Abstract
Mt. Makiling Mudspring in Laguna, Philippines is a thermophilic, acidophilic environment that previously has been shown to harbor novel microorganisms. We assessed the microbial community that exists at this volcanic mudspring using 16S rRNA-based approaches. DNA was extracted from solfataric soils and sediments taken from Mudspring. The 16S rDNA was PCR amplified using universal (519F-1392R) and archaeal-specific (23FPL-1391R) primer pairs, cloned, and sequenced. Phylogenetic analysis of the cloned 16S rDNA showed that eleven clones clustered with, and therefore related to Sulfolobus tokodaii 7 and two clones clustered with S. solfataricu, S. shibatae and S. islandicus. Three clone sequences were related to those found in thermophilic chalcopyrite (CuFeS2), a copper sulfuric ore from bioleaching reactors. One clone had low similarity (95% identity) with uncultured archaeon clone KOZ184. Fluorescence in situ hybridization (FISH) analysis revealed that about 71% of the microbial community present in the Mudspring belong to domain Archaea of which 63% were Crenarchaeota and 8% were Euryarchaeota. Seventeen percent (17%) of the population consisted of bacteria as indicated by the positive hybridization with the BACT338 probe, and the remaining 12% are unidentified. This study is the first attempt to use molecular techniques in any environment in the Philippines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mt. Makiling Mudspring is an acid solfatera with in situ temperatures ranging from 70 to 90°C, pH values ranging from 2 to 5 (Bundac et al. 1976) and a high salinity dominated by iron and sulfate (Oles and Houben 1998). Previous studies using cultivation approaches proved the existence of novel species of thermophilic Archaea in Makiling mudspring water (Itoh et al. 1999, 2003). Many other species of thermophilic organisms are expected to be present at the site. However, to date there is no published microbial community analysis of the site.
The extreme environmental conditions and the high temperature required for isolating and growing the microorganisms in Mudspring limit the in vitro cultivation of the prevailing microflora and their subsequent utilization. Moreover, the real environmental conditions that are defined by the biotic and physico-chemical features optimum for microbial growth have yet to be simulated or recreated. Thus, culture-independent molecular-based techniques are useful in exploring the microbial communities of such extreme environments.
Comparative analysis of the 16S rDNA sequence is the most widely used molecular method in microbial community analysis. Sequence analysis of the 16S rRNA gene can identify poorly described, rarely isolated, or phenotypically aberrant strains, and can lead to the recognition of novel isolates and uncultured bacteria (Bottger 1989; Kolbert and Persing 1999). Another method to study the microbial composition of Mudspring which does not rely on culture-based studies and could better represent the true microflora present is fluorescence in situ hybridization (FISH) with ribosomal RNA (rRNA)-targeted oligonucleotide probes (Langendijk et al. 1995; Amann et al. 1990a, b). This procedure allows simultaneous visualization, identification, and enumeration of individual microbial cells, permits the detection of both culturable and unculturable microorganisms, and thus help in understanding complex microbial communities (Moter and Gobel 2000).
FISH is an integral part of the rRNA approach to microbial ecology and evolution (Olsen et al. 1986). Since its first application in 1989 (DeLong et al. 1999), FISH has evolved to become a widely used tool for the direct, cultivation-independent identification of individual microbial cells in complex environmental samples (Behrens et al. 2003).
This study identified the major group of microorganisms that exists in Mudspring using sequence analysis of the 16S rDNA amplified from the extracted total community DNA and FISH using 16S rRNA-targeted probes.
Materials and methods
Sample collection and chemical analysis
Solfataric soil with hot spring water (SSW) was collected from different parts of the Mudspring pond by dipping sterile 500 ml bottles approximately 1 m below the surface of the pond. Solfataric mud (SM) was collected from several boiling mud pots. The collection bottles were placed in thermal containers and processed in the laboratory within 1 h of sample collection.
