginosa, B. cenocepacia, and S. aureus biofilms, including MRSA, in combination with conventional antibiotics. In fact, hamamelitannin can act as a QS inhibitor by increasing both the in vitro and in vivo susceptibility of MRSA biofilms, which can be associated with its ability to interact with the TraP receptor and affect the release of eDNA (Brackman et al. 2011; Yuan et al. 2020; Jiang et al. 2019).
There are more examples of studies that equally show promising results of phytochemicals regarding antiquorum sensing activity. It was demonstrated by Niu et al. that Cinnamomum cassia extract, containing cinnamaldehyde and eugenol, significantly inhibited QS of E. coli and Vibrio harveyi (Niu et al. 2006). In addition, clove, cinnamon, peppermint, and lavender also exhibited antiquorum sensing activity, according to Khan et al. (Khan et al. 2009). Iberin, which is an isothiocyanate and sulfoxide, interfered with the QS mechanism of P. aeruginosa and induced apoptosis. Organosulfur compounds from garlic, such as allicin and ajoene, were able to inhibit QS of P. aeruginosa and E. coli (Borges et al. 2013). In another study by Vikram et al. flavonoids were evaluated for their capacity to interfere with QS of V. harveyi and inhibited the development of E. coli O157:H7 and V. harveyi biofilms (Vikram et al. 2010). The results showed that kaempferol, naringenin, quercetin, and apigenin were able to act as nonspecific inhibitors of autoinducer-mediated cell–cell signaling processes. In addition, these molecules inhibited the formation of V. harveyi and E. coli O157:H7 biofilms (Vikram et al. 2010).
3.1.2 Effects of Phytochemicals on Motility
Motility, which generally depends on flagella and pili, is one of the crucial factors that contribute to biofilm formation and development, especially at an earlier stage, since these mechanisms require a multicellular movement and dispersion on a surface (Monte et al. 2014; Lemon et al. 2007; Cai et al. 2020; Kearns 2010; Köhler et al. 2000). It has been already shown that many natural compounds, extracts or pure products, have the capacity to interfere with the bacterial motility of several microorganisms (Vattem et al. 2007; Liaqat et al. 2018), which may be reflected on the reduction of biofilm formation ability. Swimming and swarming motilities of P. aeruginosa, Proteus mirabilis, and Serratia marcescens were inhibited by methanolic extracts of Cuminum cyminum, especially methyl eugenol, a compound with a well-studied antibiofilm activity. In addition, cinnamaldehyde and eugenol, from Cinnamomum cassia, were able to compromise the development of E. coli biofilms by interfering with their swimming motility (Monte et al. 2014). Gallic acid and ferulic acid displayed potential to inhibit the motility and adhesion of four pathogenic bacteria, including E. coli, P. aeruginosa, S. aureus, and Listeria monocytogenes. Identical results were obtained for isothiocyanates allyl isothiocyanate (AITC) and 2-phenylethyl isothiocyanate (PEITC) (Borges et al. 2012). Ferulic acid and salicylic acid (SA) were reported to inhibit swimming motility of Bacillus cereus and Pseudomonas fluorescens (Lemos et al. 2014).
Camellia nitidissima Chi is a well-known edible plant from China with diverse biological and medicinal properties, especially antibacterial activity. A study performed by Yang et al. assessed the inhibitory activity of the dichloromethane fraction (DF) of C. nitidissima Chi flowers on the pyocyanin production, as well as on the swarming and swimming motility of P. aeruginosa PAO1, at sub-minimum inhibitory concentrations (Yang et al. 2018). DF was associated with a concentration-dependent inhibition activity in relation to swarming and swimming motility. Moreover, the half maximal inhibitory concentrations (IC50) were determined and they found values of 0.158 ± 0.009 mg/mL for pyocyanin production, 0.139 ± 0.004 mg/mL for swarming motility, and 0.334 ± 0.049 mg/mL for swimming motility. In addition, the results of a real-time polymerase chain reaction (RT-PCR) showed that DF significantly downregulated the expression of rhlR and lasR (Yang et al. 2018). In fact, las and rhl systems are the two principal QS mechanism elements of P. aeruginosa. Finally, through the high-performance liquid chromatography (HPLC)-triple-time of flight (TOF)-MS/MS analysis, it was found that DF is mainly composed of gallic acid, catechin, ellagic acid, chlorogenic acid, quercetin, and kaempferol. These six identified compounds displayed inhibitory effects on pyocyanin production, swarming, and swimming motilities. Ellagic acid showed the strongest effect with IC50 values of 0.067 ± 0.002 mg/mL for pyocyanin production, 0.024 ± 0.008 mg/mL for swarming motility, and 0.020 ± 0.003 mg/mL for swimming motility (Yang et al. 2018). Therefore, it is possible to infer that all these alterations caused by DF may cause significant effects on the ability of P. aeruginosa to form biofilms.
In another study by Borges et al., the effect of AITC and PEITC on planktonic cell susceptibility, bacterial motility and adhesion, and biofilms of E. coli, P. aeruginosa, S. aureus, and L. monocytogenes was evaluated (Borges et al. 2014b). AITC caused complete inhibition of swimming motility of P. aeruginosa and total inhibition of swarming motility of E. coli, while PEITC caused complete inhibition of swimming motility of E. coli, P. aeruginosa, and L. monocytogenes and swarming motility of E. coli and P. aeruginosa. Moreover, the spreading motility of S. aureus was completely inhibited by PEITC. AITC and PEITC presented a preventive effect on biofilm formation, which may be related to the effects on the inhibition of bacterial motility. Moreover, AITC and PEITC showed a high potential to reduce the mass of biofilm formed by the Gram-negative bacteria (Borges et al. 2014b).
3.1.3 Effects of Phytochemicals on Adhesion
Bacterial adhesion to the surface of materials is the first step that leads to the formation of a biofilm and, from the moment the adhesion occurs, it is possible for the bacteria to communicate with each other and establish a community (Klančnik et al. 2021; Dunne 2002; Flemming et al. 2016). Therefore, since bacterial adhesion to a surface is an initial prerequisite for biofilm formation, it is essential to identify natural compounds capable of interfering with this phenomenon, which may constitute an important target when developing strategies for controlling pathogenicity (Kostakioti et al. 2013; Verderosa et al. 2019). Within this context, phenolic compounds have been shown to exhibit important roles in preventing bacterial adhesion, including the adhesion of Campylobacter jejuni (Klančnik et al. 2021). In a study of Pogačar et al., thyme ethanolic extract (TE), thyme post-hydrodistillation residue (TE-R), and olive leaf extract (OE) were evaluated for their phytochemical composition using high-performance liquid chromatography (HPLC) with photodiode array, and antimicrobial activity against C. jejuni (Šikić Pogačar et al. 2016). The analysis showed that the main components present in TE and TE-R were flavone glucuronides and rosmarinic acid derivatives, while in OE verbascoside, luteolin 7-O-glucoside and oleuroside were the major compounds. The compounds TE and TE-R were able to decrease C. jejuni adhesion to the abiotic surface by up to 35%, at a concentration range between 50–200 μg/mL, and up to 30%, at a concentration range between 0.2–12.5 μg/mL. TE-R was more effective than TE and displayed a meaningful inhibition of C. jejuni adhesion (higher than 30%), reaching up to 40%. When considering a concentration range of OE between 3.125–12.5 μg/mL, biofilm formation was affected and the adhesion inhibition reached 10% to 23%, respectively. In addition, the anti-adhesion effect of C. jejuni to cell cultures was also assessed and it was observed that the C. jejuni adhesion toward pig small intestine epithelial cell line, PSI cl1 cells, was remarkably decreased up to 30% in the presence of TE, TE-R, and OE at a concentration range between 0.78–200 μg/mL (Šikić Pogačar et al. 2016).
