Abstract
Metal corrosion is a major global concern in many economic sectors. The degradation of metal surfaces is responsible for losses in values that account for about 3% of gross domestic product (GDP) only in the US. Parts of all corrosion processes described in different environments are present mainly in marine environments. The marine environment is characterized as favoring the corrosion processes of several metallic alloys, damaging structures used in the construction of ships, ports, oil pipelines, and others. Despite chemical corrosion being the most frequently described in these environments, studies show the participation of microorganisms in direct corrosion processes or in the acceleration/influence of the corrosive action, through the formation of complex biofilms. These structures create favorable conditions for microorganisms to degrade metal surfaces, causing damage known as pitting and crevices. Currently, diverse technicians are employed in biocorrosion research, e.g. electronic microscopy, and DNA sequencing. These techniques have clarified the dynamic process of the formation of biofilm structures, allowing understanding of the succession of different species during the evolution of the structure. Improving the understanding of how this interaction between biofilm and metallic surface occurs will enable better evaluation of strategies to avoid or decelerate the degradation of metallic structures in marine environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, corrosion is a real concern and at an economic cost. Corrosion costs reach values of approximately $2.5 trillion dollars annually. Even so, these costs do not include expenses associated with training technicians, productivity loss due to corrosion damage, nor investment to solve and prevent damage caused by metallic structure corrosion. Such indirect costs are estimated at $522 billion (Kato et al. 2015).
Metal corrosion is a spontaneous process where the transfer of electrons from the zero-valent metal to a final acceptor occurs. This process happens when electron transfer from the electron-donating site, called the “anodic site”, is driven to another site, called the “cathodic site”. We can also consider these sites as oxidation and reduction reactions, either electron loss or gain respectively, in a coupled reaction called redox. Both reactions are mutually dependent on each other, and the transfer between both sites is always from the most negative to the most positive potential. This transfer of electrons from a negative pole to a positive pole will result in dissolution of the anodic site, or corrosion itself (Gadd 2004; Hamilton 2003; Watanabe et al. 2009).
In an environment where aerobic conditions prevail, oxygen plays the role of the main final electron acceptor. In this reaction, quite common to our eyes, the formation of hydroxides and iron oxide deposits (rust), are clearly visible. Further, in aqueous environments, such as a marine environment, characteristic rust formation occurs. Over time, the (hydro-) kinetic formation of oxides decreases due to the accumulation of the reaction products, which, when adhering to metal surfaces, creates an anaerobic microenvironment, making it impossible to act as an oxygen electron acceptor (Hamilton 2003). However, under anaerobic conditions, corrosion may also occur in spite of slower kinetics, although corrosion in anoxic environments is costlier from an economic viewpoint. This occurs due to the fact that structural damage induced by anaerobic corrosion is more severe. In addition, there are more mechanistic means in anaerobic corrosion processes (Beese et al. 2013). The most important of these can be found in four different groups: (1) cathodic depolarization, (2) sulphide attack, (3) direct electron capture by microorganisms, and (4) the impact of microorganisms on surface film mineralogy (Blackwood 2018).
Despite the fact that corrosion of metal alloys is best remembered as an electrochemical process, in the early twentieth century, a scientific work connected, for the first time, the bacterial action with metal corrosion. Nevertheless, only in the 1960s was the relationship between the presence of microorganisms and metal surface corrosion was widely accepted, by virtue of a study which detailed the role of sulfide-reducing bacteria (SRB) causing corrosion in carbon steel under anaerobic conditions (Booth and Tiller 1962; von Wolzogen and van der Vlugt 1964). In subsequent decades, despite the acceptance of the role of microorganisms in metal corrosion, studies of Microbiologically Influenced Corrosion (MIC) still progressed slowly. One explanation for this slow development was the difficulty in describing the microbial species directly degrading metal surfaces. Moreover, many of these microorganism strains are non-cultivable in laboratories.