Composite SSW and mud samples were analyzed at the Analytical Service Laboratory of the Agricultural Systems Cluster, University of the Philippines Los Baños and at the Soil and Plant Analysis Laboratory, Soil Science Department, University of Wisconsin, Madison, WI, USA respectively.
DNA extraction, amplification and cloning
SSW samples were centrifuged (50×g) for 5 min. About 0.5 g of sediment was used for DNA extraction using Fast DNA Spin kit for soil according to the manufacturer’s protocol (MP Biomedicals, Solon, OH). Extracted DNA was purified using the Illustra GFX PCR DNA and gel band purification kit (GE Healthcare, formerly Amersham Biosciences, Buckinghamshire, England) following the manufacturer’s protocol. DNA concentration was determined at 260 nm using the Smart Spectrophotometer 3000 (BioRad, Hercules, CA).
PCR primers used for amplifying 16S rDNA are listed in Table 1. For 16S rDNA amplification, PCR mix contained 1 μl of diluted DNA, 1X reaction buffer containing 0.15 mM MgCl2, 1.5 U Taq polymerase (Promega, Madison, WI), 0.25 mM each dNTP, 0.4 µM of each primer, and 10 μg/µl bovine serum albumin (BSA), and molecular grade water to a total reaction volume of 20 μl. For universal primers 519F and 1392R, the PCR conditions followed the protocol of Simbahan et al. (2005) with slight variations using a PTC 100 Program Thermal Controller Peltier-Effect Cycling (MJ Research, Inc. Waltham, MA). The protocol of Skirnisdottir et al. (2000) was used for the archaeal 16S rDNA 23FPL and 1391R primer set, and that of Webster et al. (2003) for bacterial primers 11F and 1492R.
PCR products were cloned directly using the pGEM-T Easy cloning kit according to the manufacturer’s instructions (Promega). Three μl of the ligation reaction mixture were transformed into 20 μl of competent E. coli JM109 (Promega). Plasmid DNA was extracted from positive clones using the plasmid miniprep protocol (Sambrook et al. 1989). Extracted plasmids were double digested using the following pairs of restriction enzymes: EcoR1-DraI and EcoR1-XhoI in a 20 µl reaction volume. After digestion, 8 μl of the digestion product was run in 1.2% agarose gel for 4 h at 50 V. The gel was stained with ethidium bromide, and visualized using the BioRad Gel Documentation system (BioRad).
DNA sequencing and in silico analysis
Representative clones from each banding pattern were submitted to Macrogen, Inc., Seoul, Korea for sequencing using T7 primer. Sequences were compared to the sequences available in the GenBank database using BLAST (www.ncbi.nlm.nih.gov) to determine their closest neighbors. Alignment and refinement of sequences were carried out using ClustalX v. 1.83 (Thompson et al. 1997) and BioEdit sequence alignment editor (Hall 1999). A total of 586 nucleotides for 16S rDNA was selected for phylogenetic analyses using default parameters in the MEGA software package v. 2.1 (Kumar et al. 2001). Phylogenetic distances were calculated by the neighbour joining (NJ) method (Saitou and Nei 1987) using the Kimura 2-parameter model in MEGA. Consensus NJ trees were evaluated by bootstrap analysis based on 1,000 reiterations of each data set. Similar branching orders were found for trees generated by maximum parsimony (MP) and maximum likelihood (ML) methods, using programs in MEGA and PHYLIP v. 3.6 (Felsenstein 2002), respectively.
