Abstract
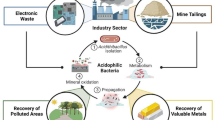
Biomining is an applied biotechnology for mineral processing and metal extraction from ores and concentrates. This alternative technology for recovering metals involves the hydrometallurgical processes known as bioleaching and biooxidation where the metal is directly solubilized or released from the matrix for further solubilization, respectively. Several commercial applications of biomining can be found around the world to recover mainly copper and gold but also other metals; most of them are operating at temperatures below 40–50 °C using mesophilic and moderate thermophilic microorganisms. Although biomining offers an economically viable and cleaner option, its share of the world´s production of metals has not grown as much as it was expected, mainly considering that due to environmental restrictions in many countries smelting and roasting technologies are being eliminated. The slow rate of biomining processes is for sure the main reason of their poor implementation. In this scenario the use of thermophiles could be advantageous because higher operational temperature would increase the rate of the process and in addition it would eliminate the energy input for cooling the system (bioleaching reactions are exothermic causing a serious temperature increase in bioreactors and inside heaps that adversely affects most of the mesophilic microorganisms) and it would decrease the passivation of mineral surfaces. In the last few years many thermophilic bacteria and archaea have been isolated, characterized, and even used for extracting metals. This paper reviews the current status of biomining using thermophiles, describes the main characteristics of thermophilic biominers and discusses the future for this biotechnology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals are useful for several and different purposes; they have industrial applications and also they are present in multiples devices in the daily life. Due to the increase in population and in the diversification of the applications of metals, their consumption has dramatically increased in recent decades; for example in the case of copper, even when it has been partially displaced for new engineering materials in some applications, the current production is 40-fold higher than it was a century ago although the world´s population only increased from 1 to about 7 billion in the same period of time. Most metals occurring as sulfides are concentrated through flotation process and finally recovered by pyrometallurgical extraction. Roasting of metal sulfides consumes great amount of energy and in addition releases polluting gases such as sulfur dioxide and eventually carbon dioxide contributing to two serious environmental problems: acid rain and greenhouse effect, respectively. Although these gases can be retained and even re-utilized, only a few modern plants around the world have incorporated such modifications. Low-grade ores (up to a cutoff typical for each metal) can not be cost-effectively concentrated by flotation and in these cases acidic solutions (very useful for leaching oxide ores) or oxidizing agent must be utilized. Depending of several factors such as market price, grade of the ore, mineralogical species and metal, hydrometallurgical extraction methods might be neither profitable nor suitable. Nowadays it is possible to enhance the efficiency of chemical leaching by microbial catalysis. These biohydrometallurgical processes (included in biomining processes) use direct and indirect microbial actions to recover metals from minerals. Most of the biomining microorganisms belong to the domains Bacteria or Archaea and present similar metabolic characteristics: iron and/or sulfur oxidizing activity, acidophilia, and in many case authotrophy (Schippers 2007). Biohydrometallurgy includes two different oxidative processes, bioleaching and biooxidation, although the microbial action in both cases is the same. During a bioleaching process the valuable metal is solubilized while in a biooxidation process it remains in the solid phase. Metal sulfides, especially those of zinc, nickel, and cobalt, are submitted to bioleaching processes for metal recovery. Biooxidation is an alternative to other more polluting technologies (roasting, pressure oxidation) for pretreating refractory ores prior the cyanidation. These pre-treatments allow gold exposure, which can not be oxidized by traditional biomining microorganisms and occurs as microparticles within a sulfidic matrix in those ores (Rawlings et al. 2003; Schippers et al. 2014; Dold 2014; Johnson 2014).
Mechanisms of bioleaching/biooxidation
The overall process involved in the bioleaching of mineral sulfides implies their oxidation to metal ions and sulfate in a reaction catalyzed by microorganisms. Usually this process occurs under aerobic conditions where oxygen is the terminal acceptor for electrons. It can be represented by:
This reaction is catalyzed by acidophilic microorganisms capable of oxidizing iron(II) and/or sulfur-compounds according to the following equations
Sand et al. (1995) proposed that during bioleaching processes iron(III) and protons are the only agents dissolving the metal sulfides. In these currently accepted mechanism microorganisms only have the role of regenerating iron(III) and/or protons.
The oxidative dissolution for sulfides non soluble in acid (such as pyrite, molybdenite, and tugstenite) proceeds through several steps (with iron(III) as oxidizing agent) where thiosulfate is the main intermediate released to the solution and in turn can be biotic or abiotically oxidized to sulfate. These processes constitute the thiosulfate mechanism (Schippers and Sand 1999) which is represented in the following equations for the case of pyrite
On the other hand, sulfides which are partially soluble in non oxidizing acid, can be oxidized by iron(III) (or even protons) in several steps that can be simplified as:
The products sulfur and iron(II) can suffer a further microbial oxidation. As polysulfide is the main intermediate species, this mechanism is known as polysulfide pathway (Schippers and Sand 1999). In this case, not only iron- but also sulfur-oxidizing microorganisms can catalyze the dissolution of such sulfides while in the first case, only iron oxidizers are suitable. More details on both mechanisms can be found in the literature (Gehrke et at. 1998; Sand and Gehrke 2006; Donati and Sand 2007; Vera et al. 2013).