Corrosion involves the participation of diverse microorganisms present on metal surfaces, especially bacteria. This community forms a complex structure called a biofilm, which allows the creation of favorable conditions for the redox process described above. To describe this complex microbial community, taxonomically or physiologically, has always been a challenge in the study of microorganisms-influenced corrosion. Only at the beginning of this century, with the advent of DNA sequencing techniques from environmental samples, without the need for microbial growth in a laboratory, was there considerable progress in understanding microbial corrosion or biocorrosion. Thus, advances in environmental DNA sequencing techniques, called metagenomics, combined with electrochemical and electron microscopy techniques, permitted glimpsing a new horizon in the study of the metal alloy biocorrosion. Today, these advances enable estimating how the succession of microorganisms can induce/accelerate the mechanics of corrosion on metallic surfaces (Blackwood 2018).
The relationship between the presence of microbial biofilms and the induction or acceleration of metal corrugation in marine environments has been increasingly proven by studies on the subject. Furthermore, the dynamic characteristics of the formation and maintenance of biofilm structures have been revealed as key factors in the corrosive process kinetics. This review aims to provide an overview of microbial biofilm evolution in marine environments and their direct role in metal corrosion.
Metal surface colonization in marine environments
Corrosion of metal alloys occurs because of the presence of sessile cells that adhere to the attacked surface, rather than planktonic cells near or in near-contact with the metal. Biofilm formation is a universal and widespread process present on different surfaces, inanimate or organic, and in distinct environmental conditions. The forces directing biofilm structure formation are diverse, including starvation, continuous water flow, unfavorable temperatures, protection against harmful chemical compounds, biocides and antibiotics (Salta et al. 2013). Additionally, biofilms allow the benefit of cooperation among microbial community members. However, this characteristic of biofilm, which involves several species living together, also configures a space of rivalry between them. High cellular density present in the biofilm grown on metal surfaces promotes intense competition for nutrients and other resources, besides the probable change of microenvironmental conditions, such as oxygen gradient and pH differences. These changes may direct the deterioration of resources and the predation of inserted cells in the biofilm (Serra and Hengge 2014). This dynamic within the biofilm establishes a stratified structure, where the deeper layers in contact with the surface are generally anoxic, and progressively only bacterial species capable of using the metal compounds as resources for their survival remain. The corrosive biofilm output includes organic compounds such as proteins, extracellular DNA, as well as mineral deposits with iron sulfide, ferric phosphate, ferrous carbonate and iron chloride (Videla and Herrera 2005).
Submerged surfaces, whether biotic or abiotic, in marine environments are rapidly colonized by multi-species consortia of microorganisms. This colonization is primarily followed by the rapid formation of biofilm structures, which provide numerous ecological advantages to these microorganisms, and support to biogeochemical cycles transpiring in seawater columns (Dang and Lovell 2015). However, the colonization of metallic surfaces by microorganisms also results in deteriorating processes on the metallic alloy, e.g. pitting, crevices. Metal corrosion has direct consequences for biofilm development, particularly in marine environments, generating significant impact on metal structures and extensive economic losses worldwide. Some examples are related to coastal environments, where bio-corrosive action is especially severe on carbon steel structures, usually in sheet-pile wall structures, in a process known as Accelerated Low Water Corrosion (ALWC). Studies on the biocorrosion of these structures demonstrate that corrosion kinetics is higher when compared to deep water corrosion, by as much as ten times higher, with corrosion rates that can exceed 1 mm/year, compared to other corrosion rates that are around 0.05 mm/year in non-ALWS sites (Melchers and Jeffrey 2013; Marty et al. 2014).
Biofilm origin of and their involvement in metal corrosion is always questioned. However, in general, studies show that initially unworthy specimens are responsible for initiating the surface adhesion process (Beech and Sunner 2004). These species are called pioneers and are responsible for the synthesis of the initial biofilm structure (Fig. 1a). The immature biofilm is usually composed of aerobic heterotrophic bacteria species, and include huge microbial diversity, especially involving bacterial groups of the phyla Proteobacteria and Bacteriodetes (Moura et al. 2018). Subsequently, the general hypothesis is that the aerobes use the dissolved oxygen around them, generating a chemical gradient, where the inner zone is anoxic, allowing the growth of anaerobic species (Fig. 1b) (Hamilton 2003; Richter et al. 2012). The maturation of the biofilm will depend on the surrounding environmental conditions. Temperature, salinity, pH level, availability of nutrients, exogenous inputs of new species, and more importantly the composition of the metal attacked will be determining factors in the composition of the species that will make up the mature biofilm. In a biofilm formed over iron and steel infrastructures, different microbial metabolic groups perform different actions on the metal. Fermentation processes, iron oxidation, iron reduction, and sulfate reduction are metabolic reactions performed by each microbial group that are co-aggregated in layers or sites other than metal surfaces (Fig. 1c). One of the reasons for this lies in numerous microhabitats, composed of innumerous chemical compounds and different redox potential sites in corrosive biofilms (Gu 2013).