Fluorescence in situ hybridization and microscopy
Probes (Table 1) were labeled at the 5′- end with Cy3 [5,5′-disulfo-1,1′-(-′-carbopentynyl)-3,3,3′,3′-tetramethylindolocarbocyanin-N-hydroxysuccinimidester]. SSW samples were fixed immediately on-site by mixing the pool water sample with formaldehyde at 1% (v/v) final concentration. The mixture was filtered using Whatman filter paper #1, and the cells were collected by centrifugation at 4,000 rpm (150×g) for 10 min at 4°C. The cell pellet was washed twice with 1× phosphate buffered saline (PBS) and stored at −20°C in a 1:1 solution of ethanol and PBS. FISH was performed as previously described (Liao et al. 2004). Two μl of the paraformaldehyde-fixed cells were transferred onto SuperCured heavy Teflon-coated slides (Cel-Line Associates Inc., Newfield, NJ). Cells were dehydrated by successive dipping in 50, 80 and 96% ethanol. Subsequently, 50 ng of probe in 8 μl of the hybridization buffer (20% formamide, 360 μl of 5 M NaCl, 40 μl of 1 M Tris–HCL, pH 8, and 2 μl of 10% sodium dodecyl sulphate [SDS]) was added to each slide. Hybridization was conducted in a saturated humidity chamber in the dark at 46°C for 2 h. After hybridization, the slides were washed twice with washing buffer (1 ml of 1 M Tris–HCL pH 8.0, 50 μl of 10% SDS, 2,150 μl of 5 M NaCl, and 500 μl of 0.5 M EDTA) at 48°C for 20 min. Dual staining with DAPI (4′ 6-diamidino-2-phenylindole dihydrochloride) solution (10 mg/ml) followed by a final washing with ice-cold water was performed, and slides were subsequently air-dried. For all FISH procedures, Sulfolobus tokodaii strain 7 was used as positive control.
Slides were visualized with a fluorescence microscope (Carl Zeiss Axioplan II, Oberkochen, Germany) equipped with filter sets for DAPI and CY3 (Carl Zeiss, Germany). Images were captured with an Olympus DP70 charge-coupled device camera (Olympus, Melville, NY). Quantitative analysis was performed using the ImagePro Plus software version 5.2 (Media Cybertic, Singapore) by counting the number of probe-positive cells in six fields. The ratio of probe-positive cells to the total number of DAPI-stained cells was calculated.
Results and discussion
DNA extraction
The high temperature and acidity of the Mudspring limit the in vitro cultivation and maintenance of the prevailing microflora. In addition, it has been estimated that cultured organisms represent less than 1% of total cell counts in soils and sediments (Amann et al. 1995). To address this problem, we used culture-independent molecular approaches to assess the microbial community in Mudspring.
Total DNA was initially extracted from solfataric mud (SM) taken from boiling mud pots. However, initial extraction procedures used (Liles et al. 2003; Simbahan et al. 2004, 2005; Zhou et al. 1996) yielded very low concentration of DNA or none at all. This was probably due to the high clay content (80%) of SM samples, low pH (Table 2), and/or the presence of high amounts of kaolinite and quartz (unpublished data). Two factors were known to complicate DNA isolation from soil: soil acidity and soil-DNA interactions (Henneberger et al. 2006). The low pH of SM could make DNA unstable due to the depurination-induced degradation of DNA (Grisham and Garrett 1999). DNA-clay binding also increases at acidic pH. Some soil components such as montmorillonite and kaolinite (Pietramellara et al. 1997), and quartz (Aardema et al. 1983) can tightly bind DNA and cells (Ogram et al. 1994) thereby making it difficult to extract DNA. Moreover, high levels of humic acid are known to inhibit PCR. Various treatments using chemicals which were supposed to remove humic acid, such as polyvinyl polypyrollidone (PVPP), polyethylene glycol (PEG), Nycodenz, SDS and combinations of these chemicals, were tried (Holben 1994; Ogram et al. 1987). However, these treatments did not result in DNA extraction, (data not shown) as the problem appeared to be the inability to separate the cells from the clay material and the adsorption of the cells and DNA to the soil particles.
The cell extraction methods of Simbahan et al. (2004, 2005) were used on the sediments collected from SSW samples. Cells were collected using 0.45 μm membrane filter paper. After the appropriate sample processing, the DNA was isolated using the Mo Bio DNA isolation kit (Simbahan et al. 2004) and the detergent lysis-phenol extraction method (Simbahan et al. 2005). None of these methods successfully extracted DNA (data not shown), even though the clay content was lower (70%) compared to that of the SM sample (Table 2).