The North American cranberry is also recognized for its high levels of phytochemicals, including phenolic acids, flavonoids, and ellagic acid (Walton 2014). The antimicrobial properties of cranberry species have been associated with high levels of polyphenol compounds, particularly proanthocyanidin (PACs). So far, several data have revealed that PACs have unique characteristics that allow the inhibition of bacterial adhesion to epithelial cells. Indeed, the studies developed by various authors have shown that there is a direct correlation between the ingestion of cranberries and the prevention of urinary tract infections (UTIs) in females (Walton 2014; Kontiokari et al. 2005; Carson and Riley 2003; Tempera et al. 2010). Several in vitro studies have already been performed and demonstrated that the anti-adhesion effects of PACs are responsible for a reduction in the bacterial adherence to urinary tract cells such as uroepithelial cells, but also to other biological materials (Walton 2014; Kontiokari et al. 2005). Furthermore, cranberry extracts also seem to have similar inhibitory effects on tissue adhesion with regard to Gram-negative bacteria, including the ones of the genera Proteus, Klebsiella, Enterobacter, and Pseudomonas. The intake of cranberry has been associated with a decrease in the incidence of gastric ulcer and gastric cancer (Walton 2014). Burger et al. showed that for cultured gastric epithelial cells, the phytochemicals found in cranberry juice can specifically interfere with a sialyllactose-specific adhesion mechanism that allows Helicobacter pylori to adhere to the gastric mucosa. Consequently, these authors inferred that the cranberry juice could also inhibit the adhesion of bacteria to the stomach in vivo and acting in the prevention of stomach ulcers that are caused by H. pylori. (Walton 2014; Burger et al. 2002).
Phenolic and polyphenolic compounds have also been shown to have substantial anti-adhesion effects. A work performed by Toivanen et al. evaluated the anti-adhesion activity of different molecular size fractions of wild cranberry Vaccinium oxycoccos polyphenols against Streptococcus pneumoniae and Streptococcus agalactiae (Toivanen et al. 2010). Moreover, according to Borges et al., there are other phytochemicals with very impressive anti-adhesion activity, namely cinnamaldehyde and eugenol, from Cinnamomum cassia, which interfered with adhesion and biofilm formation of E. coli (Borges et al. 2013). Polyanacardic acid, polysalicylic acid, catechin, epigallocatechin, and tannic acid also exhibited anti-adhesion activity and inhibited the formation of Streptococcus mutans and P. aeruginosa biofilms (Borges et al. 2013). In another work of Lee et al., the potential effects of phloretin, which can be found, for instance, in apples, were investigated (Lee et al. 2011). Phloretin reduced the attachment of E. coli O157:H7 to human colon epithelial cells and decreased the formation of E. coli O157:H7 biofilms. It was also demonstrated that phloretin was able to repress genes associated with toxicity, hlyE and stx(2); the autoinducer-2 importer genes, lsrACDBF; curli genes csgA and csgB; and also prophage genes of E. coli O157:H7 biofilms (Lee et al. 2011).
3.1.4 Effects of Phytochemicals on the EPS Production
EPS have a wide range of biological functions, being part of the carbon and energy reserves, preventing desiccation, protecting against environmental stresses, providing protection against toxins and antibiotics, as well as playing a crucial role in pathogenicity, symbiosis phenomena, and adhesion to surfaces (Singha 2012; Whitfield et al. 1993; Roberts 1996; Khan and Iqbal 2017). Hence, it is fundamental to discover and understand the role of natural compounds in inhibiting the production of EPS with regard to controlling and fighting biofilms (Mishra et al. 2020; Koo et al. 2017). Several studies described significant action of extracts and specific phytochemicals on EPS inhibition and removal. For instance, Borges et al showed that methyl eugenol interfered with EPS production (Borges et al. 2013). In a study developed by Packiavathy et al., the inhibitory properties of spices and vegetables, commonly found in South India, on the QS and EPS production of the bacteria C. violaceum were evaluated (Packiavathy et al. 2012). In the 22 samples tested, the QS inhibitory compound present in the methanolic extract of Cuminum cyminum, at a concentration of 2 mg/mL, was able to inhibit the production of violacein by C. violaceum. In addition, it was demonstrated that the C. cyminum extract highly interfered with the physiological functions regulated by AHL and the formation of biofilms, including flagellar movement and EPS production. The QS inhibitory effects shown by this plant were assigned to methyl eugenol, considering the results of molecular docking analysis. At a sub-MIC level, it promoted damage to the biofilm structure and strongly inhibited the biofilm formation of P. aeruginosa PAO1, P. mirabilis, and Serratia marcescens (Packiavathy et al. 2012). Abraham et al. assessed the inhibition of EPS production by methanolic fraction of the dried fruits of Caesalpinia spinosa. Also, the ability to inhibit biofilm formation by E. coli, P. mirabilis, S. marcescens, and P. aeruginosa PAO1 was assessed (Issac Abraham et al. 2011). In addition, its effect in inhibiting QS-dependent phenomena, such as violacein production in C. violaceum and swimming and swarming motility was also investigated. It was observed that the extract of C. spinosa interfered with swimming and swarming motility, EPS production and biofilm formation in E. coli, P. mirabilis, S. marcescens, and P. aeruginosa PAO1. Here, the extract revealed a strong QS inhibition effect, in a concentration-dependent way, however, without interfering with the microbial growth. At a concentration of 2 mg/mL, this extract significantly restricted the EPS production to 58, 46, 66, and 67%, as well as limited biofilm formation to 79, 75, 73, 70%, in S. marcescens, P. aeruginosa PAO1, E. coli, and P. mirabilis, respectively (Issac Abraham et al. 2011).
There are other phytochemicals with a proven ability to inhibit EPS production. A well-known compound is (−)-epigallocatechin gallate (EGCg), which has several antimicrobial action modes, including the ability to decrease EPS production (Borges et al. 2013). EGCg is the major component of tea catechins and displayed an inhibitory effect, EPS degradation capacity, and a destructive action against E. coli biofilms (Maeyama et al. 2005). Taking into account the EGCg effects, Maeyama et al. developed a new bactericidal surface based on a catechin polymer (Maeyama et al. 2005). The surfaces tested, containing EGCg, were prepared through photopolymerization of liquid biodegradable polyesters and the rate of releasing was potentiated by increasing the rate of surface-erosion of the polymers. Interestingly, polymers exhibiting a higher releasing ability showed a lower biofilm development on the surfaces. Moreover, EGCg induced a biofilm-destructing effect, such as EPS degradation, damage of bacterial membrane, and cell detachment (Maeyama et al. 2005). Allicin is another phytochemical with remarkable proven evidence in relation to EPS production. Lihua et al. investigated the activity of allicin against P. aeruginosa biofilm development, specifically their effect on the production of virulence factors controlling the QS mechanism and EPS (Lihua et al. 2013). The authors found that the EPS production increased over time, but decreased with the concentration of allicin used in the treatment. The study also demonstrated that although the dry weight of bacteria between the different groups was almost equal to the one registered at the beginning of the experiments, the total amount of EPS was significantly reduced when allicin was used. Moreover, bacterial adhesion was significantly reduced compared to the saline control group, when 128 μg/mL of allicin was used. Interestingly, when the biofilm was treated with 10 μg/mL of allicin, it became thinner, and its structure was compromised. Similar results were obtained for the group treated with 128 μg/mL of allicin, in which the biofilm thickness was reduced from 28.83 μm to 16.50 μm. The effect of allicin on the virulence factors exotoxin A, elastase, pyoverdine, and rhamnolipid was also assessed, being demonstrated that allicin significantly downregulated the expression of exotoxin A and elastase. In addition, there was a complete inhibition of rhamnolipid and pyoverdine production. Thus, allicin can affect the development and maturation of P. aeruginosa biofilms, suggesting that this compound may be a promising therapy for treating bacterial biofilms (Lihua et al. 2013).
4 Conclusions and Future Perspectives
Antibiotic resistance is a serious problem in modern society. It has been claimed for many years that several natural plant products have medicinal effects, which make them promising for the treatment of various diseases. The development of natural, safe, and effective therapies helps control and minimize antibiotic resistance. Within the broad therapeutic and nutritional effects of phytochemicals, the ability to impair biofilm mechanisms, damage the bacterial membrane, restrict biofilm formation and silence virulence factors make them highly sought-after, effective antimicrobial agents. Besides the interference of phytochemicals with many planktonic bacterial processes, these natural compounds also have promising antimicrobial effects on biofilm mechanisms, such as adhesion, motility, QS and EPS production, displaying a strong activity against virulence factors of both planktonic and sessile cells. Nevertheless, further research is needed to develop and tailor specific applications of phytochemicals for therapeutic and clinical purposes, to assess their safety profile, to confirm their efficacy in vivo and in humans, and to ensure appropriate and selected use to prevent the future emergence of resistances.