Phases of biofilm growth on metal surface. a Planktonic cells initiate adhesion on a metal surface. b After establishment of cells on the surface, extracellular material is produced, which will compose EPS. At this stage of maturation, the corrosion process does not yet occur and the interior of the biofilm presents satisfactory levels of oxygen for the presence of aerobic bacteria. c Biofilm mature with chemical oxygen gradient, with the innermost layer in contact with the metal presenting anoxic environment, while the most superficial layer of the biofilm still presents high levels of oxygen. At this stage there is the prevalence of anaerobic bacteria related to metal corrosion processes
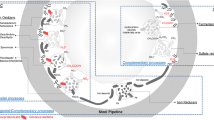
Despite an immense list of microorganisms present in corrosive biofilms, which is being constantly updated (see topic below), the classification of species into metabolic groups, such as methanogens, fermenters, acid producers, iron, manganese and sulfur reducers, can be misleading (Castelle et al. 2008; Daniels el al. 1987; De Gusseme et al. 2009). A closer look can discern that species can express different metabolites, according to what is most appropriate in a specific site or the surrounding environmental conditions. It is very common for different microbial species, and even different strains of the same species, to complement each other during biocorrosion. In actual fact, metabolic complementarity plays a significant role in corrosive biofilms. For example, the presence of fermentative bacteria and producers of syntrophic hydrogen alongside thermophilic species of the class Deltaproteobacteria is commonly described in corrosion processes in an anoxic environment (Fig. 2). These studies show that the hydrogen produced by species of the order Clostridiales is consumed by the hydrogenotrophic species of the methanogenic or sulfidogenic groups of the class Deltaproteobacteria (Iino et al. 2015; Lyles et al. 2014). Further, the presence of bacterial species with antagonistic metabolisms are also described in corrosion studies in marine environments. Potential iron-reducing strains are commonly detected alongside oxidizing iron bacteria in corrosive biofilms (Vigneron et al. 2016). In the microenvironment of the biofilm, iron-oxidant bacteria have a direct role in corrosion by means of the EMIC process (described above), whereas reducing bacteria act indirectly. This situation is possible because the corrosion products of the oxidizing iron are deposited on the surface of the metal, forming a layer of rust, which could prevent the continuity of the corrosive process. However, the specimens of iron-reducing bacteria present in the biofilms use the oxide deposits as final electron acceptors, resulting in a re-exposure of the metal surface and the continuity of the corrosion process (Videla and Herrera 2005) In this way, the highest corrosion rates are likely the consequence of complementary, antagonistic, and parallel microbial pathways between the member microorganisms within corrosive biofilms.
Microorganisms involved in marine corrosion
The role of microorganisms in deteriorating processes of metal alloys is increasingly being clarified, as demonstrated in many recent studies. Noteworthy is prokaryotes prevalence in the corrosive action of metallic surfaces, although more recently fungi have been described in metal corrosion processes. In a wide variety of environmental conditions, such as temperature variations, extreme pH levels, variations in oxygen levels, or even their complete absence, starvation situations and microbiologically competitive environments, microorganisms are able to develop biofilms, and in this way use the redox potential to attack metal surfaces. If we grouped microorganisms that oxidize/reduce iron into a single classification, even then the variability of species capable of causing corrosion would be enormous. This distribution reaches almost all bacterial groups, between Gram-negative and Gram-positive, as well as a considerable number of the Superkingdom Archaea.