DNA extraction was only successful after allowing the mud to settle for 5 min in freshly collected SSW samples. It is likely that the high levels of mud interfered with DNA isolation by binding cells and DNA. Thus, the upper layer with reduced amounts of mud was used to extract DNA using a commercially available DNA extraction kit (BIO 101 Fast DNA spin kit for soil). This resulted in a concentration (5.7 μg/g sediment) sufficient for subsequent analyses. With the use of BIO 101 kit, a direct lysis method for the isolation of DNA, the result is potentially less bias compared to methods that use cell separation prior to extraction. The use of GFX PCR DNA and gel band purification kit resulted in a purer form based on the A260/A280 ratio.
16S rDNA sequence analysis
Primer pairs extensively used in amplifying 16S rDNA of samples obtained from thermophilic, acidic environments (Bond et al. 2000; Marchant et al. 2002; Simbahan et al. 2005) were chosen. Two sets of primers specific to archaeal and bacterial domains, and one set designed for both, were used. DNA fragments of the expected molecular sizes were detected but the signal intensities differed markedly among the primers used. PCR with universal (519F and 1392R) and archaeal primer pairs (23FPL and 1391R) resulted in pronounced single bands at the 0.87 and 1.4 kb regions, respectively. Bacterial primer pair (11F and 1492R), which was expected to give a 1.3-kb fragment, produced only smeared signals using different PCR conditions. These results indicated that the extracted DNA from Mudspring mostly contained DNA from archaeal species, and that DNA from bacterial species was either few or not detected.
Both the 0.87-kb (universal) and 1.4-kb (archaeal) PCR products amplified from the purified DNA were cloned separately. A total of 69 clones with unique double digestion banding patterns (data not shown) were selected for sequencing. Initial homology search with BLAST showed that 22 of the 69 sequences were those of 16S rDNA, and were used to construct a phylogenetic tree that included closely related 16S rRNA sequences.
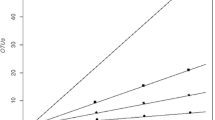
Phylogenetic analysis based on neighbor-joining (NJ) and maximum parsimony (MP) methods showed that clones from the Mudspring can be classified into five phylotypes. The first phylotype included clones N519-4, N519-42, N519-15, N519-7, D519-11, N519-2, D519-16, D519-6, N519-47, D519-2, and N519-54. They are all affiliated with Sulfolobus tokodaii strain 7 and two other Sulfolobus sp. with 97–98% sequence similarity (Fig. 1). Clones D519-12, N519-51, and N519-1 clustered with MTC-A clones isolated from a thermophilic chalcopyrite (CuFeS2), a copper sulfuric ore from bioleaching reactors (Mikkelsen et al. 2006). Clones D519-37, and D519-25 were closely related to Sulfolobus solfataricus Ron12/III with bootstrap value of 98%. These two clones are also phylogenetically related to Sulfolobus islandicus, Sulfolobus shibatae and three other Sulfolobus sp. On the other hand, clones D519-13, D519-17, N519-5, N519-28 and N23-1 were positioned outside the clusters containing other Sulfolobus sp. and the MTC-A clones. The last phylotype included clone D519-8, which was positioned outside the cluster consisting of environmental clones from Norris Geyser Basin in Yellowstone National Park (Korf et al. 2007). The presence of Sulfolobus species in the Mudspring is not surprising. The high temperature and the low pH conditions resulting from oxidation of sulfur and ferrous iron favor the growth of Sulfolobus, a sulfur-dependent thermoacidophilic archaeon. The two novel crenarchaeotes that have been previously isolated from the site, Caldivirga maquilingensis (Itoh et al. 1999) and Caldisphaera lagunensis (Itoh et al. 2003), as well as the only thermophilic bacterium belonging to the genus Thermus (Magbanua, personal communication) were not detected by 16S rDNA analysis, probably due to their low numbers in the sample.