References
Abedon S, Duffy S, Turner PE (2009) Bacteriophage ecology. Encycl Microbiol 1:42–57
Abraham SN, Sharon N, Ofek I, Schwartzman JD (2015) In: Tang Y-W, Sussman M, Liu D, Poxton I, J. B. T.-M. M. M. (Second E. Schwartzman) (eds) Chapter 24: Adhesion and colonization. Academic Press, Boston, pp 409–421
Aeschlimann JR, Dresser LD, Kaatz GW, Rybak MJ (1999) Effects of nor a inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother 43(2):335–340. https://doi.org/10.1128/AAC.43.2.335
Aggarwal S, Stewart PS, Hozalski RM (2015) Biofilm cohesive strength as a basis for biofilm recalcitrance: are bacterial biofilms overdesigned? Microbiol Insights 82:MBI.S31444. https://doi.org/10.4137/MBI.S31444
Alasil SM, Omar R, Ismail S, Yusof MY (2015) Inhibition of quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by culture extract from novel bacterial species of Paenibacillus using a rat model of chronic lung infection. Int J Bacteriol 2015:671562. https://doi.org/10.1155/2015/671562
Aliramezani A, Soleimani M, Fard RMN, Nojoomi F (2019) Virulence determinants and biofilm formation of Acinetobacter baumannii isolated from hospitalized patients. Germs 9(3):148–153. https://doi.org/10.18683/germs.2019.1171
Allen N, Mulvihill DP, Hiscock JR (2019) Novel antimicrobial agents for clinical applications. Access Microbiol 1(1A):0301. https://doi.org/10.1099/acmi.ac2019.po0250
Almaguer-Flores A (2013) 8: Biofilms in the oral environment. In: Yan MI (ed) Woodhead publishing series in biomaterials. Woodhead Publishing, pp 169–186
AL-Mamun M, Chowdhury T, Biswas B, Absar N (2018) In: Grumezescu AM, A. M. B. T.-F. S, Holban P (eds) Chapter 11: Food poisoning and intoxication: a global leading concern for human health. Academic Press, pp 307–352
Alonso B et al (2020) Characterization of the virulence of Pseudomonas aeruginosa strains causing ventilator-associated pneumonia. BMC Infect Dis 20(1):909. https://doi.org/10.1186/s12879-020-05534-1
Andrade M, Malheiro J, Borges F, Saavedra MJ, Simões M (2020) In: Simoes M, Borges A, L. B. T.-R. T. in B. S, Simoes TC (eds) Chapter 12: The potential of phytochemical products in biofilm control. Academic Press, pp 273–293
Arendt EK, Zannini E (2013) 4–Barley. In: Arendt EK, E. B. T.-C. G. for the F, Zannini BI (eds) Woodhead publishing series in food science, technology and nutrition. Woodhead Publishing, pp 155–201e
Asaduzzaman GAEE-TAE-M (2018) Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Intech Open, Rijeka. Ch. 4
Asfour HZ (2018) Anti-quorum sensing natural compounds. J Microsc Ultrastruct 6(1):1–10. https://doi.org/10.4103/JMAU.JMAU_10_18
Aung EE, Kristanti AN, Aminah NS, Takaya Y, Ramadhan R (2020) Plant description, phytochemical constituents and bioactivities of Syzygium genus: a review. Open Chem 18(1):1256–1281. https://doi.org/10.1515/chem-2020-0175
Balasubramanian D, Harper L, Shopsin B, Torres VJ (2017) Staphylococcus aureus pathogenesis in diverse host environments. Pathog Dis 75(1):ftx 005. https://doi.org/10.1093/femspd/ftx005
Balcázar JL, Subirats J, Borrego CM (2015) The role of biofilms as environmental reservoirs of antibiotic resistance. Front Microbiol 6:1216. https://doi.org/10.3389/fmicb.2015.01216
Barbieri R et al (2017) Phytochemicals for human disease: an update on plant-derived compounds antibacterial activity. Microbiol Res 196:44–68. https://doi.org/10.1016/j.micres.2016.12.003
Barbosa J, Gibbs PA, Teixeira P (2010) Virulence factors among enterococci isolated from traditional fermented meat products produced in the north of Portugal. Food Control 21(5):651–656. https://doi.org/10.1016/j.foodcont.2009.10.002
Bayir AG, Kiziltan HS, Kocyigit A (2019) Chapter 1: Plant family, carvacrol, and putative protection in gastric cancer. In: Watson RR, Preedy GD (eds) Dietary interventions in gastrointestinal diseases. Academic Press, London, pp 3–18
Beceiro A, Tomás M, Bou G (2013) Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev 26(2):185–230. https://doi.org/10.1128/CMR.00059-12
Bello-López JM et al (2019) Horizontal gene transfer and its association with antibiotic resistance in the genus Aeromonas spp. Microorganisms 7(9):363. https://doi.org/10.3390/microorganisms7090363
Berne C, Ellison CK, Ducret A, Brun YV (2018) Bacterial adhesion at the single-cell level. Nat Rev Microbiol 16(10):616–627. https://doi.org/10.1038/s41579-018-0057-5
Bi Y et al (2021) Therapeutic strategies against bacterial biofilms. Fundam Res 1(2):193–212. https://doi.org/10.1016/j.fmre.2021.02.003
Borges A, Saavedra MJ, Simões M (2012) The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 28(7):755–767. https://doi.org/10.1080/08927014.2012.706751
Borges A, Abreu A, Malheiro JF, Saavedra M, Simões M (2013) Biofilm prevention and control by dietary phytochemicals. In: Méndez-Vilas A (ed) Microbial pathogens and strategies for combating them: science, technology and education. Formatex Research Center, Badajoz, pp 32–41
Borges A, Serra S, Cristina Abreu A, Saavedra MJ, Salgado A, Simões M (2014a) Evaluation of the effects of selected phytochemicals on quorum sensing inhibition and in vitro cytotoxicity. Biofouling 30(2):183–195. https://doi.org/10.1080/08927014.2013.852542
Borges A, Simões L, Saavedra M, Simões M (2014b) The action of selected isothiocyanates on bacterial biofilm prevention and control. Int Biodeterior Biodegradation 86:25–33. https://doi.org/10.1016/j.ibiod.2013.01.015
Borges A, Abreu AC, Dias C, Saavedra MJ, Borges F, Simões M (2016) New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 21(7):877. https://doi.org/10.3390/molecules21070877
E. F. Boyd, “Chapter 4: Bacteriophage-encoded bacterial virulence factors and phage–Pathogenicity Island interactions,” in Bacteriophages, Part A, vol. 82, M. Łobocka and W. T. B. T.-A. in V. R. Szybalski, Eds. Academic Press, 2012, pp. 91–118
Brackman G, Cos P, Maes L, Nelis H, Coenye T (2011) Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother 55:2655–2661. https://doi.org/10.1128/AAC.00045-11
Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F (2011) Resistance of bacterial biofilms to disinfectants: a review. Biofouling 27(9):1017–1032. https://doi.org/10.1080/08927014.2011.626899
Britigan BE, Roeder TL, Rasmussen GT, Shasby DM, McCormick ML, Cox CD (1992) Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells. Implications for pseudomonas-associated tissue injury. J Clin Invest 90(6):2187–2196. https://doi.org/10.1172/JCI116104
Burger O, Weiss E, Sharon N, Tabak M, Neeman I, Ofek I (2002) Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit Rev Food Sci Nutr 42(3 Suppl):279–284. https://doi.org/10.1080/10408390209351916
Cai Y, Hutchin A, Craddock J, Walsh MA, Webb JS, Tews I (2020) Differential impact on motility and biofilm dispersal of closely related phosphodiesterases in Pseudomonas aeruginosa. Sci Rep 10(1):6232. https://doi.org/10.1038/s41598-020-63008-5
Caro-Astorga J et al (2020) Two genomic regions encoding exopolysaccharide production systems have complementary functions in B. cereus multicellularity and host interaction. Sci Rep 10(1):1000. https://doi.org/10.1038/s41598-020-57970-3
Carson CF, Riley TV (2003) Non-antibiotic therapies for infectious diseases. Commun Dis Intell Q Rep 27(Suppl):S143–S146
Carson CF, Mee BJ, Riley TV (2002) Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 46(6):1914–1920. https://doi.org/10.1128/AAC.46.6.1914-1920.2002
Chassagne F et al (2021) A systematic review of plants with antibacterial activities: a taxonomic and phylogenetic perspective. Front Pharmacol 11:2069. https://doi.org/10.3389/fphar.2020.586548