The phylum of Proteobacteria is the largest group among the species of the superkingdom Bacteria. Despite the difficulty in the taxonomic classification of this complex phylum, the known classes are Alpha-, Beta-, Delta-, Epsilon-, Gamma- and more recently Zetaproteobacteria description. Proteobacteria phylum is also the most diverse group metabolically, known for their medical, agricultural and industrial importance. Its members, containing chemolithotrophic, chemoorganotrophic and phototrophic species, can produce energy differently. The diverse metabolic range within Proteobacteria allows its members to thrive in different environments, such as soil, aquatic environments, including marine, abiotic surfaces and extreme environments such as hydrothermal vents or volcanic environs. Its physiology involves aerobic and anaerobic, nitrifying, methanothophic, and oxidizing and iron-reducing, sulfur and manganese species. Usually their role in metal corrosion is associated with acid production, and iron oxidation under aerobic and anoxic conditions (Fig. 2). The Proteobacteria group that is most studied is the acid-producing species and the iron oxidants. Among the acid-producing species, Acidithiobacillus ferrooxidans, a member of the class Acidithiobacillia, was the first species described, and was recently reclassified into the phylum Proteobacteria. Acidithiobacillus spp. was previously described as Thiobacillus species, and classified as belonging to the class Gammaproteobacteria. Initially Acidithiobacillus drew attention for its biotechnological application of biomining and bioleaching (Gadd 2004). Members of this class are found as single cells or occasionally in pairs or chains, depending on growth conditions. Some species are able to fix nitrogen, but most are described as lithotrophic, using iron and molecular hydrogen as the terminal electron acceptor (Drønen et al. 2014).
Species of the Proteobacteria phylum most related to iron oxidation are included in the class Betaproteobacteria. This class is composed of lithotrophic bacteria, iron oxidants, aerobics with preferences for growth in a neutral or slightly acidic environment. The main representatives are the genres Gallionella, Leptothrix and Sideroxydans. They are bacteria of difficult laboratorial cultivation, presenting slow growth and having a preference for low oxygen levels. The species Gallionella ferruginea is found in several aquatic environments, including marine. Another species commonly associated with iron oxidation in the marine environment is the Gammaproteobacteria Mariprofundus ferrooxydans (Emerson et al. 2007). Normally found next to Gallionella species in marine environments, the Mariprofundus sp. DIS-1 strain has recently been described as associated with corrosion of carbon steel under laboratory conditions (Munford et al. 2016).
Despite being difficult to establish a clear relationship between the presence of a bacterial strain and its direct role in the corrosion of the metal surface, it is widely accepted that several members of the Deltaproteobacteria class play a direct corrosive role on metal alloys (Dinh et al. 2004). Members of this class are commonly described in corrosion studies under anaerobic conditions. The metabolic group known as SRB (described above) are composed primarily of species of this class, and the main representatives are Desulfovibrio, Desulfobacter, Desulfotignum and Desulforomonas (McBeth and Emerson 2016; Park et al. 2011; Ramirez et al. 2016; Vigneron et al. 2016; Li et al. 2017). The metabolism of SRB is essentially anaerobic, so that corrosive action on metal alloys occurs primarily through iron-reduction reactions. Damage to metal structures, pitting and crevices, is also more worrying, impacting offshore pipelines in particular.
More recently a new class has been added to the phylum Proteobacteria. The Zetaproteobacteria class was suggested in 2007 by Emerson et al. (2007). Members of this class are generally isolated in hydrothermal vents or volcanic environments (Emerson and Moyer 1997; Ruehland and Dubilier 2010). The association of strains related to metallic surface corrosion has always been underestimated. Notwithstanding, more recent studies are increasingly showing the role of several species in carbon steel corrosion (Dang et al. 2011). One of the difficulties in identifying these species previously was probably due to the fact that many species are non-cultivable in laboratories.