Neighbor-joining tree showing the phylogenetic relationship of SSW clones aligned with reference strains from the domain Bacteria based on 16S rDNA sequences. Bootstrap values are shown for nodes that had 80% support in a bootstrap analysis of 1,000 replicates. Clones from this study are presented in boldface. The scale bar indicates an estimated change of 5%
Fluorescence in situ hybridization
In this study, FISH analysis was conducted using domain- and phylum- specific probes that target the 16S rRNA to determine if other phyla are present and to obtain a quantitative estimate of the domain/phylum composition of the microbial community in the Mudspring. This technique has been successfully applied for the phylogenetic identification of individual microbial cells in a number of different environmental samples (DeLong et al. 1999), determination of active populations in the archaeal community (Ng et al. 2005), and characterization of many unculturable novel archaeal lineages (Schleper et al. 1995).
Hybridization with the Archaea-specific ARCH915 (Fig. 2a) probe showed that Archaea comprised 71% of the total pool community (Table 3). This is consistent with previous work that showed that for highly thermophilic environments, a considerable number of the organisms belong to Archaea (Stetter et al. 1990).
FISH analysis of the microbial community in Mt. Makiling Mudspring, Los Baños, Philippines. Adjacent panels are the same field but stained differently. Panels a, c, e and g show all cells stained by DAPI while panels b, d, f and h were probed with ARCH915-Cy3, CREN512-Cy3, EURY514-Cy3 and BACT338-Cy3, respectively
The phylum-specific probe CREN512 showed that 63% of the Archaeal population belongs to the Crenarchaeota (Fig. 2b). The presence of the members of this group is expected since they have high tolerance to, and strong preference for, extremes of acidity and temperature that are characteristic of Mudspring. While many in the phylum prefer neutral to slightly acidic pH ranges, members of the order Sulfolobales flourish at pH 1–2 and die above pH 7. Optimum growth temperatures range from 75 to 105°C, and the maximum growth temperature can be as high as 113°C as in the case of Pyrolobus (Blochl et al. 1997). Most species are unable to grow below 70°C, although they can survive for long periods at low temperatures (Barns et al. 1996).
Eight percent (8%) of the microorganisms detected belong to the Euryarchaeota, as shown by hybridization with EURY514 probe (Fig. 2c). The presence of euryarchaeotes can be explained by the fact that in moderately thermal (40–60°C) acidic environments, the phyla Euryarchaeota, Crenarchaeota and some gram-positive bacteria may co-exist. Within Euryarchaeota, phylum Thermoplasma has been reported to be moderately thermophilic (45–67°C) and acidophilic (pH 0.5–4.0) (Bertoldo et al. 2004). Picrophilus torridus, with a temperature range of 45–65°C, has the lowest recorded pH at or below zero (Schleper et al. 1995; Futterer et al. 2004) with optimal pH for growth at 0.7.
Bacteria comprised 17% of the total microbial flora (Fig. 2d). Simbahan et al. (2004) have shown the presence of the bacteria Alicyclobacillus, with a temperature range of 35–65°C and pH range of 2–6 in Coso Hot Spring in Nebraska, USA. Acidophilic, mesophilic bacteria of the genus Acidimicrobium (Clark and Norris 1996) and Sulfobacillus (Golovacheva and Karavaiko 1978; Clark and Norris 1996; Dufresne et al. 1996) can also exist in the same environment.
The remaining 12% of cells were undetermined and unaccounted for. The validity of the FISH result was substantiated by the use of a positive control, S. tokodaii strain 7 for the Euryarchaeota and Crenarchaeota probes. S. tokodaii strain 7, a known crenarchaeote showed hybridization signals with CREN512 but not with EURY514 probe (Fig. 3a, b, respectively).
