Abstract
In offshore production facilities, large amounts of deaerated seawater are continuously injected to maintain pressure in oil reservoirs and equivalent volumes of fluids, composed of an oil/gas, and water mixture are produced. This process, brewing billions of liters of biphasic fluids particularly rich in microorganisms, goes through complex steel pipeline networks that are particularly prone to biofilm formation. Consequently, offshore facilities are frequently victims of severe microbiologically influenced corrosion. Understanding of microbiologically influenced corrosion is constantly growing. In the laboratory, the inventory of potentially corrosive microorganisms is increasing and microbial biochemical and bioelectrical processes are now recognized to be involved in corrosion. However, understanding of corrosive multispecies biofilms and the complex metabolic processes associated with corrosion remains a considerable challenge as simple laboratory biofilms comprising pure or defined mixed cultures poorly represent the complexity of in situ biofilms. Complementary, antagonistic, and parallel microbial pathways occur within the complex microbial and inorganic matrix of the biofilms which can lead to high corrosion rates. This mini-review explores models of microbiologically influenced corrosion and places them in the context of the multispecies biofilms observed in situ. Consequences of mitigation strategies on biofilm corrosiveness and dispersal are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbiologically influenced corrosion (MIC) of metals is a major problem for the oil and gas industry, leading to human and environmental risks as well as significant economic losses. In 2001, corrosion of steel infrastructure is estimated to cost the oil and gas industry in the range of $3 billion to $7 billion each year in maintenance, repairs, and replacement (Koch et al. 2005; Moiseeva and Kondrova 2005). In 2016, overall global cost of corrosion was estimated as $2.5 trillion (Koch et al. 2016). Considering inflation and the increasing proportion of aging infrastructure passing its design lifetime, costs of corrosion will likely continue to increase and represent a major economic loss worldwide. In most instances, corrosion is an abiotic electrochemical reaction of metal oxidation with oxygen and water. Under anoxic conditions, the only reactant available for iron oxidation is water-derived protons. The kinetics of this reaction are extremely slow; therefore, abiotic corrosion in the absence of oxygen is theoretically and technically insignificant (Cord-Ruwisch 2000). However, this contrasts with extreme corrosion observed in anoxic environments, demonstrating that biological processes play an important role in iron and steel corrosion. Indeed, microorganisms strongly influence corrosion reactions in anoxic conditions, such as in internal corrosion of buried or subsea pipelines containing water and/or oil (Enning and Garrelfs 2014; Usher et al. 2014). Rates of corrosion recorded in natural and engineered anoxic environments, and attributed to MIC range from 0.03 to 0.75 mm/yr. (Enning et al. 2012) and with pure cultures of sulfate-reducing bacteria the range is from 0.007 up to 0.9 mm/yr. (Enning et al. 2012; Enning and Garrelfs 2014), and more than 20% of pipeline corrosion can be attributed to microorganisms (Flemming 1994). Microbial catalysts modify the kinetics of corrosion reactions and can produce large quantities of biochemical reactants. Therefore, the vast pipeline networks that carry oil and gas in anoxic conditions (over more than 780,000 km in the USA), and offshore oil facilities provide vast, nutrient-rich environment for microorganisms. MIC or biocorrosion frequently forms pits in carbon steel pipelines leading in extreme cases to pipeline failure and oil spills (Borenstein and Lindsay 1994; Starosvetsky et al. 2007; Al-Jaroudi et al. 2011; Vigneron et al. 2016).
Offshore facilities are particularly vulnerable to MIC where reinjection of produced water (i.e., injected seawater and formation water) is common practice. During reinjection, nutrients and electron acceptors, primarily sulfate, from the seawater in conjunction with the dissolved hydrocarbons from the oil, provide the necessary carbon and energy source, as well as oxidants for the resident microorganisms. The oil and gas industry attempts to limit oxygen penetration into the pipelines linked to the injector wells by deaerating the seawater prior to injecting it. However, corrosive microorganisms, as well as oxidants which can sustain microbial life in anoxic environments such as sulfate and nitrate, are usually plentiful in oilfield fluids (Gittel et al. 2009; Vigneron et al. 2017).
Knowledge of MIC has largely relied on studies of pure, dual, or mixed cultures incubated with steel coupons or in flow-through systems like the Robbins device (Lee et al. 2006; Duan et al. 2008). Previous reviews have focused on analytic techniques (Beech 2004), mitigation strategies (Zuo 2007), specific processes involved in MIC like direct electron transfer (Kato 2016) or the role of iron-reducing (Herrera and Videla 2009) and sulfate-reducing microorganisms (Enning and Garrelfs 2014), since they are frequently detected as abundant members of microbial communities associated with instances of MIC in the field, and are often considered as the main culprits in MIC in offshore oil facilities. Although corrosion rates of the most corrosive pure culture of sulfate-reducing bacteria can match corrosion rates observed in the field with rates of 0.9 mm per year (Enning and Garrelfs 2014; Lahme and Hubert 2017), no pure culture can currently replicate the most severe corrosion rates (1 to 5.5 mm per year) observed with environmental biofilms (Beeder et al. 2007; Vik Vik et al. 2007; Vigneron et al. 2016), indicating that other processes play important roles (Kip and van Veen 2015). In this mini-review, we highlight the complexity of MIC processes and review the overall corrosive potential of microbial biofilms. After a short summary of perceptions of MIC in the oil and gas industry, this mini-review discusses the many facets of corrosive biofilms and the microorganisms that form them. Finally, the positive effects and potential pitfalls of mitigating strategies against corrosive biofilms are discussed.