From a physical point of view, contact and non-contact mechanisms can be distinguished (Sand et al. 2001; Tributsch 2001). In the first case, cells are attached to the surface of the minera through different interactions and most of them can grow and generate biofilms. Biofilm formation comprises successive phases where the irreversible attachment of cells is the next crucial step. Exopolymers (EPS) excreted by microorganisms and also some inorganic precipitates (such as jarosite—MFe3(SO4)2(OH)6—among others) form matrices where the cells are entrapped constituting the biofilms on the mineral surfaces. In the contact mode, all the processes ocurr within the microenvironment of the biofilm; in the non-contact mode cells are not in direct contact with the surfaces and the biooxidation processes take place mainly in the solution while the chemical dissolution of sulfides occur on the surface.
Commercial applications
Commercial applications can be classified in two main engineering designs; in one of them the ore is disposed in an irrigated heap or dump while in the other the ore is feed into a stirred–tank reactor (Olson et al. 2003; Rawlings et al. 2003; Watling 2006; Orell et al. 2010; Jerez 2011; Brierley and Brierley 2013; Gentina and Acevedo 2013; Schippers et al. 2013; Johnson 2014). In the first case the process is relatively inexpensive and practically does not need to be monitored; however it takes several months or years and it is usually applied only for metals with low-market value. It is typically used for leaching low-grade copper ores although it has been also used for recovering cobalt, nickel, uranium, gold, and zinc at different scales. In the case of stirred-tank reactors the process takes only a few days, however it is expensive basically because the mineral must be finely milled and results economically suitable only for recovering high-value metals. Stirred reactors are mainly used for the pretreatment process (biooxidation) in the recovery of gold from refractory concentrates. BIOX® technology was developed for treating refractory gold ores using mesophilic microorganisms (up to about 40 °C) and later the license was sold to different commercial plants around the world (Clark et al. 2006). Furthermore BIOX® technology was adapted to be applied to concentrates containing other metals (nickel and copper) although mainly at pilot scale. The most successful example of bioleaching of other metal than copper is Kasese plant, in Uganda, where BRGM process using mesophiles allows cobalt recovery from pyritic concentrates.
At the present time, approximately 10–15 % of total production of copper, 4–5 % of total production of gold, and smaller percentages for other metals (cobalt, nickel, zinc) come from biomining processes (Schippers et al. 2013; Johnson 2014). Although these amounts are not negligible, some years ago the expectations were much higher. Among other significant reasons, low bioleaching rates could be the main limiting step to extend the technology beyond the current state (Clark et al. 2006). Increasing the temperature in biomining operations would elevate the reaction rates and would allow the efficient dissolution of some refractory mineral species; besides the use of thermophiles would minimize the cooling costs needed to keep suitable temperatures for mesophilic microorganisms due to the oxidation of sulfide minerals is an exothermal process. Bioleaching at elevated temperatures requires suitable thermophilic microorganisms to replace the mesophilic and moderately thermophilic bacteria generally used in commercial applications. Also tanks and/or heaps should be aconditionated to operate at high temperature. Working with thermophiles would allow overcoming the bottleneck of copper biomining because chalcopyrite, the most abundant copper-bearing mineral (representing more than 70 % of the Earth´s copper) is highly refractory to the attack of mesophilic microorganisms (d’Hugues et al. 2002; Pradhan et al. 2008). Additionally, a succesfull bioleaching operation requires microorganisms able to develop at more acidic conditions (close to pH 1 or even lower) usually used in leaching operations to avoid ferric iron compounds (such as jarosite and goethite) precipitation (Kaksonen et al. 2014; Rawlings 2002). In conclusion, polyextremophiles capable of oxidizing iron(II) and/or sulfur at temperatures higher than 60 °C and pH values close to 1 are essencial to achieve successful leaching processes at high temperatures and low pH values.
Extremophiles
Extremophilic microorganisms, generally named extremophiles, are adapted to live in environments where one or more physicochemical parameters are outside the range considered normal for human life. The extremophiles most relevant for biomining applications are able to grow at low pH values, high temperatures and can tolerate high concentrations of heavy metals. The thermophiles are the microorganisms growing best at temperatures over 50 °C while the hyperthermophiles (mainly archaea) need temperatures over 80 °C for optimal growth. The acidophiles are the microorganisms that live at pH values lower than 5.5. The extreme acidophiles that grow at pH values lower than 3 actually need that extracellular condition to develop (Baker-Austin and Dopson 2007).