Conclusions
Analyses of the total community 16S rDNA sequences and rRNA-targeted FISH of Mt. Makiling Mudspring in Laguna, Philippines suggest that the site harbors a wide range of microorganisms that has yet to be isolated. Analysis also showed that the major population present in the Mudspring belongs to the domain Archaea particularly the Crenarchaeota, and that bacterial populations are low. Based on 16S rDNA analysis, the predominant organisms belong to the genus Sulfolobus, which are known to thrive in high temperature and low pH conditions. Most of the clones are related to S. tokodaii, a thermoacidophilic organism isolated from the Beppu Hot Spring in Kyushu, Japan. Considering the diversity of potential habitats and the great genetic diversity of microbes, the majority of the microbes from Mt. Makiling Mudspring is still unknown and could be a potential source of novel molecular structures of industrially important compounds such as enzymes. This study was the first attempt to analyze the microbial community of the site using environmental DNA. An improved understanding of the microbial diversity in the extreme environment of the Mt. Makiling Mudspring is needed if the ultimate goal is to discover and harness novel enzymes for industrial and biotechnological applications. Future work should focus on detecting the presence of thermostable enzymes, and a first step includes analysis of the non-16S rDNA clones obtained.
References
Aardema BW, Lorenz MG, Krumbein WE (1983) Protection of sediment-adsorbed transforming DNA against enzymatic inactivation. Appl Environ Microbiol 46:417–420
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl AA (1990a) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Amann R, Krumholz L, Stahl DA (1990b) Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 172:762–770
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Barns SM, Fundyga RE, Jeffries MW, Pace NR (1994) Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc Natl Acad Sci USA 91:1609–1613
Barns SM, Delwiche CF, Palmer JD, Pace NR (1996) Perspective on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc Natl Acad Sci USA 93:9188–9193
Behrens S, Ruhland C, Inacio J, Huber H, Fonseca A, Spencer-Martins I, Fuchs B, Amann R (2003) In situ accessibility of small subunit rRNA of members of the domain, bacteria, archaea and eucarya to CY3 labeled oligonucleotide probes. Appl Environ Microbiol 69:1748–1758
Bertoldo C, Dock C, Antranikian G (2004) Thermoacidophilic microorganisms and their novel biocatalyst. Eng Life Sci 4:521–532
Blochl E, Rachel R, Burggraf S, Hafenbradl D, Jannasch HW, Stetter KO (1997) Pyrolobus fumarii, gen. nov. and sp. nov., represents a novel group of archaea, extending the upper temperature limit to life to 113°C. Extremophiles 1:14–21
Bond PL, Smriga SP, Banfield JF (2000) Phylogeny of microorganisms populating a thick, subaerial predominantly lithotropic biofilm at an extreme acid mine drainage. Appl Environ Microbiol 66:3842–3849
Bottger EC (1989) Rapid determination of bacterial ribosomal RNA sequences by direct sequencing of enzymatically amplified DNA. FEMS Microbiol Lett 53:171–176
Bundac AE, Carreon VR, Fernandez A (1976) Report of the thermal and geochemical survey of the geothermal areas in Los Baños and vicinity. Commission on Volcanology, Tech Report Quezon City
Clark DA, Norris PR (1996) Acidimicrobium ferrooxidans gen. nov., sp. nov.: mixed-culture ferrous iron oxidation with Sulfobacillus species. Microbiology 142:785–790
DeLong EF, Taylor LT, Marsh TL, Preston CM (1999) Visualization and enumeration of marine planktonic archaea and bacteria by using polyribonucleotide probes and fluorescent in situ hybridization. Appl Environ Microbiol 65:5554–5563