Historic and actual perception of microbiologically influenced corrosion
Recent advances in characterization of microbial communities as well as oil industry investment in corrosion mitigation has progressively changed industry’s view of MIC. Although pioneer studies from as long ago as 1934 had already associated some microorganisms with corrosion (see review by (Iverson 2001)), our understanding of MIC is blurred by the plethora of microorganisms detected in corrosive biofilms and the large range of metabolic activities they may potentially express. Initially, cathodic depolarization of the metal surface was considered as the main process in MIC in anoxic and acid conditions (Iverson 1965; King and Miller 1971). In this model, oxidation of molecular hydrogen at the metal surface by hydrogen-scavenging microorganisms was considered to be the main driver of iron corrosion. However, the validity of this model has been widely questioned (Hamilton and Lee 1995). Previous studies highlighted that incubation of hydrogen-scavenging bacteria on steel coupons did not result in significant corrosion activity (Venzlaff et al. 2013), and on the basis of thermodynamic and kinetic considerations (production of cathodic hydrogen is slow), the cathodic hydrogen consumption model conflicted with the rapid corrosion rates observed in situ (Cord-Ruwisch 2000). Based on pure culture experimentation and molecular investigations, two important mechanisms are currently recognized as being involved in MIC:
-
1.
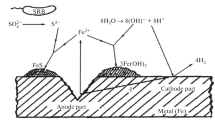
Metal can be indirectly attacked by microorganisms through the production of corrosive metabolites (Fig. 1). In this process called chemical microbiologically influenced corrosion (CMIC) or type II corrosion (Xu and Gu 2011), iron reacts with corrosive compounds such as hydrogen sulfide, generating loose deposits (iron sulfide in case of reaction with hydrogen sulfide) (Enning et al. 2012; Venzlaff et al. 2013). Sulfidogenic microorganisms such as sulfate, sulfite, thiosulfate, and sulfur reducers but also sulfur oxidizers that produce hydrogen sulfide directly as an end-product of their metabolism also can be involved in CMIC. Depending on their concentrations and operating conditions, these sulfur derivatives can be more or less potent in causing CMIC (Zhang et al. 2012). In addition to sulfidogenic microorganisms, fermentative bacteria producing hydrogen that will react with elemental sulfur leading to H2S formation can also be implicated in CMIC. Corrosion via other corrosive metabolic products (e.g., CO2, nitrite, oxidized sulfur compounds, or volatile fatty acids produced by acid-producing fermentative bacteria) are also implicated in CMIC (Fig. 1). However, typical corrosive metabolites such as H2S or FeS do not reproduce the dramatic pitting corrosion observed in situ, indicating that additional processes are involved (Cord-Ruwisch 2000; Enning and Garrelfs 2014).
-
2.
Metal can be directly attacked by specific microorganisms via direct electron uptake. In this process referred as electrical microbiologically influenced corrosion (EMIC) or type I MIC, metabolism of the bacteria is directly fueled by electrons from iron and steel oxidation (Fig. 1). This specific capacity has been observed in physiologically and phylogenetically diverse microorganisms including some Deltaproteobacteria (Desulfovibrio ferrophilus and Desulfopila corrodens (Dinh et al. 2004), Desulfovibrio vulgaris (Xu and Gu 2014)), Euryarchaeota (Methanococcus maripaludis (Uchiyama et al. 2010), and Methanobacterium sp. IM1 (Dinh et al. 2004)), Firmicutes (Bacillus licheniformis and Sporomusa sp. strain GT1) (Xu et al. 2013; Kato et al. 2015), and Bacteroidetes (Proxilibacter sp. strain MIC1-1) (Iino et al. 2015) lineages (Fig. 1). The exact process involved in electron transfer remains poorly understood (Kato 2016). Direct electrons transfer from Fe0 or electroconductive iron sulfide deposits to microorganisms has been suspected in deltaproteobacterial lineages in a process potentially involving multiheme cytochromes (Venzlaff et al. 2013; Enning and Garrelfs 2014), conductive pili (nanowires) (Sherar et al. 2011), and/or extracellular electron shuttles (e.g., riboflavin, quinone-containing molecules) (Zhang et al. 2015). Extracellular enzymes such as hydrogenases and formate-dehydrogenases were also observed as potential mediators of the reaction in Methanococcus maripaludis incubations (Deutzmann et al. 2015). Therefore, it is likely that different strategies are used by taxonomically distant microbial lineages with EMIC capacity.
Conceptual model (not to scale) of microbial communities and processes involved in microbiologically influenced corrosion of the iron and steel infrastructures. Each microbial group is characterized by their potential metabolic functions: fermentation, iron oxidation, iron reduction, sulfate reduction. EMIC, electrical microbiologically influenced corrosion; CMIC, chemical microbiologically influenced corrosion. Corrosive products and reactions are labeled in red
Analysis of corrosion products was proposed as an indicator of which process is the main culprit for corrosion (Enning and Garrelfs 2014). However, EMIC by sulfate-reducing deltaproteobacteria produce only FeS whereas EMIC by nitrate-reducing Bacteroidetes led to FePO4 and FeCO3 deposits (Iino et al. 2015). Therefore, corrosion products generated by EMIC depend on the coupled metabolism (e.g., sulfate, nitrate reduction) and the local ionic environment. Consequently, diagnosis of MIC mechanisms by analysis of loose corrosion deposits appears to be challenging, and it remains unclear what controls the balance of EMIC or CMIC as the main culprit for corrosion. CMIC greatly depends on the stochiometric reaction of organic matter/hydrocarbon degradation coupled to sulfate reduction, whereas EMIC is driven by microorganisms that can grow autotrophically with direct electron uptake as an energy source, suggesting that the predominant process might probably depend on carbon source availability. Indeed, after 7 days of organic carbon starvation, Desulfovibrio vulgaris biofilm colonizing carbon steel shifted from CMIC to EMIC metabolisms, leading to more aggressive corrosion (Xu and Gu 2014). However, these conclusions are mainly based on single species incubations in the laboratory, and it remains unclear to what extend this can be extrapolated to corrosion in the field, where numerous and diverse microbial species interact within surface biofilms.