Microorganisms with such characteristics can be found in natural environments, typically geothermal areas, or in sites that had become acidic and/or of elevated temperature as a result of anthropogenic activities (Fuchs et al. 1995; Ehrlich et al. 2015). In the first case there are many examples, especially reports of assessments done by molecular ecology techniques, where acidophilic and thermophilic species dominate hot acidic natural environments all over the world (Chan et al. 2015; Lantican et al. 2011; Reigstad et al. 2010). Volcanic areas are rich in a variety of sulfur compounds due to their geological origin (Ehrlich et al. 2015) and are the natural habitats of acidophiles and thermophiles with potential applications in biomining. In Argentina the geothermal area of Caviahue-Copahue, located in Cordillera de Los Andes, is dominated by the still active Copahue volcano and presents a great diversity of pools, ponds and hot springs of different pH and temperature conditions where acidophiles and thermoacidophiles have been detected by molecular ecology techniques (Urbieta et al. 2012, 2014) and also by culturing (Urbieta et al. 2015; Giaveno et al. 2013). Copahue has proven to be the habitat of novel species of archaea with enormous potential in biomining applications (Giaveno et al. 2011). Such is the case of Acidianus copahuensis, a novel thermoacidophilic crenarchaeota autochthonous of Copahue, capable of oxidizing sulfur compounds and ferrous iron at high temperatures and at rates that make it an excellent candidate for bioleaching operations (Giaveno et al. 2013).
An interesting feature of extremophiles is the genetic, biochemical, and metabolical adaptations they present in other to thrive with the unfriendly environmental conditions of their habitats. In the case of thermophiles and hyperthermophiles the major challenge is to maintain proteins, especially enzymes, stabilized and functional at elevated temperatures. To achieve this goal thermophiles present a series of modifications in their proteins, such as an increase in overall superficial charge by increasing the use of charged residues and decreasing uncharged polar ones, increase of ionic interactions in the structure and hydrophobicity in the core of the protein, substitution of thermolabile amino acids (cysteine, asparagines, aspartic acid) in the surface of the protein, increase in the volume of residues that provide tighter packing, higher isoelectric point and more salt bridges, ion pairs and networks of salt bridges (Reed et al. 2013; Taylor and Vaisman 2010; Zhou et al. 2008). These modifications are not essentially at the primary structure level; it has been proven that only few changes in key locations can modify protein structure and folding to make them stable at high temperatures. Thermophiles also have to ensure the stability and the semifluid state of their cellular membranes at high temperatures; in this case the modifications, comparing with mesophiles, involve the use of ether bond between glycerol and fatty acids that is much more resistant to heat and acid hydrolysis than ester bond and the inclusion of more saturated fatty acids which form a stronger hydrophobic structure (Koga 2012; Driessen and Albers 2007). Most of the hyperthermophiles are archaea which do not present fatty acids in the conformation of their membranes but have hydrocarbons of 40 carbon atoms (C40) made of repetitions of isoprene units (C5) named biphytanyl that form tetraether lipids. These C40 lipids are long and pass across the membrane bilayer covalently linking the two sides thus it can be considered as a monolayer (Madigan et al. 2010). This kind of structure is more rigid and allows membranes to tolerate extreme conditions. However, high temperature adaptation does not require the tetraether lipids, while the adaptation of thermophiles to acidic environment requires the tetraether polar lipids (Ulrih et al. 2009).
Acidophiles also present modifications in their cells constituents to avoid damage by the high protons concentration of their environments. Among the most studied representatives it can be highlighted the fact that they have shorter genomes which decrease acid damaging of the genetic material, the existence of many genes with DNA repairing function and an enormous metabolic effort to maintain the cytoplasm pH near neutrality. The last point is achieved by cellular membranes more impermeable to protons by increased content of cyclopropane, an inverse membrane potential (positive in the inner side generated by the influx of potassium ions to the cytoplasm) which acts as a chemostatic barrier for the entrance of protons, an increase in concentration of buffering compounds in the cytoplasm, the activation of degradative pathways of organic acids, and a great number of bombs that pump protons out of the cell (Dhakar and Pandey 2016; Slonczewski et al. 2009; Baker-Austin and Dopson 2007). For most acidophiles the maintenance of a pH gradient of approximately 5 units across the membrane (environmental pH of 2, intracellular pH of 6.5–7) is fundamental for their energetic balance as it is the main component of the proton motive force that guide ATP synthesis by F0F1 ATPase (Baker-Austin and Dopson 2007).
Biomining operations entail the accumulation of metals, especially heavy metals, in the liquors; consequently tolerance and/or resistance to these conditions are essential for biomining microorganisms. The cellular mechanisms to such tolerance/resistance are not well known, particularly for thermophilic microorganisms, although it is established that they implicate diverse groups of proteins and enzymes involved in mobilization, chelation, or modification of the metal species that are not coded in defined cluster of genes (Dopson et al. 2003). Even more, the exposure to toxic metals might trigger stress responses that are not metal specific, but improve the protection of the cell against their noxious effects (Bini 2010). Resistance to copper is of special interest as thermoacidophilic microorganisms are promising candidates for effective bioleaching of different copper sulfides including chalcopyrite (Abdollahi et al. 2014; Qin et al. 2013). The cop gene cluster, described by the first time in S. solfataricus (Ettema et al. 2006), encodes a potential copper resistance system formed by a P-type cation transporting ATPase (CopA), which has been proposed to mediate efflux of copper, among other heavy metal cations such as zinc and cadmium (Nies 2003), a putative metallochaperone (CopM), and an archaeal specific transcriptional regulator (CopT). The same kind of system was found in S. metallicus and Ferroplasma acidarmanus (Orell et al. 2013). In them the polycistronic copMA transcript accumulates in response to growth-inhibiting concentrations of copper and cadmium and CopT binds to copMA promoter in response to copper concentrations, suggesting that the resistance mechanism is related to copper efflux (Orell et al. 2010). An alternative mechanism of metal tolerance is the sequestration of metal cations by inorganic polyphosphates (polyP) and the posterior excretion of the complex (Navarro et al. 2013). The genes involved in polyP metabolism in Crenarchaeota (the phylogenetic group that includes most of the thermoacidophilic archaea) include inorganic polyphosphate/ATP-NAD kinases, exopolyphosphatases and a series of proteins capable of exporting the polyP-metal complex together with phosphate transport regulation proteins and phosphate uptake regulators. An enzyme with polyP synthase activity has not been reported in Crenarchaeota but it has been fully characterized in Acidithiobacillus ferrooxidans and other bacteria known to cope with high concentrations of metals (Orell et al. 2012).