Chen Y et al (2014) Phytochemical profiles and antioxidant activities in six species of ramie leaves. PLoS One 9(9):e108140. https://doi.org/10.1371/journal.pone.0108140
Chiba A, Sugimoto S, Sato F, Hori S, Mizunoe Y (2015) A refined technique for extraction of extracellular matrices from bacterial biofilms and its applicability. Microb Biotechnol 8(3):392–403. https://doi.org/10.1111/1751-7915.12155
Chouhan S, Sharma K, Guleria S (2017) Antimicrobial activity of some essential oils-present status and future perspectives. Medicines (Basel, Switzerland) 4(3):58. https://doi.org/10.3390/medicines4030058
Ćirić AD et al (2019) Natural products as biofilm formation antagonists and regulators of quorum sensing functions: a comprehensive review update and future trends. South African J Bot 120:65–80. https://doi.org/10.1016/j.sajb.2018.09.010
Clark DP, Pazdernik NJ, McGehee MR (2019) Chapter 16: Regulation of transcription in prokaryotes. In: Clark DP, Pazdernik NJ, McGehee MR (eds) Molecular biology. Academic Press, London, pp 522–559
Costa OYA, Raaijmakers JM, Kuramae EE (2018) Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol 9:1636. https://doi.org/10.3389/fmicb.2018.01636
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318–1322. https://doi.org/10.1126/science.284.5418.1318
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12(4):564–582. https://doi.org/10.1128/CMR.12.4.564
Cox CD (1986) Role of pyocyanin in the acquisition of iron from transferrin. Infect Immun 52(1):263–270. https://doi.org/10.1128/iai.52.1.263-270.1986
Cox SD et al (2000) The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol 88(1):170–175. https://doi.org/10.1046/j.1365-2672.2000.00943.x
Cushnie TPT, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26(5):343–356. https://doi.org/10.1016/j.ijantimicag.2005.09.002
de Carvalho CCCR (2018) Marine biofilms: a successful microbial strategy with economic implications. Front Mar Sci 5:126. https://doi.org/10.3389/fmars.2018.00126
Del Pozo JL (2018) Biofilm-related disease. Expert Rev Anti-Infect Ther 16(1):51–65. https://doi.org/10.1080/14787210.2018.1417036
Desai SD, Saheb S, Das K, Haseena S (2015) Phytochemical analysis of Nigella sativa and it’s antidiabetic effect. J Pharm Sci Res 7:527–532
Di Martino P (2018) Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol 4(2):274–288. https://doi.org/10.3934/microbiol.2018.2.274
Diep CS, Baranowski J, Baranowski T (2015) 4–The impact of fruit and vegetable intake on weight management. In: T. B. T.-M, Gill PO (eds) Woodhead publishing series in food science, technology and nutrition. Woodhead Publishing, pp 59–78
Diggle SP, Whiteley M (2020) Microbe profile: Pseudomonas aeruginosa: opportunistic pathogen and lab rat. Microbiology 166(1):30–33. https://doi.org/10.1099/mic.0.000860
Dinges MM, Orwin PM, Schlievert PM (2000) Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13(1):16–34. https://doi.org/10.1128/CMR.13.1.16
Dunne WM (2002) Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev 15(2):155–166. https://doi.org/10.1128/CMR.15.2.155-166.2002
Eberl L, Molin S, Givskov M (1999) Surface motility of serratia liquefaciens MG1. J Bacteriol 181(6):1703–1712. https://doi.org/10.1128/JB.181.6.1703-1712.1999
Etter D, Schelin J, Schuppler M, Johler S (2020) Staphylococcal enterotoxin C—an update on SEC variants, their structure and properties, and their role in foodborne intoxications. Toxins 12(9):584. https://doi.org/10.3390/toxins12090584
Fair RJ, Tor Y (2014) Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem 6:25–64. https://doi.org/10.4137/PMC.S14459
Feldberg RS et al (1988) In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob Agents Chemother 32(12):1763–1768. https://doi.org/10.1128/AAC.32.12.1763
Ferri M, Ranucci E, Romagnoli P, Giaccone V (2017) Antimicrobial resistance: a global emerging threat to public health systems. Crit Rev Food Sci Nutr 57(13):2857–2876. https://doi.org/10.1080/10408398.2015.1077192
Fisher EL, Otto M, Cheung GYC (2018) Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front Microbiol 9:436. https://doi.org/10.3389/fmicb.2018.00436
Flannagan RS, Heit B, Heinrichs DE (2015) Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens 4(4):826–868. https://doi.org/10.3390/pathogens4040826
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633. https://doi.org/10.1038/nrmicro2415
Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575. https://doi.org/10.1038/nrmicro.2016.94
Floyd KA, Eberly AR, Hadjifrangiskou M (2017) In: Deng Y, W. B. T.-B. and I. M. D. Lv (eds) 3- Adhesion of bacteria to surfaces and biofilm formation on medical devices. Woodhead Publishing, pp 47–95
Forni C et al (2019) Beneficial role of phytochemicals on oxidative stress and age-related diseases. Biomed Res Int 2019:8748253. https://doi.org/10.1155/2019/8748253
Francolini I, Norris P, Piozzi A, Donelli G, Stoodley P (2004) Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob Agents Chemother 48(11):4360–4365. https://doi.org/10.1128/AAC.48.11.4360-4365.2004
M. Frederix and J. A. Downie, “Chapter 2: Quorum sensing: regulating the regulators,” vol. 58, R. K. B. T.-A. in M. P. Poole, Ed. Academic Press, 2011, pp. 23–80
Frieri M, Kumar K, Boutin A (2017) Antibiotic resistance. J Infect Public Health 10(4):369–378. https://doi.org/10.1016/j.jiph.2016.08.007
Gao L et al (2019) Mediation of extracellular polymeric substances in microbial reduction of hematite by Shewanella oneidensis MR-1. Front Microbiol 10:575. https://doi.org/10.3389/fmicb.2019.00575
Garrett TR, Bhakoo M, Zhang Z (2008) Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 18(9):1049–1056. https://doi.org/10.1016/j.pnsc.2008.04.001
Ghoshal G (2018) Chapter 2: Biotechnology in food processing and preservation: an overview. In: Holban AM, A. M. B. T.-A. in B. for F. I. Grumezescu (eds) Handbook of Food Bioengineering. Academic Press, pp 27–54
Giaouris E, Simões M (2018) Pathogenic biofilm formation in the food industry and alternative control strategies. In: Dodd CER (ed) Foodborne diseases. Elsevier, London, pp 309–377
Giulia O, O'Toole GA (2021) ‘It takes a village’: mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J Bacteriol 202(1):e00530–e00519. https://doi.org/10.1128/JB.00530-19
Górniak I, Bartoszewski R, Króliczewski J (2019) Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 18(1):241–272. https://doi.org/10.1007/s11101-018-9591-z
E. F. Goulden, L. Høj, and M. R. Hall, “8–Microbial management for bacterial pathogen control in invertebrate aquaculture hatcheries,” in Woodhead publishing series in food science, technology and nutrition, G. Allan and G. B. T.-A. in A. H. T. Burnell, Eds. Woodhead Publishing, 2013, pp. 246–285
Grainha T, Jorge P, Alves D, Lopes SP, Pereira MO (2020) Unraveling Pseudomonas aeruginosa and Candida albicans communication in coinfection scenarios: insights through network analysis. Front Cell Infect Microbiol 10:676. https://doi.org/10.3389/fcimb.2020.550505
Guan R et al (2021) A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 271:129499. https://doi.org/10.1016/j.chemosphere.2020.129499
Haraguchi H, Oike S, Muroi H, Kubo I (1996) Mode of antibacterial action of totarol, a diterpene from Podocarpus nagi. Planta Med 62(2):122–125. https://doi.org/10.1055/s-2006-957832