Biofilms, the organ of microbiologically influenced corrosion
Biofilm formation is a universal and fundamental survival mechanism that provides microorganisms with critical advantages over planktonic growth, including greater access to nutrients and other resources, enhanced interaction between organisms, greater environmental stability, and protection from predators, virus, antibiotics, and other biocidal compounds (Dang and Lovell 2016). Offshore infrastructure harbors a complex network of pipes, pipelines, and other engineered structures that are often prone to biofilm formation and pitting corrosion (Duncan et al. 2017). The flow regimen of multiphase fluids greatly influences the location of the biofilm formation (Bryers and Characklis 1982) and associated corrosion risk (Nešić 2007). Low velocity flow on production lines allows sediment and bacteria to settle at the bottom of the pipe and at retention points, providing a favorable site for biofilm colonization (Vigneron et al. 2016). This can lead to a vicious circle where biofilm formation will further slow fluid flow (biofouling) and promote bacterial sedimentation (Coetser and Cloete 2005). Therefore, biofilms associated with high corrosion rates have been observed in heat affected zones of welds, pipe elbows, separators, or valves present in steel infrastructure which represent areas of locally reduced flow (Duncan et al. 2017). Additionally, the inner surfaces of storage tank are also susceptible to biofilm formation as a result of stagnant flow conditions (Duncan et al. 2017).
Corrosive biofilms generally include extensive mineral deposits (e.g., FeS, FePO4, FeCO3, FeCl2) and bacterial exopolysaccharides or other polymers (e.g., proteins, extracellular DNA) (Videla and Herrera 2005). The main role of extracellular polymeric substances (EPS) in biofilms is to enhance adhesion of the cells and to protect against environmental stresses (Sutherland 2001). However, when bound to a metallic surface, EPS has been shown to bind iron particles, stimulating interactions between microorganisms and metal ions (Beech 2004; Kumar and Mody 2009). Therefore, EPS-bound metal ions can potentially act as electron shuttles and transfer electrons to more distant microorganisms (Beech and Sunner 2004).
Numerous micro-habitats with different redox potential and chemical gradients are present within corrosive biofilms (Schwermer et al. 2008). Presence of these micro-habitats and gradients in key biogeochemical parameters (e.g., pH, electron donors and acceptors) allows the establishment of microorganisms with complementary but also antagonistic physiologies and enhances the metabolic versatility of the resident microbial community (Lewandowski 2000). Indeed, the spatial heterogeneity of biofilms contributes to the formation of optimal, suboptimal, and adverse micro-habitats for a given microorganism within the biofilm three-dimensional structure (Dang and Lovell 2016). Changes in the distribution of chemical species in a biofilm (e.g., decrease of fresh iron availability after formation of an iron sulfide layer) may also change the microenvironments and thus influence the activities of microorganisms involved in MIC (Lewandowski 2000; Keresztes et al. 2001; Little and Lee 2014).
Naturally occurring biofilms generally exhibit higher corrosiveness than biofilms of pure cultures indicating that high corrosion rates are the results of multiple processes and interactions between taxonomically and metabolically different microorganisms (Beech and Sunner 2004; Kip and van Veen 2015). Each biofilm is unique and its composition and corrosiveness can vary depending on environmental factors (e.g., temperature, salinity, biocide treatment, oil composition, nitrate, and sulfate concentration in fluids). Furthermore, molecular investigation of microbial community composition by multigenic high-throughput amplicon sequencing in corrosive biofilms indicated that most members of a corrosive biofilm can potentially have a direct or indirect corrosive effect on the steel surface (Vigneron et al. 2016).
The origin of biofilm-forming microorganisms is frequently questioned. Pioneer biofilm-forming bacteria may originate from indigenous oil reservoir microbes (especially in lower temperature reservoirs (< ca. 90 °C) or exogenous inputs (e.g., injected seawater). The frequent detection of thermophilic microorganisms in biofilms in facilities connected to high temperature oil reservoirs suggested that at least some of the corrosive microorganisms are indigenous to the oil field (Duncan et al. 2009; Davidova et al. 2012; Lenhart et al. 2014). However, distinct microbial communities have been observed in biofilms and circulating fluids, indicating selection in the biofilm community following co-aggregation of the cells (Hernández Gayosso et al. 2004; Okoro et al. 2016). Different bacterial and archaeal lineages have been identified in corrosive biofilms depending on environmental conditions (e.g., temperature, salinity, biocide treatment).