The use of extremophilic microorganisms in biotechnological processes, especially novel species of environmental origin, requires their isolation and thorough characterization. Culturing and isolation of extremophilic microorganisms is not always an easy and successful task; growth conditions (temperature, pH, media composition) are most of the times uncertain which makes it a matter of trial and error. Besides growth can be very slow increasing contamination risk and/or deterioration of culture media. The cultivation of thermophiles and acidophiles in solid media, fundamental to obtain single colonies, represents an extra challenge. For the former the use of regular gelling agents, such as agar or agarose, it is not possible because they melt over 55 °C. To overcame this problem Lindström and Sehlin (1989) described a procedure using a double layer of Gelrite as a solidifying agent (a supporting gel of 0.8 % wt/vol and an overlay soft gel of 0.4 % wt/vol in which the colonies are grown) that increased the plating efficiency to 100 % and it is still used (Giaveno et al. 2013). Acidophiles also cannot be grown in agar plates because they are susceptible to the hydrolysis products of agar caused by the acidic media. In this case the overlay technique described by Johnson and Hallberg (2007) improved the plating efficiency of a variety of acidophiles by incorporating a lower layer with a heterotrophic acidophile that eliminates toxic organic material produced by the acidic hydrolysis. More recently Tsudome et al. (2009) developed a solidified media that employ a porous matrix of nanofibrous cellulose that can support the growth of diverse extremophiles, including thermoacidophiles. The authors claim that use of nanofibrous cellulose plates have several advantages for culturing polyextremophiles over conventional solidified media using agar or gellan gum highlighting versatility, stability and simplicity in preparation.
Biomining using thermophiles
Numerous studies of bioleaching by mesophilic microorganisms in pure cultures or in consortia, at laboratory and field scale, have been reported in the last 30 years and excellent reviews of the main results have been published (Rawlings et al. 2003; Watling 2006; Jerez 2011; Schippers et al. 2013; Vera et al. 2013; Johnson 2014; Watling 2014). Although increasing in recent years, the number of papers about studies on bioleaching by moderate or extreme thermophiles is markedly lower.
Moderately thermophilic bacteria such as At. caldus and some Leptospirillum species are used in some biomining operations at moderate temperatures. Many BIOX® stirred tanks operated at 42–45 °C in different locations are dominated by species of Acidiplasma and Ferroplasma, while Sulfobacillus species were found to dominate in several reactors at the BIOX® plant in Ghana operated at 45 °C (van Hille et al. 2011). Furthermore, different moderate thermophilic consortia have been used in monophasic or biphasic bioleaching studies on monometallic and polymetallic concentrates (Brierley and Brierley 2001; Patel et al. 2012; Brierley and Brierley 2013; Watlings 2014).
Biomining processes above 60 °C generally involve extremely thermophilic sulfur and iron oxidizing archaeal species. They belong mainly to the genera Sulfolobus, Acidianus, Metallosphaera, and Sulfurisphaera. Thermophilic archaea have shown to be advantageous for bioleaching copper from primary copper sulfides, such as chalcopyrite, enargite, and covellite (Castro and Donati 2016a; Takatsugi et al. 2011; Watling et al. 2016). The passivation and formation of diffusional layers over chalcopyrite surface appear to be reduced using extremely thermoacidophilic microorganisms (Li et al. 2013; Norris et al. 2013; Panda et al. 2015). At redox potentials higher than 600–700 mV (vs SHE), the surface of chalcopyrite is passivated, even at high temperatures. Although there is no general consent about the nature of the passivation, the formation of one or more layers limits the reactivity of the surface and decreases the copper extraction rate (Watling 2006). Elemental sulfur, metal-deficient sulfides, polysulfides, and iron precipitates (Córdoba et al. 2008) have been proposed as the components of the limiting layers. To alleviate this problem of passivation and to allow a faster chalcopyrite leaching rate, the redox potential should be maintained below 600 mV (vs SHE) (Córdoba et al. 2008; Gericke et al. 2010; Li et al. 2013; Watling 2013; Zhao et al. 2015). Recently, bioleaching studies with A. copahuensis have demonstrated that this novel archaeal species is able to keep low redox potential achieving a high copper recovery from chalcopyrite (Castro and Donati 2016a). The low capability of A. copahuensis to oxidize ferrous iron keeps a low redox potential where chalcopyrite dissolution is favored without the necessity of external redox potential control.