Harapanahalli AK, Chen Y, Li J, Busscher HJ, van der Mei HC (2015) Influence of adhesion force on Ica a and cid a gene expression and production of matrix components in Staphylococcus aureus biofilms. Appl Environ Microbiol 81(10):3369–3378. https://doi.org/10.1128/AEM.04178-14
Harriott BS (2019) Biofilms and antibiotics. In: Caplan MJ (ed) Reference module in biomedical sciences. Elsevier, Amsterdam
Harshey RM (2003) Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol 57:249–273. https://doi.org/10.1146/annurev.micro.57.030502.091014
Harvey J, Gilmour A (1999) Staphylococcus/Staphylococcus Aureus. In: Robinson FM (ed) Encyclopedia of food microbiology. Elsevier, Oxford, pp 2066–2071
Hawkings NJ, Wood F, Butler CC (2007) Public attitudes towards bacterial resistance: a qualitative study. J Antimicrob Chemother 59(6):1155–1160. https://doi.org/10.1093/jac/dkm103
Hentzer M, Givskov M (2003) Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest 112(9):1300–1307. https://doi.org/10.1172/JCI20074
Hentzer M et al (2002) Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148(Pt 1):87–102. https://doi.org/10.1099/00221287-148-1-87
Holmes AH et al (2016) Understanding the mechanisms and drivers of antimicrobial resistance. Lancet (London, England) 387(10014):176–187. https://doi.org/10.1016/S0140-6736(15)00473-0
Howes M-JR, Simmonds MSJ (2014) The role of phytochemicals as micronutrients in health and disease. Curr Opin Clin Nutr Metab Care 17(6):558–566. [Online]. Available: https://journals.lww.com/co-clinicalnutrition/Fulltext/2014/11000/The_role_of_phytochemicals_as_micronutrients_in.12.aspx
Huang Y, Xiao D, Burton-Freeman B, Edirisinghe I (2016) Chemical changes of bioactive phytochemicals during thermal processing. In: Reference module in food science
Huber B, Eberl L, Feucht W, Polster J (2003) Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Zeitschrift für Naturforsch C 58(11–12):879–884. https://doi.org/10.1515/znc-2003-11-1224
Huycke MM, Sahm DF, Gilmore MS (1998) Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis J 4(2):239. https://doi.org/10.3201/eid0402.980211
Ike Y (2017) Pathogenicity of enterococci. Nihon Saikingaku Zasshi 72(2):189–211. https://doi.org/10.3412/jsb.72.189
Issac Abraham SVP, Palani A, Ramaswamy BR, Shunmugiah KP, Arumugam VR (2011) Antiquorum sensing and antibiofilm potential of Capparis spinosa. Arch Med Res 42(8):658–668. https://doi.org/10.1016/j.arcmed.2011.12.002
Jachlewski S, Jachlewski WD, Linne U, Bräsen C, Wingender J, Siebers B (2015) Isolation of extracellular polymeric substances from biofilms of the Thermoacidophilic archaeon Sulfolobus acidocaldarius. Front Bioeng Biotechnol 3:123. https://doi.org/10.3389/fbioe.2015.00123
Jamal M et al (2018) Bacterial biofilm and associated infections. J Chinese Med Assoc 81(1):7–11. https://doi.org/10.1016/j.jcma.2017.07.012
Jiang Q, Chen J, Yang C, Yin Y, Yao K (2019) Quorum sensing: a prospective therapeutic target for bacterial diseases. Biomed Res Int 2019:2015978. https://doi.org/10.1155/2019/2015978
Jiao Y et al (2010) Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl Environ Microbiol 76(9):2916–2922. https://doi.org/10.1128/AEM.02289-09
Jimenez-Garcia SN et al (2018) Chapter 13: Phytochemical and pharmacological properties of secondary metabolites in berries. In: Holban AM, Grumezescu AMBT-TF (eds) Handbook of food bioengineering. Academic Press, pp 397–427
Jindal AK, Pandya K, Khan ID (2015) Antimicrobial resistance: a public health challenge. Med Journal, Armed Forces India 71(2):178–181. https://doi.org/10.1016/j.mjafi.2014.04.011
Julian KG, Blumberg EA (2017) In: Cohen J, Powderly WG, S. M. B. T.-I. D. Fourth E. Opal (eds) Practice point 19–urinary tract infections in kidney transplant recipients. Elsevier, pp 557–558.e1
Kali A, Bhuvaneshwar D, Charles P, Seetha K (2016) Antibacterial synergy of curcumin with antibiotics against biofilm producing clinical bacterial isolates. J Basic Clin Pharm 7:93. https://doi.org/10.4103/0976-0105.183265
Karlowsky JA, Hoban DJ, Hackel MA, Lob SH, Sahm DF (2017) Antimicrobial susceptibility of gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Asia-Pacific countries: SMART 2013-2015. J Med Microbiol 66(1):61–69. https://doi.org/10.1099/jmm.0.000421
Kearns DB (2010) A field guide to bacterial swarming motility. Nat Rev Microbiol 8(9):634–644. https://doi.org/10.1038/nrmicro2405
Khan MSA, Iqbal MMA (2017) Chemical nature of biofilm matrix and its significance. In: Ahmad I (ed) Biofilms in plant and soil health. Wiley, Chichester, pp 151–177
Khan IA, Mirza ZM, Kumar A, Verma V, Qazi GN (2006) Piperine, a phytochemical potentiator of ciprofloxacin against Staphylococcus aureus. Antimicrob Agents Chemother 50(2):810–812. https://doi.org/10.1128/AAC.50.2.810-812.2006
Khan MSA, Zahin M, Hasan S, Husain FM, Ahmad I (2009) Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett Appl Microbiol 49(3):354–360. https://doi.org/10.1111/j.1472-765X.2009.02666.x
Khatoon Z, McTiernan CD, Suuronen EJ, Mah T-F, Alarcon EI (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4(12):e01067–e01067. https://doi.org/10.1016/j.heliyon.2018.e01067
Kibe M, Konyole S, Oloo M, Ochieng N, Kathure D (2017) The role of phytochemicals in prevention and control of chronic diseases. Int J Curr Res 9:62540–62543
Klančnik A, Šimunović K, Sterniša M, Ramić D, Smole Možina S, Bucar F (2021) Anti-adhesion activity of phytochemicals to prevent campylobacter jejuni biofilm formation on abiotic surfaces. Phytochem Rev 20(1):55–84. https://doi.org/10.1007/s11101-020-09669-6
Koche D, Shirsat R, Kawale M (2018) An overerview of major classes of phytochemicals: their types and role in disease prevention. Hislopia 9:2016
Köhler T, Curty LK, Barja F, van Delden C, Pechère J-C (2000) Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol 182(21):5990–5996. https://doi.org/10.1128/JB.182.21.5990-5996.2000
Kongkham B, Prabakaran D, Puttaswamy H (2020) Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia 147:104762. https://doi.org/10.1016/J.FITOTE.2020.104762
Kontiokari T, Salo J, Eerola E, Uhari M (2005) Cranberry juice and bacterial colonization in children--a placebo-controlled randomized trial. Clin Nutr 24(6):1065–1072. https://doi.org/10.1016/j.clnu.2005.08.009
Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L (2017) Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15(12):740–755. https://doi.org/10.1038/nrmicro.2017.99
Kostakioti M, Hadjifrangiskou M, Hultgren SJ (2013) Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 3(4):a010306. https://doi.org/10.1101/cshperspect.a010306
Lade H, Paul D, Kweon JH (2014) N-acyl homoserine lactone-mediated quorum sensing with special reference to use of quorum quenching bacteria in membrane biofouling control. Biomed Res Int 2014:162584. https://doi.org/10.1155/2014/162584
Lahlou M (2013) The success of natural products in drug discovery. Pharmacol Pharm 04:17–31. https://doi.org/10.4236/pp.2013.43A003
Lami R (2019) In: Tommonaro GBT-QS (ed) Chapter 3: Quorum sensing in marine biofilms and environments. Academic Press, pp 55–96
Lebeaux D, Ghigo J-M, Beloin C (2014) Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 78(3):510–543. https://doi.org/10.1128/MMBR.00013-14
Lee J-H et al (2011) Apple flavonoid phloretin inhibits Escherichia coli O157:H7 biofilm formation and ameliorates colon inflammation in rats. Infect Immun 79(12):4819–4827. https://doi.org/10.1128/IAI.05580-11
Lee J-H, Park J-H, Cho HS, Joo SW, Cho MH, Lee J (2013) Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 29(5):491–499. https://doi.org/10.1080/08927014.2013.788692