Although not exhaustive, the list of microbial lineages associated with corrosive biofilms generally include sulfate-reducing microorganisms (Enning et al. 2012; Enning and Garrelfs 2014), methanogens (Dinh et al. 2004; Davidova et al. 2012; Liang et al. 2015; Vigneron et al. 2016), iron reducers (Herrera and Videla 2009), iron oxidizers (Dinh et al. 2004), and fermenters (Gu and Galicia 2012). However, the classification of MIC-related microorganisms by overall metabolic function can be misleading. Most of these lineages are versatile and can express pathways other than those which are commonly attributed to them. For instance, numerous studies have reported methanogens in corrosive biofilms (Dinh et al. 2004; Davidova et al. 2012; Liang et al. 2015; Vigneron et al. 2016) and methane formation has been found to be correlated with metal weight loss in incubation experiments (Mand et al. 2015; Okoro et al. 2016). Methane is inert toward iron and steel; therefore, corrosiveness of methanogens must rely on processes other than methane production. It has been proposed that corrosion observed with cultures of Methanobacterium sp. IM1 and Methanococcus maripaludis resulted from coupling methanogenesis with direct iron oxidation, i.e., EMIC (Dinh et al. 2004; Kato 2016) or that extracellular hydrogenases were involved in the consumption of hydrogen generated by CO2 corrosion (Deutzmann et al. 2015). Still, various other lineages of methanogens have been found in corrosive biofilms, and it remains unclear whether the ability to directly withdraw electrons from iron and thus capacity for EMIC is widespread among other methanogenic lineages (Davidova et al. 2012; Vigneron et al. 2016). Another hypothesis that may explain the contribution of methanogens to corrosion is their capacity for sulfidogenic dissimilatory sulfur reduction in S0-rich environments (Stetter and Gaag 1983). Sulfur can be naturally present in reservoir fluids or result from oxidation of hydrogen sulfide by oxides (e.g., iron oxides) or sulfur oxidizing microorganisms present in corrosive biofilms (Schmitt 1991). Therefore, methanogens can potentially produce hydrogen sulfide within petroleum production lines. Alternatively, methanogens can contribute indirectly to MIC by through interactions with syntrophic bacteria that generate fatty acids (Lyles et al. 2014; Liang et al. 2015; Ozuolmez et al., 2015). Interestingly, this seems to be a result of CMIC where fatty acids produced by the syntrophic partner lead to corrosion and the degree of corrosion was generally greater when the syntrophic fatty acid producers were grown in the absence of methanogens and if the methanogen in syntrophic co-cultures was replaced by a sulfate-reducing bacterium, the degree of corrosion was greater than in a methanogenic co-culture (Lyles et al. 2014). Pelobacter spp., may also catalyze processes not conventionally attributed to them but which may contribute to MIC. Cultivated species of Pelobacter bacteria are non-sulfidogenic hydrogen producers able to grow by fermentation of various hydrocarbon-derived substrates (Stackebrandt et al. 1989). However, in the presence of iron oxides, sulfide and/or elemental sulfur, Pelobacter can grow by indirect reduction of iron coupled to sulfide oxidation to S0 (Lovley et al. 1995; Haveman et al. 2008), a potent corrosive metabolite (Schmitt 1991).
Microbiologically influenced corrosion: Complementary, antagonist, and parallel microbial pathways
The examples above illustrate clearly the complexity of the microbial processes potentially involved in MIC-associated biofilms since a given microbial population can express different metabolic traits depending on the physicochemical conditions at the scale of the biofilm. Microbial intra- and interspecies interactions, including cooperation and competition, shape the routes of community succession, biofilm development, and functional maturation of a corrosive biofilm, resulting ultimately in complex microbial communities with potentially high corrosion rates (above 1 mm per year) (Beeder et al. 2007; Vik et al. 2007; Vigneron et al. 2016). The highest corrosion rates are likely the consequence of complementary, antagonistic, and parallel microbial pathways within corrosive biofilms (Fig. 1).
Indeed, complementary metabolic pathways play an important role in corrosive biofilms. Fermentative bacteria and syntrophic hydrogen producers such as members of the order Clostridiales (e.g., Acetobacterium, Syntrophobacterium), the thermophilic order Thermotogales and some Deltaproteobacteria (e.g., Pelobacter sp., Desulfovibrio sp.) are frequently observed in corrosive biofilms (Moiseeva and Kondrova 2005; Uchiyama et al. 2010; Davidova et al. 2012; Cote et al. 2014; Vigneron et al. 2016). These bacteria can grow by anaerobic oxidation of volatile fatty acids, alcohols, or hydrocarbons that are abundant in produced waters from oil production facilities. Hydrogen produced during fermentation is consumed by the hydrogenotrophic members of the biofilm such as methanogens or sulfidogenic Deltaproteobacteria (Lyles et al. 2014). Therefore, fermentative and acetogenic bacteria, through the production of hydrogen but also acetate and/or lactate, can indirectly enhance iron corrosion via stimulation of growth and activity of CMIC and/or EMIC bacteria (Cord-Ruwisch 2000; Kato 2016). In addition, fermentative bacteria can contribute to CMIC by the production of weak acids such as acetate and formate that can chemically attack the iron surface (Gu and Galicia 2012). Finally, hydrogen produced during fermentation can also reduce elemental sulfur and forms large amount of corrosive hydrogen sulfide (Fig. 1).
Antagonistic and complimentary metabolisms may also be important in corrosive biofilms. For example, potential iron reducers (e.g., Pseudomonas sp., Desulfuromonadales) and iron oxidizers co-occur within corrosive biofilms (Rao et al. 2000; Vigneron et al. 2016). Iron oxidizers are directly involved in corrosion through the EMIC process whereas iron reducers play an indirect role in corrosion. Corrosion products (iron oxides and iron sulfides) precipitated after metal oxidation can form a protective layer and a diffusion barrier against corrosion, limiting the contact between metal surface and microorganisms and/or chemical reactants (Beech and Sunner 2004). However, iron reducers can use iron oxide deposits as an electron acceptor and therefore degrade the protective layer (Videla et al. 2008; Herrera and Videla 2009). Iron oxide reduction can lead to the re-exposure of the metal surface to corrosive products and microorganisms, thus enhancing MIC (Fig. 1).
In addition to complementary and antagonistic pathways, parallel pathways can also occur in corrosive biofilms. Iron, the electron donor in EMIC is not limiting in the system. Furthermore, iron sulfides in the biofilm can serve as electron conductors, distributing electrons to distal members of the biofilm (Beech 2004). Therefore, multiple EMIC pathways can occur in parallel throughout the thickness and in different microenvironments in the biofilm. The presence of microorganisms with EMIC capacity depends on electron acceptor distribution (e.g., sulfate, nitrate, CO2), and all these are likely to be present in produced fluids from offshore oil production facilities.