Liang et al. (2010) showed a great dissolution of copper in chalcopyrite bioleaching by A. manzaensis YN-25 with the addition of l-cysteine. The addition of l-cysteine greatly improves the bioleaching rate of a Cu-Ni sulfide by a mixture of four acidophilic thermophiles, A. brierleyi, A. manzaensis, M. sedula, and S. metallicus (Li et al. 2014). Recently, Watling et al. (2016) showed that the oxidation rates of ferrous iron and tetrathionate by thermophilic microorganisms were reduced in the presence of chloride levels below chloride concentrations in seawater, limiting the applicability of these microorganisms in the bioleaching of chalcopyrite with saline water.
In the case of chalcopyrite-bearing molybdenite, thermoacidophiles can selectively remove copper leading to improvement of molybdenite flotation concentrates, with very low molybdenum dissolution (Abdollahi et al. 2014). Assays performed to study the bioleaching of enargite using thermoacidophilic archaea showed that these species can selectively solubilize copper, while the arsenite (very toxic form of arsenic) released from the enargite is immediately oxidized to arsenate (the lesser toxic form of arsenic) precipitating as scorodite, thus alleviating the toxic effect of arsenic (Takatsugi et al. 2011).
A pilot-scale bioleaching of chalcopyrite using thermophiles was carried out at Chuquicamata Mine (Chile), the process operated at 78 °C with concentrates not suitable for pyrometallurgy due to the high content of arsenic (Batty and Rorke 2006). Also, bioleaching of low-grade nickel-copper sulfide concentrate in a pilot-scale was demonstrated at the Aguablanca Mine in Spain (Neale et al. 2009). BHP Billiton thermophilic tank bioleaching process (BioCyn™) has been licensed to GoldFields for application at the Fairview mine in Barberton, South Africa (Du Plessis et al. 2007).
Most reports on biomining by thermophiles refer to copper recovery, however there are some interesting and successful studies on the use of thermophilic microorganisms in the bioleaching of low-grade and polymetallic ores to recover nickel (Cruz et al. 2010; Norris et al. 2012; Li et al. 2014) and zinc (Castro and Donati 2016b) and in the biooxidation of refractory gold-bearing ores (Lindström et al. 2003; Clark et al. 2006; Astudillo and Acevedo 2009; Watling 2014).
The advatage of thermophilic conditions in biomining is the higher rate of oxidation resulting in smaller reactor size and favorable reactor operating costs. The use of thermophilic consortia in commercial operations could have other advantages; for example, in the application to metal sulfide concentrates, since thermophiles seem to be more tolerant to heavy metal concentrations than mesophiles, major product concentrations in the liquor could be achieved before extracting the metal with solvents and electrowinning. Besides, using higher temperatures in biooxidation plants would allow decrease the formation of thiocianate and consequently the cyanide comsumption (Clark et al. 2006).
Biomining operations at high temperature entail certain difficulties; materials such as ceramics or special stainless steels are needed for the reactors in order to avoid the steel corrosion under oxidizing conditions at high temperature, the low gas solubility requires the replacement of air by oxygen (in order to increase its dissolution) and the addition of carbonate rock to provide carbon dioxide for authotrophs, also special design is required to reduce evaporation, etc. Finally, thermophiles seem to be more sensitive to stress under high pulp densities than mesophiles.
Due to the above mentioned problems, new developments are being implemented in heaps (much cheaper to operate than tank reactors) in order to make them suitable for working at high temperatures with thermophiles and consequently improve the metal solubilization rate. Within these new developments, the use of thermofilms to cover and isolate the heap allows increasing the temperature for an adequate thermophiles growth. Moreover aeration and irrigation can be controlled to provide enough oxygen and carbon dioxide with low evaporation losses. The improvement of heap-leaching processes could substantially reduce the time for metal solubilization and it could even make it suitable for high-value metals. A modification of the traditional heap technology intercalating layers of milled ore within layers of inert rock has been successfully proved not only for the pretreatment of refractory gold minerals but also for the bioleaching of nickel, copper, cobalt and zinc (Clark et al. 2006; Brierley and Brierley 2013). In this technology the inoculum is sprayed during the stacking of the heap over the layer of the ore of interest. Although aeration with oxygen or oxygen-enriched gas instead of air could increase solubility of oxygen at high temperatures, part of the heap could be under anaerobic conditions; however, even in such conditions, chemical attack by iron(III) and protons would continue and also certain microorganisms, such as A. copahuensis, could enhance the dissolution by oxidizing sulfur with iron(III) as the final electron acceptor.
Summarizing, although the number of studies at laboratory and pilot scale is increasing, commercial application of thermophilic bioleaching is still in development stage. Screening at extreme natural environments to detect and isolate novel thermoacidophilic species and deeper studies on thermophiles are still necessary to achieve a flexible and robust biotechnology for leaching ores at high temperatures.