Dufresne S, Bousquet J, Boissinot M, Guay R (1996) Sulfobacillus disulfidooxidans sp. nov., a new acidophilic, disulfide oxidizing, Gram-positive, spore-forming bacterium. Int J Syst Bacteriol 46:1056–1064
Felsenstein J (2002) Phylip, phylogeny inference package version 3.6a3, July 2002. Department of Genome Sciences, University of Washington, Seattle, Wash. (http://evolution.gs.washington.edu/phylip.html)
Futterer O, Angelov A, Liesegang H, Gottchalk G, Schleper C, Schepers B (2004) Genome sequence of Picrophilus torridus and its implications for life around pH 0. Proc Natl Acad Sci USA 101:9091–9096
Golovacheva R, Karavaiko S (1978) A new genus of thermophilic spore-forming bacteria, Sulfobacillus. Microbiology 47:658–665
Grisham CM, Garrett RH (1999) Biochemistry, 2nd edn. Saunders College Publishing, Texas, pp 371–372
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis. Department of Microbiology, North Carolina State University, North Carolina
Henneberger RM, Walter RM, Anitoria RP (2006) Extraction of DNA from acidic, hydrothermally modified volcanic soils. Environ Chem 3:100–104
Holben WE (1994) Isolation and purification of bacterial DNA from soil. In: Weawer RW (ed) Methods of soil analysis, vol 2. Soil Science Society of America Inc, Madison, pp 727–751
Itoh T, Suzuki K, Sanchez PC, Nakase T (1999) Caldivirga maquilingensis gen. nov., sp. nov., a new genus of rod-shape crenarchaeote isolated from hot spring in the Philippines. Int J Syst Bact 49:1157–1163
Itoh T, Suzuki K, Sanchez PC, Nakase T (2003) Caldisphaera lagunensis gen. nov., sp. nov., a novel thermoacidophilic crenarchaeote isolated from a hot spring at Mt. Maquiling, Philippines. Int J Syst Evol Microbiol 53(4):1149–1154
Jurgens G, Glöckner FO, Amann R, Saano A, Montonen L, Likolammi M, Münster U (2000) Identification of novel Archaea in bacterioplankton of a boreal forest lake by phylogenetic analysis and fluorescent in situ hybridization. FEMS Microbiol Ecol 34:45–56
Kolbert CP, Persing DH (1999) Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr Opin Microbiol 2:299–305
Korf SE, Inskeep WP, Macur RE, Kozubal MQ, Taylor WP, Nagy A (2007) Microbial population distribution at Norris Geyser Basin in Yellowstone National Park. As cited by Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. 2008. Genbank. Nucleic Acids Res 36 (Database issue): D25–30
Kumar S, Tamura K, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245
Langendijk PS, Schut F, Jansen GJ, Raangs GC, Kamphuis GR, Wilkinson MHF, Welling GW (1995) Quantitative fluorescencein situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in faecal samples. Appl Environ Microbiol 61:3069–3075
Liao J, Lou IC, Delos Reyes III FL (2004) Relationship of species-specific filament levels to filamentous bulking in activated sludge. Appl Environ Microbiol 70:2420–2428
Liles MR, Manske BF, Bintrim SB, Handelsman J, Goodman RM (2003) A census of rRNA genes and linked genomic sequences within a soil metagenomic library. Appl Environ Microbiol 69:2684–2691
Marchant R, Banat IM, Rahman TJ, Berzona M (2002) The frequency and characteristics of highly thermophilic bacteria in cool soil environments. Environ Microbiol 4:595–602
Mikkelsen D, Kappler U, Mcewan AG, Sly LI (2006) Archaeal diversity in two thermophilic chalcopyrite bioleaching reactors. Environ Microbiol 8:2050–2056
Moter A, Gobel UB (2000) Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microbiol Methods 41:85–112
Ng CC, Chang CC, Shyu YT (2005) Archeal community revealed by 16S rRNA and fluorescence in situ hybridization in a sulphuric hydrothermal hot spring, Northern Taiwan. World J Microbiol Biotech 21:933–939