Species of Gram-positive bacteria have been described in biocorrosion studies in recent years. In spite of being historically unassociated with corrosive processes, metagenomic studies have described strains of this phylum present in corrosive biofilms, usually on offshore pipeline surfaces (Lenhart 2014). The main representatives are species of the phylum Firmicutes, especially the genus Bacillus, and species of the phylum Bacteriodetes. The endospore-forming genera Bacillus is the most studied among those cited. Bacillus are facultative or obligatory aerobic bacteria, widely distributed in the environment, associated with several diseases in humans. Recently, in marine environments, the species Bacillus cereus and Bacillus subtilis were detected in corrosive biofilms on oil pipeline surfaces (Guo 2017). More recently, studies of isolated strains and their corrosion capacity on different metals have been studied under laboratory conditions. The phylum Bacteroidetes includes three large bacterial groups, which have two important taxonomically associated classes of corrosion, Flavobacteriia and Sphingobacteriia. The representatives of this phylum, despite a direct redox action on metal surfaces not yet being established, are believed to play an important role in corrosive biofilm formation and maintenance in marine environments, thus acting indirectly in biocorrosion (Dang et al. 2011; Rajala et al. 2015).
Alongside Bacteria, members of the superkingdom Archaea are the most important microorganisms in the corrosion process of metallic alloys in marine environments. Group Members, described as direct or indirect participants in corrosive processes, are included in the phylum Euryarchaeota. Euryarchaeota comprise a very diverse group metabolically and morphologically, being found in extreme environments, including at deep and anoxic oceans level. Among the Euryarchaeota, the methanogenic strains are most associated with metal corrosion in seawater. Physiologically, methanogens are mandatory anaerobes, and it appears that these species and strains produce methane during the corrosion process while using CO2 as the final electron acceptor. As a result of the difficulty of culturing these microorganisms, the main approaches are metagenomic studies, identifying several associated species in carbon steel pipe wall corrosion processes. Specimens are mainly distributed among the orders Methanobacteriales, Methanococcales, Methanomicrobiales, Methanosarcinales and Methanomassiliicoccales (Vigneron et al. 2016; Zhang et al. 2003).
Techniques to study MIC
One of the major challenges in the study of microorganism corrosion has always been establishing the microorganism-metal relationship. Usually, in marine environment corrosion processes, the process takes place through the formation and maintenance of a corrosive microbial biofilm on the attacked surface. This complex structure discussed above presents an abundance of organic and inorganic compounds, and of course, living organisms that are constantly changing due to the natural microbiological succession that occurs over time or by changing environmental factors around them.
The use of electrochemical techniques is still of extreme importance in biofilm studies, although new and exciting techniques have appeared in recent years. One technique that is promising for the characterization of microorganisms adhered to metal surfaces is Atomic Force Microscopy (AFM). AFM allows quantifying the forces between the AFM tip and the sample being analyzed, as well as providing images of the interaction of the corrosive biofilm structure. Allied to AFM, the use of Atomic Force Spectroscopy (AFS) assists in the determining the physical properties of the biofilm associated with the surface or even of free cells near the surface of the analyzed material (Beech and Sunner 2004).
One of the most important compounds in the biofilm is the proteins, either as a consequence of their structural role or acting in several compounds-maintenance reactions or participating in redox processes during metal corrosion. The most powerful technique currently employed to study proteins present in biofilms is Mass Spectrometry (MS). The use of MS in biofilm study has significantly expanded the understanding of the functioning of these complex structures. MS allows large protein detection and even deals with complex mixtures. Allied to the analytical tools, the use of high-thought techniques, such as genomic, metagenomic, metatranscriptomic and metabolomic are emergent. The dawn of the genomic era began with the sequencing of Haemophilus influenza in 1995 employing the Sanger sequencing technique. Hence, numerous advances in DNA sequencing techniques have emerged, and as a result of this advance, numerous microbial genomes have been described. In addition, there was a rapid improvement in methodologies that allowed the sequencing results of whole genomes to be generated in weeks, unlike in the beginning years when they took years. Another barrier that has been overcome is cost. Currently, costs for sequencing a bacterial genome run at around $1000, well below the prohibitive values at the inception of the genomic era.