Dangers of microbiologically influenced corrosion mitigation and prevention—new routes for corrosion
One of the most widely used treatments to combat internal MIC of pipelines is pigging, a mechanical procedure for cleaning the interior of pipelines, combined with a non-oxidative biocide treatment such as batch injection of tetrakis(hydroxymethyl)phosphonium sulfate (THPS) or glutaraldehyde (Videla 2002; Morris and van der Kraan 2017). Biocide treatment alone appears to be less efficient since often biofilms are resistant to chemical treatments. Therefore, pigging of the pipeline removes most of the biofilms and deposits, and improves the biocide action (Enning et al. 2016). Nonetheless this practice is not without risks. Monitoring of biofilm formation on steel coupons in a simulated seawater injection system highlighted that community composition of corrosive biofilm can change after mechanical pipeline cleaning when a different biocide treatment is added (Enning et al. 2016). This strategy might lead to the selection of fast growing and biocide-resistant microorganisms, and there is no guarantee that the new microbial community will be less corrosive than the initial microbial community. Furthermore, mechanical destruction of the biofilm can potentially promote the dispersion of corrosive organisms from the biofilm to other sections of the pipeline. Dispersion is particularly exacerbated when fluid is recycled and reinjected in the pipeline network without further treatment (Duncan et al. 2017). Finally, pigging also removes the protective layer formed after iron oxides precipitation, re-exposing a fresh metal surface.
Nitrate injection in oil fields is considered as an efficient and low cost approach to limit corrosion and H2S production (souring) issues in offshore oil facilities (Lahme and Hubert 2017). The injection of nitrate provides an alternative electron acceptor and theoretically limits the growth and activity of sulfate-reducing prokaryotes by diverting and inhibiting their sulfidogenic metabolism and promoting competition for electron donors between sulfate- and nitrate-reducing bacteria (Schwermer et al. 2008). Since energy yield of nitrate reduction is greater than sulfate reduction, the nitrate-reducing bacteria should theoretically outcompete sulfate reducers until nitrate becomes limiting. Experimental and in situ monitoring of nitrate-amended oil fields have demonstrated that the treatment leads to modification of the microbial community structure and metabolic potential of the oil field microbial community with a decrease in sulfate reducer abundance and selection for Deferribacteres and Epsilonproteobacteria with the capacity for nitrate reduction (Hubert et al. 2005; Vigneron et al. 2017). However, the effect on corrosion remains unclear. Comparison of iron lost from pipes between nitrate treated and non-treated oil fields in the North Sea has shown no significant effect of nitrate treatment on corrosion (Gittel et al. 2009). By contrast, nitrate amendment appears in some case to increase corrosion (Nemati et al. 2001; Beeder et al. 2007; Vik et al. 2007). Isolation of iron-oxidizing nitrate-reducing bacteria from the phylum Firmicutes, from an oil well, also suggest that there is potential for nitrate injection to lead to biocorrosion via the selection of specific bacteria with EMIC capacity (Xu et al. 2013; Iino et al. 2015). Furthermore, nitrite, the product of nitrate reduction can be corrosive at low concentrations (Kielemoes et al. 2000). Nitrate injection can also stimulate sulfate reducers with metabolic capacity for nitrate reduction, such as Desulfovibrio species (Marietou 2016; Vigneron et al. 2017). However, upon nitrate depletion these versatile microorganisms will shift their activity toward sulfate reduction potentially leading to re-emergence of damaging hydrogen sulfide production. Nitrate injection has also been shown to select for microorganisms coupling sulfur-oxidation to nitrate reduction such as some members of the Epsilonproteobacteria (Callbeck et al. 2011; Gittel et al. 2012; Vigneron et al. 2017). The metabolic activity of these bacteria leads to the formation of intermediate oxidation state sulfur species (e.g., S0, thiosulfate, polysulfides) that can be highly corrosive (Lahme and Hubert 2017). These bacteria were almost undetectable before nitrate injection (Gittel et al. 2012; Vigneron et al. 2017), suggesting nitrate injections can open new routes for MIC.
Perspectives
Despite recent advance in our understanding of MIC, numerous aspects of the phenomenon remain to be explored. A major difficulty for MIC investigation is access to representative samples such as fresh biofilms from corroded pipelines (Lenhart et al. 2014). Conversely, information from laboratory experiments is not easily extrapolated to in situ conditions, studies of pure, dual, or mixed cultures have revealed the large taxonomic range of microorganisms with EMIC and CMIC capacities. However, it is likely that the diversity of microorganisms capable of EMIC is much larger in situ and that new EMIC lineages will be isolated in the future. Identification of enzymatic and genetic markers that can be directly correlated with MIC is a key challenge for MIC studies. Finally, the complexity of MIC which couples physical, electrochemical, and microbiological processes and is influenced by material properties, suggests that MIC would be an excellent candidate for microfluidic, lab-on-a-chip based investigations. Real-time corrosion rate could be estimated through measurement of electrochemical impedance and the electric current between metal electrodes (Kotu et al. 2016), while biofilm function could be observed in a flow-controlled microfluidic device. This would allow high-throughput screening of biocides and coatings to develop better, more effective corrosion control strategies.