References
Abdollahi H, Shafaei SZ, Noaparast M, Manafi Z, Niemelä SI, Tuovinen OH (2014) Mesophilic and thermophilic bioleaching of copper from chalcopyrite-containing molybdenite concentrate. Int J Miner Process 128:25–32
Astudillo C, Acevedo F (2009) Effect of CO2 air enrichment in the biooxidation of a refractory gold concentrate by Sulfolobus metallicus adapted to high pulp densities. Hydrometallurgy 97:94–97
Baker-Austin C, Dopson M (2007) Life in acid: pH homeostasis in acidophiles. Trends Microbiol 15:165–171
Batty JD, Rorke GV (2006) Development and commercial demonstration of the BioCOP™ thermophile process. Hydrometallurgy 83:83–89
Bini E (2010) Archaeal transformation of metals in the environment. FEMS Microbiol Ecol 73:1–16
Brierley JA, Brierley CL (2001) Present and future commercial applications of biohydrometallurgy. Hydrometallurgy 59:233–239
Brierley LC, Brierley JA (2013) Progress in bioleaching: part B: applications of microbial processes by minerals industries. Appl Microbiol Biot 97:7543–7552
Castro C, Donati E (2016a) Effects of different energy sources on cell adhesion and bioleaching of a chalcopyrite concentrate by extremophilic archaeon Acidianus copahuensis. Hydrometallurgy 162:49–56
Castro C, Donati E (2016b) Improving zinc recovery by the thermoacidophilic archaeon Acidianus copahuensis using tetrathionate. T Nonferr Metal Soc, in press
Chan CS, Chan KG, Tay YL, Chua YH, Goh KM (2015) Diversity of thermophiles in a Malaysian hot spring determined using 16S rRNA and shotgun metagenome sequencing. Front Microbiol 6:177–183
Clark ME, Batty JD, van Buuren CB, Dew DW, Eamon MA (2006) Biotechnology in minerals processing: technological breakthroughs creating value. Hydrometallurgy 83:3–9
Córdoba EM, Muñoz JA, Blázquez ML, González F, Ballester A (2008) Leaching of chalcopyrite with ferric ion. Part IV: the role of redox potential in the presence of mesophilic and thermophilic bacteria. Hydrometallurgy 93:106–115
Cruz FLS, Oliveira VA, Guimarães D, Souza AD, Leão VA (2010) High-temperature bioleaching of nickel sulfides: thermodynamic and kinetic implications. Hydrometallurgy 105:103–109
d’Hugues P, Foucher S, Galle’-Cavalloni P, Morin D (2002) Continuous bioleaching of chalcopyrite using a novel extremely thermophilic mixed culture. Int J Min Process 66:107–119
Dhakar K, Pandey A (2016) Wide pH range tolerance in extremophiles: towards understanding an important phenomenon for future biotechnology. Appl Microbiol Biot 100:2499–2510
Dold B (2014) Mineralogical and geochemical controls in biomining and bioremediation. In: Parmar N, Singh A (eds) Geomicrobiology and biogeochemistry soil biology. Springer, Heidelberg, pp 119–138
Donati E, Sand W (2007) Microbial processing of metal sulfides. Springer, Heidelberg
Dopson M, Baker-Austin C, Koppineedi PR, Bond PL (2003) Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic microorganisms. Microbiology 149:1959–1970
Driessen AJM, Albers SV (2007) Membrane adaptations of (hyper)thermophiles to high temperatures. In: Gerday C, Glansdorff N (eds) Physiology and biochemistry of extremophiles. ASM Press, Washington, pp 104–116
Du-Plessis CA, Batty JD, Dew DW (2007) Commercial applications of thermophile bioleaching. In: Rawlings D, Johnson B (eds) Biomining. Springer, Berlin
Ehrlich HL, Newman DK, Kappler A (2015) Ehrlich’s Geomicrobiology. CRC Press, Florida
Ettema TJ, Brinkman AB, Lamers PP, Kornet NG, de Vos WM, van der Oost J (2006) Molecular characterization of a conserved archaeal copper resistance (cop) gene cluster and its copper-responsive regulator in Sulfolobus solfataricus P2. Microbiology 152:1969–1979
Fuchs T, Huber H, Teiner K, Burggraf S, Stetter KO (1995) Metallosphaera prunae, sp. nov., a novel metal-mobilizing, thermoacidophilic archaeum, isolated from a uranium mine in Germany. Syst Appl Microbiol 18:560–566
Gehrke T, Telegdi J, Thierry D, Sand W (1998) Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl Environ Microbiol 64:2743–2747
Gentina JC, Acevedo F (2013) Application of bioleaching to copper mining in Chile. Electronic J Biotechnol. doi:10.2225/vol16-issue3-fulltext-12
Gericke M, Govender Y, Pinches A (2010) Tank bioleaching of low-grade chalcopyrite concentrates using redox control. Hydrometallurgy 104:414–419
Giaveno MA, Pettinari G, Toril EG, Aguilera A, Urbieta MS, Donati E (2011) The influence of two thermophilic consortia on troilite (FeS) dissolution. Hydrometallurgy 106:19–25
Giaveno MA, Urbieta MS, Ulloa JR, Toril EG, Donati ER (2013) Physiologic versatility and growth flexibility as the main characteristics of a novel thermoacidophilic Acidianus strain isolated from Copahue geothermal area in Argentina. Microbial Ecol 65:336–346