However, another challenge was faced by researchers: the overwhelming majority of microorganisms are uncultivable in the laboratory. Without growing microorganisms it was impossible to obtain sufficient genetic material for DNA sequencing. The emergence of the metagenome technique, which allowed the sequencing of DNA directly from environmental samples without the need to grow microorganisms in the laboratory, nor to have in hand quantities of genetic material, opened possibilities to taxonomically identify microbial communities present in biofilms acting in metal surface corrosion. Several studies of the composition of the bacterial community in corrosive processes in marine environments have been published (Moura et al. 2018). Metagenomics has brought a new paradigm of the participation in metal corrosion processes of members of Bacteria and Archaea in particular, thus helping in biocorrosion understanding and in proposing new means to mitigate this process.
Metagenomics answered the question of “who is corroding the metal,” but another quest arose: “how is the metal being corroded.” The answers to this second question may have come to light through metatranscriptome and metabolomic techniques. Metatranscriptome allows the sequencing of the messenger RNA (mRNA) pool present in the environmental sample. Once we know the mRNA transcription is a physiological response for the cell to live and explore the possibilities around it, the results produced by the metatranscriptome allow us to know the physiology of the biofilm community during the metal surface corrosion process. Another technique that brings information about the dynamics of the community's physiology in biofilms is metabolomics. This technique describes the metabolites produced by the cell in active metabolism, which could assist in assembling the puzzle of complex physiology at work during corrosion, including by microorganisms.
Conclusions
None of these techniques described here is sufficient to answer many biocorrosion questions. Not even the use of these tools together would suffice. For example, techniques such as proteomics and metabolomics have the limitations that many of the proteins and metabolites of microbial origin, and especially of non-cultivable microbes, have no notes yet published. Whereas the metagenomic technique, besides limiting the description of the taxonomic groups present in the sample, suffers other limitations. One of these limitations would be the fact that metagenomics produces only relative abundance counts, thus hindering quantitative insights. Biocorrosion is an immensely complex process, involving not only microbiological agents, but also environmental factors, which change throughout the process. In addition to the time factor, it is already known that, over time, succession microbiology has a considerable impact on biocorrosion.
References
Beech IB, Sunner J (2004) Biocorrosion: towards understanding interactions between biofilms and metals. Curr Opin Biotechnol 15(3):181–186. https://doi.org/10.1016/j.copbio.2004.05.001
Beese P, Venzlaff H, Srinivasan J, Garrelfs J, Stratmann M, Mayrhofer KJJ (2013) Monitoring of anaerobic microbially influenced corrosion via electrochemical frequency modulation. Electrochim Acta 105:239–247. https://doi.org/10.1016/j.electacta.2013.04.144
Blackwood DJ (2018) An electrochemist perspective of microbiologically influenced corrosion. Corros Mater Degrad 1:59–76. https://doi.org/10.3390/cmd1010005
Booth GH, Tiller AK (1962) Polarization studies of mild steel in cultures of sulphate-reducing bacteria. Part 2. Thermophilic organisms. Transactions of the Faraday Society 58:110–115. https://doi.org/10.1039/TF9625800110
Castelle C, Guiral M, Malarte G, Ledgham F, Leroy G, Brugna M, Giudici-Orticoni MT (2008) A new iron-oxidizing/O2-reducing supercomplex spanning both inner and outer membranes, isolated from the extreme acidophile Acidithiobacillus ferrooxidans. J Biol Chem 283(38):25803–25811. https://doi.org/10.1038/ismej.2010.200