References
Al-Jaroudi SS, Ul-Hamid A, Al-Gahtani MM (2011) Failure of crude oil pipeline due to microbiologically induced corrosion. Corros Eng Sci Technol 46(4):568–579. https://doi.org/10.1179/147842210X12695149033819
Beech IB (2004) Corrosion of technical materials in the presence of biofilms—current understanding and state-of-the art methods of study. Int Biodeterior Biodegrad 53(3):177–183. https://doi.org/10.1016/S0964-8305(03)00092-1
Beech IB, Sunner J (2004) Biocorrosion: towards understanding interactions between biofilms and metals. Curr Opin Biotechnol 15(3):181–186. https://doi.org/10.1016/j.copbio.2004.05.001
Beeder J, Andersen TR, Liengen T, Drønen K, Torsvik T (2007) Corrosion as a side effect during nitrate treatment of produced water and aquifer water injection. In: NACE-07512. NACE International, NACE. https://www.onepetro.org/conference-paper/NACE-07512
Borenstein SW, Lindsay PB (1994) Mic failure analysis. Mater Perform U S 33:4
Bryers JD, Characklis WG (1982) Processes governing primary biofilm formation. Biotechnol Bioeng 24(11):2451–2476. https://doi.org/10.1002/bit.260241111
Callbeck CM, Dong X, Chatterjee I, Agrawal A, Caffrey SM, Sensen CW, Voordouw G (2011) Microbial community succession in a bioreactor modeling a souring low-temperature oil reservoir subjected to nitrate injection. Appl Microbiol Biotechnol 91(3):799–810. https://doi.org/10.1007/s00253-011-3287-2
Coetser SE, Cloete TE (2005) Biofouling and biocorrosion in industrial water systems. Crit Rev Microbiol 31(4):213–232. https://doi.org/10.1080/10408410500304074
Cord-Ruwisch R (2000) Microbially influenced corrosion of steel. In: Environmental microbe-metal interactions. American Society of Microbiology (7):159–173. https://doi.org/10.1128/9781555818098.ch7
Cote C, Rosas O, Sztyler M, Doma J, Beech I, Basseguy R (2014) Corrosion of low carbon steel by microorganisms from the “pigging” operation debris in water injection pipelines. Biocorrosion 97:97–109
Dang H, Lovell CR (2016) Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev 80(1):91–138. https://doi.org/10.1128/MMBR.00037-15
Davidova IA, Duncan KE, Perez-Ibarra BM, Suflita JM (2012) Involvement of thermophilic archaea in the biocorrosion of oil pipelines. Environ Microbiol 14(7):1762–1771. https://doi.org/10.1111/j.1462-2920.2012.02721.x
Deutzmann JS, Sahin M, Spormann AM (2015) Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. MBio 6
Dinh HT, Kuever J, Muszmann M, Hassel AW, Stratmann M, Widdel F (2004) Iron corrosion by novel anaerobic microorganisms. Nature 427(6977):829–832. https://doi.org/10.1038/nature02321
Duan J, Wu S, Zhang X, Huang G, Du M, Hou B (2008) Corrosion of carbon steel influenced by anaerobic biofilm in natural seawater. Electrochim Acta 54(1):22–28. https://doi.org/10.1016/j.electracta.2008.04.085
Duncan KE, Gieg LM, Parisi VA, Tanner RS, Tringe SG, Bristow J, Suflita JM (2009) Biocorrosive thermophilic microbial communities in Alaskan North Slope oil facilities. Environ Sci Technol 43(20):7977–7984. https://doi.org/10.1021/es9013932
Duncan KE, Davidova IA, Nunn HS, Stamps BW, Stevenson BS, Souquet PJ, Suflita JM (2017) Design features of offshore oil production platforms influence their susceptibility to biocorrosion. Appl Microbiol Biotechnol 101(16):6517–6529. https://doi.org/10.1007/s00253-017-8356-8
Enning D, Garrelfs J (2014) Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microbiol 80(4):1226–1236. https://doi.org/10.1128/AEM.02848-13
Enning D, Venzlaff H, Garrelfs J, Dinh HT, Meyer V, Mayrhofer K, Hassel AW, Stratmann M, Widdel F (2012) Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ Microbiol 14(7):1772–1787. https://doi.org/10.1111/j.1462-2920.2012.02778.x
Enning D, Smith R, Stolle J (2016) Evaluating the efficacy of weekly THPS and glutaraldehyde batch treatment to control severe microbial corrosion in a simulated seawater injection system. In: NACE-2016-7322. NACE international, NACE. https://www.onepetro.org/conference-paper/NACE-2016-7322
Flemming H-C (1994) Microbial deterioration of materials: fundamentals, economical and technical overview. Mater Corros 45(1):5–9. https://doi.org/10.1002/maco.19940450105
Gittel A, Sørensen KB, Skovhus TL, Ingvorsen K, Schramm A (2009) Prokaryotic community structure and sulfate reducer activity in water from high-temperature oil reservoirs with and without nitrate treatment. Appl Environ Microbiol 75(22):7086–7096. https://doi.org/10.1128/AEM.01123-09
Gittel A, Kofoed MVW, Sørensen KB, Ingvorsen K, Schramm A (2012) Succession of Deferribacteres and Epsilonproteobacteria through a nitrate-treated high-temperature oil production facility. Syst Appl Microbiol 35(3):165–174. https://doi.org/10.1016/j.syapm.2012.01.003
Gu T, Galicia B (2012) Can acid producing bacteria be responsible for very fast MIC pitting? In: NACE-2012-1214. NACE International, NACE. https://www.onepetro.org/conference-paper/NACE-2012-1214
Hamilton WA, Lee W (1995) Biocorrosion. In: Barton LL (ed) Sulfate-reducing bacteria. Springer US, Boston, MA, pp 243–264. https://doi.org/10.1007/978-1-4899-1582-5_9
Haveman SA, DiDonato RJ, Villanueva L, Shelobolina ES, Postier BL, Xu B, Liu A, Lovley DR (2008) Genome-wide gene expression patterns and growth requirements suggest that Pelobacter carbinolicus reduces Fe(III) indirectly via sulfide production. Appl Environ Microbiol 74(14):4277–4284. https://doi.org/10.1128/AEM.02901-07