Jerez CA (2011) Bioleaching and biomining for the industrial recovery of metals. In: Murray M-Y (ed) Comprehensive biotechnology, 2nd edn. Elsevier, Amsterdam, pp 717–729
Johnson DB (2014) Biomining—biotechnologies for extracting and recovering metals from ores and waste materials. Curr Opin Biotech 30:24–31
Johnson DB, Hallberg KB (2007) Techniques for detecting and identifying acidophilic mineral-oxidizing microorganisms. In: Rawlings DE, Johnson DB (eds) Biomining. Springer, Berlin, pp 237–261
Kaksonen AH, Morris C, Rea S, Li J, Wylie J, Usher KM, Ginige MP, Cheng KY, Hilario F, du Plessis C (2014) Biohydrometallurgical iron oxidation and precipitation: part I—effect of pH on process performance. Hydrometallurgy 147–148:255–263
Koga Y (2012) Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea. doi:10.1155/2012/789652
Lantican NB, Diaz MGQ, Cantera JJL, Francis L, Raymundo AK (2011) Microbial community of a volcanic mudspring in the Philippines as revealed by 16S rDNA sequence analysis and fluorescence in situ hybridization. World J Microbiol Biot 27:859–867
Li Y, Kawashima N, Li J, Chandra AP, Gerson AR (2013) A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv Colloid Interfac 197–198:1–32
Li S, Zhong H, Hu Y, Zhao J, He Z, Gu G (2014) Bioleaching of a low-grade nickel-copper sulfide by mixture of four thermophiles. Bioresour Technol 153:300–306
Liang CL, Xia JL, Zhao XJ, Yang Y, Gong SQ, Nie ZY, Ma CY, Zheng L, Zhao YD, Qiu GZ (2010) Effect of activated carbon on chalcopyrite bioleaching with extreme thermophile Acidianus manzaensis. Hydrometallurgy 105:179–185
Lindström EB, Sehlin HM (1989) High efficiency of plating of the thermophilic sulfur-dependent archaebacterium Sulfolobus acidocaldarius. Appl Environ Microbiol 55:3020–3021
Lindström EB, Sandström A, Sundkvist JE (2003) A sequential two-step process using moderately and extremely thermophilic cultures for biooxidation of refractory gold concentrates. Hydrometallurgy 71:21–30
Madigan MT, Clark DP, Stahl D, Martinko JM (2010) Brock biology of microorganisms. Pearson, London
Navarro CA, von Bernath D, Jerez CA (2013) Heavy metal resistance strategies of acidophilic bacteria and their acquisition: importance for biomining and bioremediation. Biol Res 46:363–371
Neale JW, Robertson SW, Muller HH, Gericke M (2009) Integrated piloting of a thermophilic bioleaching process for the treatment of a low-grade nickel-copper sulphide concentrate. J South Afr Inst Min Metall 109:273–293
Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27:313–339
Norris PR, Brown CF, Caldwell PE (2012) Ore column leaching with thermophiles: iI, polymetallic sulfide ore. Hydrometallurgy 127–128:70–76
Norris PR, Burton NP, Clark DA (2013) Mineral sulfide concentrate leaching in high temperature bioreactors. Miner Eng 48:10–19
Olson GJ, Brierley JA, Brierley CL (2003) Bioleaching review part B: progress in bioleaching: applications of microbial processes by the minerals industries. Appl Microbiol Biot 63:249–257
Orell A, Navarro CA, Arancibia R, Mobarec JC, Jerez CA (2010) Life in blue: copper resistance mechanisms of bacteria and archaea used in industrial biomining of minerals. Biotech Adv 28:839–848
Orell A, Navarro CA, Rivero M, Aguilar JS, Jerez CA (2012) Inorganic polyphosphates in extremophiles and their possible functions. Extremophiles 16:573–583
Orell A, Remonsellez F, Arancibia R, Jerez CA (2013) Molecular characterization of copper and cadmium resistance determinants in the biomining thermoacidophilic archaeon Sulfolobus metallicus. Archaea. doi:10.1155/2013/289236
Panda S, Akcil A, Pradhan N, Deveci H (2015) Current scenario of chalcopyrite bioleaching: a review on the recent advances to its heap-leach technology. Bioresour Technol 196:694–706
Patel BC, Tipre DR, Dave SR (2012) Development of Leptospirillum ferriphilum dominated consortium for ferric iron regeneration and metal bioleaching under extreme stresses. Bioresour Technol 118:483–489
Pradhan N, Nathsarma KC, Srinivasa Rao K, Sukla LB, Mishra BK (2008) Heap bioleaching of chalcopyrite; a review. Miner Eng 21:355–365
Qin W, Yang C, Lai S, Wang J, Liu K, Zhang B (2013) Bioleaching of chalcopyrite by moderately thermophilic microorganisms. Bioresour Technol 129:200–208
Rawlings DE (2002) Heavy metal mining using microbes. Annu Rev Microbiol 56:65–91
Rawlings DE, Dew D, du Plessis C (2003) Biomineralization of metal-containing ores and concentrates. Trends Biotechnol 21:38–44