Lee C-R et al (2017) Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front Cell Infect Microbiol 7:55. https://doi.org/10.3389/fcimb.2017.00055
Lemon KP, Higgins DE, Kolter R (2007) Flagellar motility is critical for listeria monocytogenes biofilm formation. J Bacteriol 189(12):4418–4424. https://doi.org/10.1128/JB.01967-06
Lemos M et al (2014) The effects of ferulic and salicylic acids on Bacillus cereus and Pseudomonas fluorescens single-and dual-species biofilms. Int Biodeterior Biodegradation 86:42–51. https://doi.org/10.1016/j.ibiod.2013.06.011
Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10(12):S122–S129. https://doi.org/10.1038/nm1145
Li Y-H, Tian X (2012) Quorum sensing and bacterial social interactions in biofilms. Sensors (Basel) 12(3):2519–2538. https://doi.org/10.3390/s120302519
Liaqat I et al (2018) Flagellar motility plays important role in biofilm formation of Bacillus cereus and Yersinia enterocolitica. Pak J Pharm Sci 31(5):2047–2052
Lihua L, Jianhuit W, Jialini Y, Yayin L, Guanxin L (2013) Effects of allicin on the formation of Pseudomonas aeruginosa biofinm and the production of quorum-sensing controlled virulence factors. Polish J Microbiol 62(3):243–251
Lin YT, Kwon YI, Labbe RG, Shetty K (2005) Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl Environ Microbiol 71(12):8558–8564. https://doi.org/10.1128/AEM.71.12.8558-8564.2005
Liu RH (2013) Health-promoting components of fruits and vegetables in the diet. Adv Nutr 4(3):384S–392S. https://doi.org/10.3945/an.112.003517
Liu Y, Tong Z, Shi J, Li R, Upton M, Wang Z (2021) Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics 11(10):4910–4928. https://doi.org/10.7150/thno.56205
Llor C, Bjerrum L (2014) Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 5(6):229–241. https://doi.org/10.1177/2042098614554919
López Y, Soto SM (2020) The usefulness of microalgae compounds for preventing biofilm infections. Antibiotics 9(1):9. https://doi.org/10.3390/antibiotics9010009
Maeyama R, Kwon IK, Mizunoe Y, Anderson JM, Tanaka M, Matsuda T (2005) Novel bactericidal surface: Catechin-loaded surface-erodible polymer prevents biofilm formation. J Biomed Mater Res A 75(1):146–155. https://doi.org/10.1002/jbm.a.30346
Mandal P, Sinha Babu SP, Mandal NC (2005) Antimicrobial activity of saponins from Acacia auriculiformis. Fitoterapia 76(5):462–465. https://doi.org/10.1016/j.fitote.2005.03.004
Manganaris GA, Goulas V, Mellidou I, Drogoudi P (2018) Antioxidant phytochemicals in fresh produce: exploitation of genotype variation and advancements in analytical protocols. Front Chem 5:95. https://doi.org/10.3389/fchem.2017.00095
Martínez A, Stinco CM, Meléndez-Martínez AJ (2014) Free radical scavenging properties of phytofluene and phytoene isomers as compared to lycopene: a combined experimental and theoretical study. J Phys Chem B 118(33):9819–9825. https://doi.org/10.1021/jp503227j
Mascini EM et al (2006) Genotyping and preemptive isolation to control an outbreak of vancomycin-resistant enterococcus faecium. Clin Infect Dis an Off Publ Infect Dis Soc Am 42(6):739–746. https://doi.org/10.1086/500322
Meireles A, Gonçalves AL, Gomes IB, Simões LC, Manuel S (2015) Methods to study microbial adhesion on abiotic surfaces. AIMS Bioeng 2(4):297–309. https://doi.org/10.3934/bioeng.2015.4.297
Melander R, Basak A, Melander C (2020) Natural products as inspiration for the development of bacterial antibiofilm agents. Nat Prod Rep 37:1454–1477. https://doi.org/10.1039/D0NP00022A
Melzig MF, Bader G, Loose R (2001) Investigations of the mechanism of membrane activity of selected triterpenoid saponins. Planta Med 67(1):43–48. https://doi.org/10.1055/s-2001-10632
Memariani H, Memariani M, Ghasemian A (2019) An overview on anti-biofilm properties of quercetin against bacterial pathogens. World J Microbiol Biotechnol 35(9):143. https://doi.org/10.1007/s11274-019-2719-5
Meredith HR, Srimani JK, Lee AJ, Lopatkin AJ, You L (2015) Collective antibiotic tolerance: mechanisms, dynamics and intervention. Nat Chem Biol 11(3):182–188. https://doi.org/10.1038/nchembio.1754
Micol V, Mateo CR, Shapiro S, Aranda FJ, Villalaín J (2001) Effects of (+)-totarol, a diterpenoid antibacterial agent, on phospholipid model membranes. Biochim Biophys Acta-Biomembr 1511(2):281–290. https://doi.org/10.1016/S0005-2736(01)00284-X
Micoli F, Bagnoli F, Rappuoli R, Serruto D (2021) The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol 19(5):287–302. https://doi.org/10.1038/s41579-020-00506-3
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55:165–199. https://doi.org/10.1146/annurev.micro.55.1.165
Mirzoeva OK, Grishanin RN, Calder PC (1997) Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol Res 152(3):239–246. https://doi.org/10.1016/S0944-5013(97)80034-1
Mishra R, Panda AK, De Mandal S, Shakeel M, Bisht SS, Khan J (2020) Natural anti-biofilm agents: strategies to control biofilm-forming pathogens. Front Microbiol 11:2640. https://doi.org/10.3389/fmicb.2020.566325
Monte J, Abreu AC, Borges A, Simões LC, Simões M (2014) Antimicrobial activity of selected phytochemicals against Escherichia coli and Staphylococcus aureus and their biofilms. Pathogens (Basel, Switzerland) 3(2):473–498. https://doi.org/10.3390/pathogens3020473
Mori A, Nishino C, Enoki N, Tawata S (1987) Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 26(8):2231–2234. https://doi.org/10.1016/S0031-9422(00)84689-0
Moshynets O, Spiers A (2016) Viewing biofilms within the larger context of bacterial aggregations. IntechOpen, London
Muhammad MH et al (2020) Beyond risk: bacterial biofilms and their regulating approaches. Front Microbiol 11:928. https://doi.org/10.3389/fmicb.2020.00928
Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR (2019) Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol 10:539. https://doi.org/10.3389/fmicb.2019.00539
Mundy LM, Sahm DF, Gilmore M (2000) Relationships between enterococcal virulence and antimicrobial resistance. Clin Microbiol Rev 13(4):513–522. https://doi.org/10.1128/CMR.13.4.513
Munita JM, Arias CA (2016) Mechanisms of antibiotic resistance. Microbiol Spectr 4(2):11. https://doi.org/10.1128/microbiolspec.VMBF-0016-2015
Nandakumar V, Chittaranjan S, Kurian VM, Doble M (2013) Characteristics of bacterial biofilm associated with implant material in clinical practice. Polym J 45(2):137–152. https://doi.org/10.1038/pj.2012.130
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V (2013) Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel) 6(12):1451–1474. https://doi.org/10.3390/ph6121451
Neu HC (1992) The crisis in antibiotic resistance. Science 257(5073):1064–1073. https://doi.org/10.1126/science.257.5073.1064
Nigam A, Gupta D, Sharma A (2014) Treatment of infectious disease: beyond antibiotics. Microbiol Res 169(9):643–651. https://doi.org/10.1016/j.micres.2014.02.009
Niu C, Afre S, Gilbert ES (2006) Subinhibitory concentrations of cinnamaldehyde interfere with quorum sensing. Lett Appl Microbiol 43(5):489–494. https://doi.org/10.1111/j.1472-765X.2006.02001.x
Oussalah M, Caillet S, Lacroix M (2006) Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and listeria monocytogenes. J Food Prot 69(5):1046–1055. https://doi.org/10.4315/0362-028x-69.5.1046
Packiavathy IASV, Agilandeswari P, Musthafa KS, Pandian SK, Ravi AV (2012) Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against gram negative bacterial pathogens. Food Res Int 45(1):85–92. https://doi.org/10.1016/j.foodres.2011.10.022