Hernández Gayosso M, Zavala Olivares G, Ruiz Ordaz N, Juárez Ramirez C, Garcia Esquivel R, Padilla Viveros A (2004) Microbial consortium influence upon steel corrosion rate, using polarisation resistance and electrochemical noise techniques. Electrochim Acta 49:4295–4301
Herrera LK, Videla HA (2009) Role of iron-reducing bacteria in corrosion and protection of carbon steel. 14th Int Biodeterior Biodegrad 63(7):891–895. https://doi.org/10.1016/j.ibiod.2009.06.003
Hubert C, Nemati M, Jenneman G, Voordouw G (2005) Corrosion risk associated with microbial souring control using nitrate or nitrite. Appl Microbiol Biotechnol 68(2):272–282. https://doi.org/10.1007/s00253-005-1897-2
Iino T, Ito K, Wakai S, Tsurumaru H, Ohkuma M, Harayama S (2015) Iron corrosion induced by nonhydrogenotrophic nitrate-reducing Prolixibacter sp. strain MIC1-1. Appl Environ Microbiol 81(5):1839–1846. https://doi.org/10.1128/AEM.03741-14
Iverson WP (1965) Direct evidence for the cathodic depolarization theory of bacterial corrosion. Science 151(3713):986–8. https://doi.org/10.1126/science.151.3713.986
Iverson WP (2001) Research on the mechanisms of anaerobic corrosion. Int Biodeter & Biodegr 47(2):63–70. https://doi.org/10.1016/S0964-8305(00)00111-6
Kato S (2016) Microbial extracellular electron transfer and its relevance to iron corrosion. Microb Biotechnol 9(2):141–148. https://doi.org/10.1111/1751-7915.12340
Kato S, Yumoto I, Kamagata Y (2015) Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl Environ Microbiol 81(1):67–73. https://doi.org/10.1128/AEM.02767-14
Keresztes Z, Felhősi I, Kálmán E (2001) Role of redox properties of biofilms in corrosion processes. Electrochim Acta 46(24-25):3841–3849. https://doi.org/10.1016/S0013-4686(01)00671-5
Kielemoes J, De Boever P, Verstraete W (2000) Influence of denitrification on the corrosion of iron and stainless steel powder. Environ Sci Technol 34(4):663–671. https://doi.org/10.1021/es9902930
King RA, Miller JDA (1971) Corrosion by the sulphate-reducing bacteria. Nature 233(5320):491–492. https://doi.org/10.1038/233491a0
Kip N, van Veen JA (2015) The dual role of microbes in corrosion. ISME J 9(3):542–551. https://doi.org/10.1038/ismej.2014.169
Koch GH, Brongers MP, Thomson N, Virmanio Y, Payer JH (2005) Cost of corrosion in the United States. Handb Environ Degrad Mater 1:3–24. https://doi.org/10.1016/B978-081551500-5.50003-3
Koch GH, Varney J, Thompson NO, Moghissi O, Gould M, Payer JH (2016) NACE International IMPACT report 2016. https://impact.nace.org
Kotu SP, Erbay C, Sobahi N, Han A, Mannan S, Jayaraman A (2016) Integration of electrochemical impedance spectroscopy and microfluidics for investigating microbially influenced corrosion using co-culture biofilms. In: NACE-2016-7793. NACE international, NACE. https://www.onepetro.org/conference-paper/NACE-2016-7793
Kumar AS, Mody K (2009) Microbial exopolysaccharides: variety and potential applications. Microb Prod Biopolym Polym Precursors Appl Perspect 10:229–253
Lahme S, Hubert C (2017) Corrosion risks associated with (bio) chemical processes in sour systems due to nitrate injection or oxygen ingress. In: Microbiologically influenced corrosion in the upstream oil and gas industry. Routledge, pp 87–110. https://doi.org/10.1201/9781315157818-6
Lee AK, Buehler MG, Newman DK (2006) Influence of a dual-species biofilm on the corrosion of mild steel. Corros Sci 48(1):165–178. https://doi.org/10.1016/j.corsci.2004.11.013
Lenhart TR, Duncan KE, Beech IB, Sunner JA, Smith W, Bonifay V, Biri B, Suflita JM (2014) Identification and characterization of microbial biofilm communities associated with corroded oil pipeline surfaces. Biofouling 30(7):823–835. https://doi.org/10.1080/08927014.2014.931379
Lewandowski Z (2000) MIC and biofilm heterogeneity. Proc Corros 400:1–7
Liang B, Wang L-Y, Mbadinga SM, Liu J-F, Yang S-Z, Gu J-D, Mu B-Z (2015) Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation. AMB Express 5(1):37. https://doi.org/10.1186/s13568-015-0117-4
Little BJ, Lee JS (2014) Microbiologically influenced corrosion: an update. Int Mater Rev 59(7):384–393. https://doi.org/10.1179/1743280414Y.0000000035
Lovley DR, Phillips EJ, Lonergan DJ, Widman PK (1995) Fe(III) and S0 reduction by Pelobacter carbinolicus. Appl Environ Microbiol 61(6):2132–2138
Lyles CN, Le HM, Beasley WH, McInerney MJ, Suflita JM (2014) Anaerobic hydrocarbon and fatty acid metabolism by syntrophic bacteria and their impact on carbon steel corrosion. Front Microbiol 5:114
Mand J, Park HS, Okoro C, Lomans BP, Smith S, Chiejina L, Voordouw G (2015) Microbial methane production associated with carbon steel corrosion in a Nigerian oil field. Front Microbiol 6:1538
Marietou A (2016) Nitrate reduction in sulfate-reducing bacteria. FEMS Microbiol Lett 363:fnw155
Moiseeva LS, Kondrova OV (2005) Biocorrosion of oil and gas field equipment and chemical methods for its suppression. I. Prot Met 41(4):385–393. https://doi.org/10.1007/s11124-005-0054-8
Morris BE, van der Kraan GM (2017) Application of biocides and chemical treatments to both combat microorganisms and reduce (bio) corrosion. Microbiol Influ Corros Upstream Oil Gas Ind 11:229-38. https://doi.org/10.1201/9781315157818-12
Nemati M, Jenneman GE, Voordouw G (2001) Impact of nitrate-mediated microbial control of souring in oil reservoirs on the extent of corrosion. Biotechnol Prog 17(5):852–859. https://doi.org/10.1021/bp010084v