Reed JC, Lewis H, Trejo E, Winston V, Evilia C (2013) Protein adaptations in archaeal extremophiles. Archaea. doi:10.1155/2013/373275
Reigstad LJ, Jorgensen SL, Schleper C (2010) Diversity and abundance of Korarchaeota in terrestrial hot springs of Iceland and Kamchatka. ISME J 4:346–356
Sand W, Gehrke T (2006) Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res Microbiol 157:49–56
Sand W, Gehrke T, Hallmann R, Schippers A (1995) Sulfur chemistry, biofilm, and the (in)direct attack mechanism—a critical evaluation of bacterial leaching. Appl Microbiol Biotechnol 43:961–966
Sand W, Gehrke T, Jozsa PG, Schippers A (2001) (Bio) chemistry of bacterial leaching—direct vs. indirect bioleaching. Hydrometallurgy 59:159–175
Schippers A (2007) Microorganisms involved in bioleaching and nucleic acid-based molecular methods for their identification and quantification. In: Donati E, Sand W (eds) Microbial processing of metal sulfides. Springer, Heildelberg, pp 3–33
Schippers A, Sand W (1999) Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl Environ Microbial 65:319–321
Schippers A, Hedrich S, Vasters J, Drobe M, Sand W, Willscher S (2013) Biomining: metal recovery from ores with microorganisms. Adv Biochem Eng/Biotechnol 141:1–47
Schippers A, Glombitza F, Sand W (2014) Geobiotechnology I metal-related issues. Springer, Heidelberg
Slonczewski JL, Fujisawa M, Dopson M, Krulwich TA (2009) Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv Microb Physiol 55:1–79
Takatsugi K, Sasaki K, Hirajima T (2011) Mechanism of the enhancement of bioleaching of copper from enargite by thermophilic iron-oxidizing archaea with the concomitant precipitation of arsenic. Hydrometallurgy 109:90–96
Taylor TJ, Vaisman II (2010) Discrimination of thermophilic and mesophilic proteins. BMC Struct Biol 10:55–65
Tributsch H (2001) Direct versus indirect bioleaching. Hydrometallurgy 59:177–185
Tsudome M, Deguchi S, Tsujii K, Ito S, Horikoshi K (2009) Versatile solidified nanofibrous cellulose-containing media for growth of extremophiles. Appl Environ Microbiol 75:4616–4619
Ulrih NP, Gmajner D, Raspor P (2009) Structural and physicochemical properties of polar lipids from thermophilic archaea. Appl Microbiol Biot 84:249–260
Urbieta MS, Toril EG, Aguilera A, Giaveno MA, Donati E (2012) First prokaryotic biodiversity assessment using molecular techniques of an acidic river in Neuquén, Argentina. Microbial Ecol 64:91–104
Urbieta MS, Toril EG, Giaveno MA, Bazán AA, Donati ER (2014) Archaeal and bacterial diversity in five different hydrothermal ponds in the Copahue region in Argentina. Syst Appl Microbiol 37:429–441
Urbieta MS, Porati GW, Segretín AB, González-Toril E, Giaveno MA, Donati ER (2015) Copahue geothermal system: a volcanic environment with rich extreme prokaryotic biodiversity. Microorganisms 3:344–363
Van Hille RP, van Wyk N, Harrison STL (2011) Review of the microbial ecology of BIOX® reactors illustrates the dominance of the genus Ferroplasma in many commercial reactors. In: Qiu G, Jiang T, Qin W, Liu X, Yang Y, Wang H (eds) Biohydormetallurgy: biotech key to unlock minerals resources value. Central South University Press, Changsha, pp 1021–1031
Vera M, Schippers A, Sand W (2013) Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation – part A. Appl Microbiol Biot 97:7529–7541
Watling HR (2006) The bioleaching of sulphide minerals with emphasis on copper sulphides—a review. Hydrometallurgy 84:81–108
Watling HR (2013) Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 140:163–180
Watling HR (2014) Review of biohydrometallurgical metals extraction from polymetallic mineral resources. Minerals 5:1–60
Watling HR, Collinson DM, Corbett MK, Shiers DW, Kaksonen AH, Watkin LJ (2016) Saline-water bioleaching of chalcopyrite with thermophilic, iron(II)—and sulfur-oxidizing microorganisms. Res Microbiol 167:546–554
Zhao H, Wang J, Yang C, Hu M, Gan X, Tao L, Qin W, Qiu G (2015) Effect of redox potential on bioleaching of chalcopyrite by moderately thermophilic bacteria: an emphasis on solution compositions. Hydrometallurgy 151:141–150
Zhou XX, Wang YB, Pan YJ, Li WF (2008) Differences in amino acids composition and coupling patterns between mesophilic and thermophilic proteins. Amino Acids 34:25–33
Acknowledgments
Dr. ER Donati and Dr. MS Urbieta are researchers from CONICET. This research was supported by ANPCyT (PICT 2013-630).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donati, E.R., Castro, C. & Urbieta, M.S. Thermophilic microorganisms in biomining. World J Microbiol Biotechnol 32, 179 (2016). https://doi.org/10.1007/s11274-016-2140-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-016-2140-2