Packiavathy IASV, Sasikumar P, Pandian SK, Veera Ravi A (2013) Prevention of quorum-sensing-mediated biofilm development and virulence factors production in vibrio spp. by curcumin. Appl Microbiol Biotechnol 97(23):10177–10187. https://doi.org/10.1007/s00253-013-4704-5
Packiavathy IASV, Priya S, Pandian SK, Ravi AV (2014) Inhibition of biofilm development of uropathogens by curcumin–an anti-quorum sensing agent from Curcuma longa. Food Chem 148:453–460. https://doi.org/10.1016/j.foodchem.2012.08.002
Pan S-Y et al (2013) New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Based Complement Alternat Med 2013:627375. https://doi.org/10.1155/2013/627375
Patra AK (2012) An overview of antimicrobial properties of different classes of phytochemicals. Diet Phytochem Microbes 1:1–32. https://doi.org/10.1007/978-94-007-3926-0_1
Pena RT et al (2019) Relationship between quorum sensing and secretion systems. Front Microbiol 10:1100. https://doi.org/10.3389/fmicb.2019.01100
Phillips PL, Schultz GS (2012) Molecular mechanisms of biofilm infection: biofilm virulence factors. Adv Wound Care 1(3):109–114. https://doi.org/10.1089/wound.2011.0301
Pidwill GR, Gibson JF, Cole J, Renshaw SA, Foster SJ (2021) The role of macrophages in Staphylococcus aureus infection. Front Immunol 11:3506. https://doi.org/10.3389/fimmu.2020.620339
Pollitt EJG, Diggle SP (2017) Defining motility in the Staphylococci. Cell Mol Life Sci 74(16):2943–2958. https://doi.org/10.1007/s00018-017-2507-z
Potera C (2010) Antibiotic Resistance: biofilm dispersing agent rejuvenates older antibiotics. Environ Health Perspect 118(7):A288. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2920928/
Power E (2006) Impact of antibiotic restrictions: the pharmaceutical perspective. Clin Microbiol Infect 12:25–34. https://doi.org/10.1111/j.1469-0691.2006.01528.x
Prestinaci F, Pezzotti P, Pantosti A (2015) Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 109(7):309–318. https://doi.org/10.1179/2047773215Y.0000000030
Quave CL et al (2012) Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS One 7(1):e28737. https://doi.org/10.1371/journal.pone.0028737
Ray S, Das S, Suar M (2017) In: Arora G, Sajid A, Kalia VC (eds) Molecular mechanism of drug resistance BT–drug resistance in bacteria, fungi, malaria, and cancer. Springer International Publishing, Cham, pp 47–110
Roberts IS (1996) The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol 50:285–315. https://doi.org/10.1146/annurev.micro.50.1.285
Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9(1):522–554. https://doi.org/10.1080/21505594.2017.1313372
Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2(11):a012427. https://doi.org/10.1101/cshperspect.a012427
Sakarikou C, Kostoglou D, Simões M, Giaouris E (2020) Exploitation of plant extracts and phytochemicals against resistant Salmonella spp. in biofilms. Food Res Int 128:108806. https://doi.org/10.1016/j.foodres.2019.108806
Santajit S, Indrawattana N (2016) Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int 2016:2475067. https://doi.org/10.1155/2016/2475067
Saranraj P, Sivasakthi S, Deepa M (2016) Phytochemistry of pharmacologically important medicinal plants–a review. Int J Curr Res Chem Pharm Sci 3:56–66. https://doi.org/10.22192/ijcrcps.2016.03.11.009
Sarshar M, Behzadi P, Scribano D, Palamara AT, Ambrosi C (2021) Acinetobacter baumannii: an ancient commensal with weapons of a pathogen. Pathogens 10(4):387. https://doi.org/10.3390/pathogens10040387
Schmitz FJ et al (1998) The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in-vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother 42(6):807–810. https://doi.org/10.1093/jac/42.6.807
Schultz F et al (2020) Targeting ESKAPE pathogens with anti-infective medicinal plants from the greater Mpigi region in Uganda. Sci Rep 10(1):11935. https://doi.org/10.1038/s41598-020-67572-8
Sekyere JO, Asante J (2018) Emerging mechanisms of antimicrobial resistance in bacteria and fungi: advances in the era of genomics. Future Microbiol 13(2):241–262. https://doi.org/10.2217/fmb-2017-0172
Septembre-Malaterre A, Remize F, Poucheret P (2017) Fruits and vegetables, as a source of nutritional compounds and phytochemicals: changes in bioactive compounds during lactic fermentation. Food Res Int 104:86–99. https://doi.org/10.1016/j.foodres.2017.09.031
Sharma AK et al (2017) Bacterial virulence factors: secreted for survival. Indian J Microbiol 57(1):1–10. https://doi.org/10.1007/s12088-016-0625-1
Sharma D, Misba L, Khan AU (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 8(1):76. https://doi.org/10.1186/s13756-019-0533-3
Sheng G-P, Yu H-Q, Li X-Y (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol Adv 28(6):882–894. https://doi.org/10.1016/j.biotechadv.2010.08.001
Šikić Pogačar M, Klančnik A, Bucar F, Langerholc T, Smole Možina S (2016) Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene and intestine epithelial cells. J Sci Food Agric 96(8):2723–2730. https://doi.org/10.1002/jsfa.7391
Simões M, Bennett R, Rosa E (2009) Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep 26:746–757. https://doi.org/10.1039/b821648g
Singh MK, Singh SK, Singh AV, Verma H, Singh PP, Kumar A (2020) In: B. B. T.-F. and P. P. of P. Prakash (ed) 12–Phytochemicals: intellectual property rights. Academic Press, pp 363–375
Singha T (2012) Microbial extracellular polymeric substances: production, isolation and applications. IOSR J Pharm 2:276–281. https://doi.org/10.9790/3013-0220276281
Sinha Babu SP, Sarkar D, Ghosh NK, Saha A, Sukul NC, Bhattacharya S (Dec. 1997) Enhancement of membrane damage by saponins isolated from Acacia auriculiformis. Jpn J Pharmacol 75(4):451–454. https://doi.org/10.1254/jjp.75.451
Skariyachan S, Garka S (2018) In: Grumezescu Graphenes AMBT-F, Nanotubes (eds) Chapter 1: Exploring the binding potential of carbon nanotubes and fullerene towards major drug targets of multidrug resistant bacterial pathogens and their utility as novel therapeutic agents. William Andrew Publishing, pp 1–29
Skindersoe ME et al (2008) Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 52(10):3648–3663. https://doi.org/10.1128/AAC.01230-07
Smith RP, Barraza I, Quinn RJ, Fortoul MC (2020) Chapter One: The mechanisms and cell signaling pathways of programmed cell death in the bacterial world. In: Spetz JKE, L. B. T.-I. R. of C. and M. B. Galluzzi (eds) Cell death regulation in health and disease-part B, vol 352. Academic Press, pp 1–53
Spellberg B (2014) The future of antibiotics. Crit Care 18(3):228. https://doi.org/10.1186/cc13948
Srinivasan R, Santhakumari S, Poonguzhali P, Geetha M, Dyavaiah M, Xiangmin L (2021) Bacterial biofilm inhibition: a focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front Microbiol 12:1106. [Online]. Available: https://www.frontiersin.org/article/10.3389/fmicb.2021.676458
Stones DH, Krachler AM (2016) Against the tide: the role of bacterial adhesion in host colonization. Biochem Soc Trans 44(6):1571–1580. https://doi.org/10.1042/BST20160186
Suarez M et al (2005) Structure-function characterization and optimization of a plant-derived antibacterial peptide. Antimicrob Agents Chemother 49(9):3847–3857. https://doi.org/10.1128/AAC.49.9.3847-3857.2005
Sundar L, Chang FN (1992) The role of guanosine-3′,5′-bis-pyrophosphate in mediating antimicrobial activity of the antibiotic 3, 5-dihydroxy-4-ethyl-trans-stilbene. Antimicrob Agents Chemother 36(12):2645–2651. https://doi.org/10.1128/AAC.36.12.2645
Tam K, Torres VJ (2019) Staphylococcus aureus secreted toxins and extracellular enzymes. Microbiol Spectr 7(2):315. https://doi.org/10.1128/microbiolspec.GPP3-0039-2018