Nešić S (2007) Key issues related to modelling of internal corrosion of oil and gas pipelines – a review. Corros Sci 49(12):4308–4338. https://doi.org/10.1016/j.corsci.2007.06.006
Okoro CC, Samuel O, Lin J (2016) The effects of Tetrakis-hydroxymethyl phosphonium sulfate (THPS), nitrite and sodium chloride on methanogenesis and corrosion rates by methanogen populations of corroded pipelines. Corros Sci 112:507–516
Ozuolmez D, Na H, Lever MA, Kjeldsen KU, Jørgensen BB, Plugge CM (2015) Methanogenic archaea and sulfate reducing bacteria co-cultured on acetate: teamwork or coexistence? Front Microbiol 6:492
Rao TS, Sairam TN, Viswanathan B, Nair KVK (2000) Carbon steel corrosion by iron oxidising and sulphate reducing bacteria in a freshwater cooling system. Corros Sci 42(8):1417–1431. https://doi.org/10.1016/S0010-938X(99)00141-9
Schmitt G (1991) Effect of elemental sulfur on corrosion in sour gas systems. Corrosion 47(4):285–308. https://doi.org/10.5006/1.3585257
Schwermer CU, Lavik G, Abed RMM, Dunsmore B, Ferdelman TG, Stoodley P, Gieseke A, de Beer D (2008) Impact of nitrate on the structure and function of bacterial biofilm communities in pipelines used for injection of seawater into oil fields. Appl Environ Microbiol 74(9):2841–2851. https://doi.org/10.1128/AEM.02027-07
Sherar BWA, Power IM, Keech PG, Mitlin S, Southam G, Shoesmith DW (2011) Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corros Sci 53:955–960
Stackebrandt E, Wehmeyer U, Schink B (1989) The phylogenetic status of Pelobacter acidigallici, Pelobacter venetianus, and Pelobacter carbinolicus. Syst Appl Microbiol 11(3):257–260. https://doi.org/10.1016/S0723-2020(89)80022-0
Starosvetsky J, Starosvetsky D, Armon R (2007) Identification of microbiologically influenced corrosion (MIC) in industrial equipment failures. Pap Present Second Int Conf Eng Fail Anal Tor Can 14:1500–1511 12–15 Sept. 2006 Part II
Stetter KO, Gaag G (1983) Reduction of molecular sulphur by methanogenic bacteria. Nature 305(5932):309–311. https://doi.org/10.1038/305309a0
Sutherland IW (2001) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147(1):3–9. https://doi.org/10.1099/00221287-147-1-3
Uchiyama T, Ito K, Mori K, Tsurumaru H, Harayama S (2010) Iron-corroding methanogen isolated from a crude-oil storage tank. Appl Environ Microbiol 76(6):1783–1788. https://doi.org/10.1128/AEM.00668-09
Usher KM, Kaksonen AH, Cole I, Marney D (2014) Critical review: microbially influenced corrosion of buried carbon steel pipes. Int Biodeterior Biodegrad 93:84–106. https://doi.org/10.1016/j.ibiod.2014.05.007
Venzlaff H, Enning D, Srinivasan J, Mayrhofer KJJ, Hassel AW, Widdel F, Stratmann M (2013) Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros Sci 66:88–96. https://doi.org/10.1016/j.corsci.2012.09.006
Videla HA (2002) Prevention and control of biocorrosion. Biodeterior Constr Mater 49:259–270
Videla HA, Herrera LK (2005) Microbiologically influenced corrosion: looking to the future. Int Microbiol Off J Span Soc Microbiol 8:169–180
Videla HA, Le Borgne S, Panter C, Singh Raman RK (2008) Mic of steels by iron reducing bacteria. In: NACE-08505. NACE international, NACE. https://www.onepetro.org/conference-paper/NACE-08505
Vigneron A, Alsop EB, Chambers B, Lomans BP, Head IM, Tsesmetzis N (2016) Complementary microorganisms in highly corrosive biofilms from an offshore oil production facility. Appl Environ Microbiol 82(8):2545–2554. https://doi.org/10.1128/AEM.03842-15
Vigneron A, Alsop EB, Lomans BP, Kyrpides NC, Head IM, Tsesmetzis N (2017) Succession in the petroleum reservoir microbiome through an oil field production lifecycle. Isme J 11:2141
Vik EA, Janbu AO, Garshol FK, Henninge LB, Engebretsen S, Kuijvenhoven C, Oilphant D, Hendriks WP (2007) Nitrate based souring mitigation of produced water - side effects and challenges from the Draugen produced water re-injection pilot. In: SPE-106178-MS. Society of Petroleum Engineers, SPE. https://www.onepetro.org/conference-paper/SPE-106178-MS
Xu D, Gu T (2011) Bioenergetics explains when and why more severe MIC pitting by SRB can occur. In: NACE-2011-11426. NACE International, NACE.
Xu D, Gu T (2014) Carbon source starvation triggered more aggressive corrosion against carbon steel by the Desulfovibrio vulgaris biofilm. Int Biodeterior Biodegrad 91:74–81. https://doi.org/10.1016/j.ibiod.2014.03.014
Xu D, Li Y, Song F, Gu T (2013) Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros Sci 77:385–390. https://doi.org/10.1016/j.corsci.2013.07.044
Zhang L, Wang X, Wen Z, Liu Z, Li X, Lu M (2012) Interactive effects of H2S and elemental sulfur on corrosion of steel. In: NACE-2012-1575. NACE International, NACE. https://www.onepetro.org/conference-paper/NACE-2012-1575
Zhang P, Xu D, Li Y, Yang K, Gu T (2015) Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 101:14–21. https://doi.org/10.1016/j.bioelechem.2014.06.010
Zuo R (2007) Biofilms: strategies for metal corrosion inhibition employing microorganisms. Appl Microbiol Biotechnol 76(6):1245–1253. https://doi.org/10.1007/s00253-007-1130-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Vigneron, A., Head, I.M. & Tsesmetzis, N. Damage to offshore production facilities by corrosive microbial biofilms. Appl Microbiol Biotechnol 102, 2525–2533 (2018). https://doi.org/10.1007/s00253-018-8808-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8808-9