Dang H, Lovell CR (2015) Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev 80(1):91–138. https://doi.org/10.1146/annurev-marine-120709-142753
Dang H, Chen R, Wang L, Shao S, Dai L, Ye Y, Guo L, Huang G, Klotz MG (2011) Molecular characterization of putative biocorroding microbiota with a novel niche detection of Epsilon- and Zetaproteobacteria in Pacific Ocean coastal seawaters. Environ Microbiol 13(11):3059–3074
Daniels L, Belay N, Rajagopal BS, Weimer PJ (1987) Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons. Science 237(4814):509–511. https://doi.org/10.1126/science.237.4814.509
De Gusseme B, De Schryver P, De Cooman M, Verbeken K, Boeckx P, Verstraete W, Boon N (2009) Nitrate-reducing, sulfide-oxidizing bacteria as microbial oxidants for rapid biological sulfide removal. FEMS Microbiol Ecol 67(1):151–161. https://doi.org/10.1111/j.1574-6941.2008.00598.x
Dinh HT, Kuever J, Mußmann M, Hassel AW, Stratmann M, Widdel F (2004) Iron corrosion by novel anaerobic microorganisms. Nature 427(6977):829–832. https://doi.org/10.1038/nature02321
Drønen K, Roalkvam I, Beeder J, Torsvik T, Steen IH, Skauge A, Liengen T (2014) Modeling of heavy nitrate corrosion in anaerobe aquifer injection water biofilm: a case study in a flow rig. Environ Sci Technol 48(15):8627–8635. https://doi.org/10.1021/es500839u
Emerson D, Moyer C (1997) Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol 63(12):4784–4792
Emerson D, Rentz JA, Lilburn TG, Davis RE, Aldrich H, Chan C, Moyer CL (2007) A novel lineage of proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS ONE 2(7):e667. https://doi.org/10.1371/journal.pone.0000667
Enning D, Venzlaff H, Garrelfs J, Dinh HT, Meyer V, Mayrhofer K, Hassel AW, Stratmann M, Widdel F (2012) Marine sulfatereducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ Microbiol 14(7):1772–1787. https://doi.org/10.1111/j.1462-2920.2012.02778.x
Gadd GM (2004) Microbial influence on metal mobility and application for bioremediation. Geoderma 122:109–119. https://doi.org/10.1016/j.geoderma.2004.01.002
Gralnick JA, Newman DK (2007) Extracellular respiration. Mol Microbiol 65(1):1–11. https://doi.org/10.1111/j.1365-2958.2007.05778.x
Gu T (2013) Theoretical modeling of the possibility of acid producing bacteria causing fast pitting biocorrosion. J Microb Biochem Technol 6:67–73. https://doi.org/10.4172/1948-5948.1000124
Guo Z, Liu T, Cheng YF, Guo N, Yin Y (2017) Adhesion of Bacillus subtilis and Pseudoalteromonas lipolytica to steel in a seawater environment and their effects on corrosion. Colloids Surf B Biointerfaces 157:157–165. https://doi.org/10.1016/j.colsurfb.2017.05.045
Hamilton WA (2003) Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling 19(1):65–76. https://doi.org/10.1080/0892701021000041078
Iino T, Ito K, Wakai S, Tsurumaru H, Ohkuma M, Harayama S (2015) Iron corrosion induced by nonhydrogenotrophic nitrate-reducing Prolixibacter sp. strain MIC1–1. Appl Environ Microbiol 81(5):1839–1846.https://doi.org/10.1128/AEM.03741-14
Kato S, Yumoto I, Kamagata Y (2015) Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl Environ Microbiol 81(1):67–73. https://doi.org/10.1128/AEM.02767-14
Lenhart TR, Duncan KE, Beech IB, Sunner JA, Smith W, Bonifay V, Biri B, Suflita JM (2014) Identification and characterization of microbial biofilm communities associated with corroded oil pipeline surfaces. Biofouling 30(7):823–835. https://doi.org/10.1080/08927014.2014.931379
Li X, Duan J, Xiao H, Li Y, Liu H, Guan F, Zhai X (2017) Analysis of bacterial community composition of corroded steel immersed in Sanya and Xiamen seawaters in China via method of Illumina MiSeq sequencing. Front Microbiol 8:1737. https://doi.org/10.3389/fmicb.2017.01737
Lyles CN, Le HM, Beasley WH, McInerney MJ, Suflita JM (2014) Anaerobic hydrocarbon and fatty acid metabolism by syntrophic bacteria and their impact on carbon steel corrosion. Front Microbiol 5:114. https://doi.org/10.3389/fmicb.2014.00114