Ogram A, Sayler GS, Barkay T (1987) The extraction and purification of microbial DNA from sediments. J Microbiol Methods 7:57–66
Ogram AV, Mathot ML, Harsh JB, Boyle J, Pettigrew CA (1994) Effects of DNA polymer length on its adsorption to soils. Appl Environ Microbiol 60:393–396
Oles D, Houben G (1998) Greigite (Fe3S4) in an acid mudpool at Makiling Volcano, the Philippines. J Southeast Asian Earth Sci 16:513–517
Olsen GJ, Lane DJ, Giovannoni SJ, Pace NR, Stahl DA (1986) Microbial ecology and evolution: a ribosomal RNA approach. Ann Rev Microbiol 40:337–365
Pace NR, Stahl DA, Lane DJ, Olsen GJ (1986) The analysis of natural microbial populations by ribosomal RNA sequences. Adv Microb Ecol 9:1–55
Pietramellara G, Dal Canto L, Vettori C, Gallori E, Nannipieri P (1997) Effects of air-drying and wetting cycles on the transforming ability of DNA bound on clay minerals. Soil Biol Biochem 29:55–61
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York 9.16
Schleper C, Puehler G, Holz I, Gambacorta A, Janekovic D, Santarius U, Klenk H-P, Zillig W (1995) Picrophilus gen. nov., fam. nov.: a novel aerobic heterotrophic thermoacidophilic genus and family comprising archaea capable of growing around pH 0. J Bact 177:7050–7059
Simbahan J, Drijber R, Blum P (2004) Alicyclobacillus vulcanalis sp. nov., a thermophilic acidophilic bacterium isolated from Coso hot springs, California, USA. Int J Syst Evol Microbiol 54:1703–1707
Simbahan J, Kurth E, Schelert J, Dillman A, Moriyama E, Jovanovich S, Blum P (2005) Community analysis of a mercury hot spring supports occurrence of domain specific forms of mercuric reductase. Appl Environ Microbiol 71:8836–8845
Skirnisdottir S, Hreggvidsson GO, Rleifsdottir SH, Marteinsson VT, Petursdottir SK, Holst O, Kristjansson JK (2000) Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Appl Environ Microbiol 66:2835–2841
Stahl DA, Amann R (1991) Development and application of nucleic acid probes. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 205–248
Stetter KO, Fiala G, Huber G, Huber R, Segerer A (1990) Hyperthermophilic microorganisms. FEMS Microbiol Rev 75:117–124
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Webster G, Newberry CJ, Fry JC, Weightman AJ (2003) Assessment of bacterial community structure in the deep sub-seafloor biosphere by 16S rDNA-based techniques: a cautionary tale. J Microbiol Methods 55:155–164
Zhou J, Bruns MA, Tiedje JM (1996) DNA recovery for soils of diverse composition. Appl Environ Microbiol 62:316–322
Acknowledgments
This study was funded by the Philippine Council for the Advanced Science and Technology Research and Development of the Department of Science and Technology and University of the Philippines Modernization Program—Doctoral Studies Fund. The support and technical inputs of Dr. Robert Goodman of the University of Wisconsin-Madison, Dr. Casiana M. Vera Cruz, her staff, and Rowena Oane of the International Rice Research Institute, Dr. Jessica Simbahan of the National Institute of Biotechnology and Applied Microbiology, University of the Philippines Los Baños (UPLB) and Mr. Andrew D. Montecillo of the Institute of Biological Sciences, UPLB are hereby acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
The 16S rRNA sequence data derived in this study have been submitted to the GenBank database with accession numbers HM449090 to HM449111.
Rights and permissions
About this article
Cite this article
Lantican, N.B., Diaz, M.G.Q., Cantera, J.J.L. et al. Microbial community of a volcanic mudspring in the Philippines as revealed by 16S rDNA sequence analysis and fluorescence in situ hybridization. World J Microbiol Biotechnol 27, 859–867 (2011). https://doi.org/10.1007/s11274-010-0528-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0528-y