Tempera G, Corsello S, Genovese C, Caruso FE, Nicolosi D (2010) Inhibitory activity of cranberry extract on the bacterial adhesiveness in the urine of women: an ex-vivo study. Int J Immunopathol Pharmacol 23(2):611–618. https://doi.org/10.1177/039463201002300223
Thakur M, Singh K, Khedkar R (2020) In: B. B. T.-F. and P. P. of P. Prakash (ed) 11–Phytochemicals: extraction process, safety assessment, toxicological evaluations, and regulatory issues. Academic Press, pp 341–361
Thatoi HN, Patra JK, Das SK (2014) Free radical scavenging and antioxidant potential of mangrove plants: a review. Acta Physiol Plant 36(3):561–579. https://doi.org/10.1007/s11738-013-1438-z
Toivanen M et al (2010) Screening of binding activity of Streptococcus pneumoniae, Streptococcus agalactiae and Streptococcus suis to berries and juices. Phytother Res 24(Suppl 1):S95–S101. https://doi.org/10.1002/ptr.2939
Tomada S, Puopolo G, Perazzolli M, Musetti R, Loi N, Pertot I (2016) Pea broth enhances the biocontrol efficacy of Lysobacter capsici AZ78 by triggering cell motility associated with biogenesis of type IV pilus. Front Microbiol 7:1136. https://doi.org/10.3389/fmicb.2016.01136
Trombetta D et al (2005) Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother 49(6):2474–2478. https://doi.org/10.1128/AAC.49.6.2474-2478.2005
Ultee A, Kets EP, Smid EJ (1999) Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 65(10):4606–4610. https://doi.org/10.1128/AEM.65.10.4606-4610.1999
N. Upmanyu and V. N. Malviya, “16–Multidrug resistance in pathogenic microorganisms,” P. Chowdhary, A. Raj, D. Verma, Y. B. T.-M. for S. E. H. Akhter, Eds. Elsevier, 2020, pp. 329–341
Vattem DA, Mihalik K, Crixell SH, McLean RJC (2007) Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 78(4):302–310. https://doi.org/10.1016/j.fitote.2007.03.009
Venkatesan N, Perumal G, Doble M (2015) Bacterial resistance in biofilm-associated bacteria. Future Microbiol 10(11):1743–1750. https://doi.org/10.2217/fmb.15.69
Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. P T 40(4):277–283. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/25859123
Verderosa AD, Totsika M, Fairfull-Smith KE (2019) Bacterial biofilm eradication agents: a current review. Front Chem 7:824. https://doi.org/10.3389/fchem.2019.00824
Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS (2010) Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J Appl Microbiol 109(2):515–527. https://doi.org/10.1111/j.1365-2672.2010.04677.x
Visioli F, Borsani L, Galli C (2000) Diet and prevention of coronary heart disease: the potential role of phytochemicals. Cardiovasc Res 47(3):419–425. https://doi.org/10.1016/S0008-6363(00)00053-5
Waheed Janabi AH et al (2020) Flavonoid-rich foods (FRF): a promising nutraceutical approach against lifespan-shortening diseases. Iran J Basic Med Sci 23(2):140–153. https://doi.org/10.22038/IJBMS.2019.35125.8353
Walton EW (2014) Topical phytochemicals: applications for wound healing. Adv Skin Wound Care 27(7):324–328. https://doi.org/10.1097/01.ASW.0000450101.97743.0f
Watkins RR, David MZ, Salata RA (2012) Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus. J Med Microbiol 61(Pt 9):1179–1193. https://doi.org/10.1099/jmm.0.043513-0
C. Whitfield and M. A. Valvano, “Biosynthesis and expression of cell-surface polysaccharides in gram-negative bacteria,” vol. 35, A. H. B. T.-A. in M. P. Rose, Ed. Academic Press, 1993, pp. 135–246
Wintola OA, Afolayan AJ (2015) The antibacterial, phytochemicals and antioxidants evaluation of the root extracts of Hydnora africanaThunb. Used as antidysenteric in Eastern Cape Province, South Africa. BMC Complement Altern Med 15(1):307. https://doi.org/10.1186/s12906-015-0835-9
Wu H, Moser C, Wang H-Z, Høiby N, Song Z-J (2015) Strategies for combating bacterial biofilm infections. Int J Oral Sci 7(1):1–7. https://doi.org/10.1038/ijos.2014.65
Xu T et al (2014) Mutations of flagellar genes fli C12, fli A and flh DC of Edwardsiella tarda attenuated bacterial motility, biofilm formation and virulence to fish. J Appl Microbiol 116(2):236–244. https://doi.org/10.1111/jam.12357
Yan X, Gu S, Shi Y, Cui X, Wen S, Ge J (2017) The effect of emodin on Staphylococcus aureus strains in planktonic form and biofilm formation in vitro. Arch Microbiol 199(9):1267–1275. https://doi.org/10.1007/s00203-017-1396-8
Yang R et al (2018) Phytochemicals from Camellia nitidissima chi flowers reduce the Pyocyanin production and motility of Pseudomonas aeruginosa PAO1. Front Microbiol 8:2640. https://doi.org/10.3389/fmicb.2017.02640
Yin W, Wang Y, Liu L, He J (2019) Biofilms: the microbial ‘protective clothing’ in extreme environments. Int J Mol Sci 20(14):3423. https://doi.org/10.3390/ijms20143423
Yoo S, Kim K, Nam H, Lee D (2018) Discovering health benefits of phytochemicals with integrated analysis of the molecular network, chemical properties and ethnopharmacological evidence. Nutrients 10(8):1042. https://doi.org/10.3390/nu10081042
Yu M, Gouvinhas I, Rocha J, Barros AIRNA (2021) Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep 11(1):10041. https://doi.org/10.1038/s41598-021-89437-4
Yuan Z et al (2020) Thymol inhibits biofilm formation, eliminates pre-existing biofilms, and enhances clearance of methicillin-resistant Staphylococcus aureus (MRSA) in a mouse peritoneal implant infection model. Microorganisms 8(1):99. https://doi.org/10.3390/microorganisms8010099
Zhang J, Rui X, Wang L, Guan Y, Sun X, Dong M (2014) Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control 42:125–131. https://doi.org/10.1016/j.foodcont.2014.02.001
Zhang Y-J et al (2015) Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 20(12):21138–21156. https://doi.org/10.3390/molecules201219753
Zhang K, Li X, Yu C, Wang Y (2020a) Promising therapeutic strategies against microbial biofilm challenges. Front Cell Infect Microbiol 10:359. https://doi.org/10.3389/fcimb.2020.00359
Zhang L et al (2020b) Is combined medication with natural medicine a promising therapy for bacterial biofilm infection? Biomed Pharmacother 128:110184. https://doi.org/10.1016/j.biopha.2020.110184
Zhao Z, Ma L (2015) In: Tang Y-W, Sussman M, Liu D, Poxton I, J. B. T.-M. M. M. Second E. Schwartzman (eds) Chapter 45: Skin and soft tissue infections: a clinical overview. Academic Press, Boston, pp 825–835
Acknowledgments
This work was financially supported by: Base Funding—UIDB/00511/2020 of the Laboratory for Process Engineering, Environment, Biotechnology and Energy—LEPABE—funded by national funds through the FCT/MCTES (PIDDAC); Projects Biocide_for_Biofilm—TDC/BII-BTI/30219/2017—POCI-01-0145-FEDER-030219; Germirrad—POCI-01-0247-FEDER-072237 funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES; Project “HealthyWaters—Identification, Elimination, Social Awareness and Education of Water Chemical and Biological Micropollutants with Health and Environmental Implications,” with reference NORTE-01-0145-FEDER-000069, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sousa, M., Gomes, I.B., Simões, L.C., Simões, M., Ribeiro, M. (2022). The Action of Phytochemicals in the Control of Pathogenic Biofilms. In: Richter, K., Kragh, K.N. (eds) Antibiofilm Strategies. Springer Series on Biofilms, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-031-10992-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-10992-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10991-1
Online ISBN: 978-3-031-10992-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)