Marty F, Gueuné H, Malard E, Sánchez-Amaya JM, Sjögren L, Abbas B, Quillet L, van Loosdrecht MC, Muyzer G (2014) Identification of key factors in accelerated low water corrosion through experimental simulation of tidal conditions: influence of stimulated indigenous microbiota. Biofouling 30(3):281–297. https://doi.org/10.1080/08927014.2013.864758
McBeth JM, Emerson D (2016) In situ microbial community succession on mild steel in estuarine and marine environments: exploring the role of iron-oxidizing bacteria. Front Microbiol 7:767. https://doi.org/10.3389/fmicb.2016.00767
Melchers RE, Jeffrey RJ (2013) Accelerated low water corrosion of steel piling in harbours. Corros Eng Sci Technol 48(7):496–505. https://doi.org/10.1179/1743278213Y.0000000103
Moura V, Ribeiro I, Moriggi P, Capão A, Salles C, Bitati S, Procópio L (2018) The influence of surface microbial diversity and succession on microbiologically influenced corrosion of steel in a simulated marine environment. Arch Microbiol 200(10):1447–1456. https://doi.org/10.1007/s00203-018-1559-2
Mumford AC, Adaktylou IJ, Emerson D (2016) Peeking under the iron curtain: development of a microcosm for imaging the colonization of steel surfaces by Mariprofundus sp. strain DIS-1, an oxygen-tolerant Fe-Oxidizing Bacterium. Appl Environ Microbiol 82(22):6799–6807. https://doi.org/10.1128/AEM.01990-16
Park HS, Chatterjee I, Dong X, Wang SH, Sensen CW, Caffrey SM, Jack TR, Boivin J, Voordouw G (2011) Effect of sodium bisulfite injection on the microbial community composition in a brackish-water-transporting pipeline. Appl Environ Microbiol. https://doi.org/10.1128/AEM.05891-11
Rajala P, Carpén L, Vepsäläinen M, Raulio M, Sohlberg E, Bomberg M (2015) Microbially induced corrosion of carbon steel in deep groundwater environment. Front Microbiol 6:647. https://doi.org/10.3389/fmicb.2015.00647
Ramírez GA, Hoffman CL, Lee MD, Lesniewski RA, Barco RA, Garber A, Toner BM, Wheat CG, Edwards KJ, Orcutt BN (2016) Assessing marine microbial induced corrosion at Santa Catalina Island California. Front Microbiol 7:1679. https://doi.org/10.3389/fmicb.2016.01679
Richter K, Schicklberger M, Gescher J (2012) Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl Environ Microbiol 78(4):913–921. https://doi.org/10.1128/AEM.06803-11
Ruehland C, Dubilier N (2010) Gamma- and epsilonproteobacterial ectosymbionts of a shallow-water marine worm are related to deep-sea hydrothermal vent ectosymbionts. Environ Microbiol 12(8):2312–2326. https://doi.org/10.1111/j.1462-2920.2010.02256.x
Salta M, Wharton JA, Blache Y, Stokes KR, Briand JF (2013) Marine biofilms on artificial surfaces: structure and dynamics. Environ Microbiol 15(11):2879–2893. https://doi.org/10.1111/1462-2920
Serra DO, Hengge R (2014) Stress responses go three dimensional—the spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ Microbiol 16(6):1455–1471. https://doi.org/10.1111/1462-2920
Videla HA, Herrera LK (2005) Microbiologically influenced corrosion: looking to the future. Int Microbiol 8(3):169–180
Vigneron A, Alsop EB, Chambers B, Lomans BP, Head IM, Tsesmetzis N (2016) Complementary microorganisms in highly corrosive biofilms from an offshore oil production facility. Appl Environ Microbiol 82(8):2545–2554. https://doi.org/10.1128/AEM.03842-15
von Wolzogen Kuhr CAH, van der Vlugt LS (1964) The graphitization of cast iron as an electrobiochemical process in anaerobic soils. Water 18(16):147–165
Watanabe K, Manefield M, Lee M, Kouzuma A (2009) Electron shuttles in biotechnology. Curr Opin Biotechnol 20(6):633–641. https://doi.org/10.1016/j.copbio.2009.09.006
Zhang T, Fang HH, Ko BC (2003) Methanogen population in a marine biofilm corrosive to mild steel. Appl Microbiol Biotechnol 63(1):101–106
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Procópio, L. The role of biofilms in the corrosion of steel in marine environments. World J Microbiol Biotechnol 35, 73 (2019). https://doi.org/10.1007/s11274-019-2647-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2647-4