Abstract
Forty agricultural soils were collected from Chiang Mai and Lampang provinces in northern Thailand. Bacteria, actinomycetes and fungi were isolated and screened for their ability to degrade polylactic acid (PLA), polycaprolactone (PCL) and poly(butylene succinate) (PBS) by the agar diffusion method. Sixty-seven actinomycetes, seven bacteria and five fungal isolates were obtained. The majority of actinomycetes were Streptomyces based on morphological characteristic, chemotaxonomy and 16S rRNA gene data. Seventy-nine microorganisms were isolated from 40 soil samples. Twenty-six isolates showed PLA-degradation (32.9 %), 44 isolates showed PBS-degradation (55.7 %) and 58 isolates showed PCL-degradation (73.4 %). Interestingly, 16 isolates (20.2 %) could degrade all three types of bioplastics used in this study. The Amycolatopsis sp. strain SCM_MK2-4 showed the highest enzyme activity for both PLA and PCL, 0.046 and 0.023 U/mL, respectively. Moreover, this strain produced protease, esterase and lipase on agar plates. Approximately, 36.7 % of the PLA film was degraded by Amycolatopsis sp. SCM_MK2-4 after 7 days of cultivation at 30 °C in culture broth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Production of plastic has increased from 204 million tonnes in 2002–299 million tonnes in 2013, representing a 46.6 % increase (Plastic Europe 2015). World plastics production has been continuously increasing for more than 50 years. These synthetic polymers cannot be degraded and waste plastics lead to environmental pollution. Biodegradable biopolymer production was studied and bioplastics-degrading microorganisms were reported about 20 years ago. The biodegradable biopolymers are referred to as bioplastics and they can be divided into two groups, renewable resource-based polymers and petroleum-based polymers. Polyhydroxyalkanoates (PHAs) and poly(lactic acid) (PLA) are renewable source-based polymers which are produced from biomass and are thus biodegradable polymers. On the other hand, polycaprolactone (PCL) and poly(butylene succinate) (PBS) are petroleum based, but, they can be degraded by microorganisms (Tokiwa et al. 2009). Degradation of these plastics in soil depends on the environment. So, analysis and isolation of plastics degrading microbes were carried out.
There are many species of microorganisms which can degrade PLA, PCL and PBS such as actinomycetes, bacteria, fungi and yeast. Several actinomycetes including Amycolatopsis sp. 3118 (Ikura and Kudo 1999), Amycolatopsis sp. HT-6 (Pranamuda et al. 1999), Saccharothrix waywayandensis JMC9114 (Jarerat and Tokiwa 2003), Kibdelosporangium aridum JMC7912 (Jarerat et al. 2003), Actinomadura keratinilytica T16-1 (Sukkhum et al. 2011), Amycolatopsis thailandensis PLA07 (Chomchoei et al. 2011), Streptomyces bangladeshensis 77T-4 (Hsu et al. 2012), Streptomyces thermoviolaceus subsp. thermoviolaceus 76T-2 (Chua et al. 2013) were reported as bioplastic degraders. In addition, Bacillus brevis 93 (Tomita et al. 1999), Acidovorax delafieldii BS-3 (Uchida et al. 2000), Paenibacillus amyloticus TB-13 (Teeraphatpornchai et al. 2003), Bacillus pumilus 1-A (Hayase et al. 2004), Bordetella petrii PLA-3 (Kim and Park 2010), Pseudomonas aeruginosa PBSA-2 (Lee and Kim 2010), Shewanella sp. CT01 (Sekiguchi et al. 2010) are examples of bioplastic-degrading bacteria. Many studies on fungal degradation of the bioplastic have also been performed including Paecilomyces lilacinus D218 (Oda et al. 1995), Fusarium moniliforme Fmm (Torres et al. 1996), Aspergillus flavus ATCC9643 (Benedict et al. 1983), Thermoascus aurantiacus IFO31910 (Sanchez et al. 2000), Tritirachium album ATCC22563 (Jarerat and Tokiwa 2001), Paecilomyces verrucosum (Szumigaj et al. 2008) and Aspergillus sp. XH0501-a (Li et al. 2011). Cryptococcus sp. S-2 (Masaki et al. 2005) and Pseudozyma antarctica JCM10317 (Shinozaki et al. 2013) were reported to be bioplastic-degrading yeasts.
There are many types of enzymes reported to be involved in bioplastics degradation. For example, Williams (1981) was the first to report the enzymatic degradation of PLA using proteinase K, bromelain and pronase. Among these enzymes, proteinase K from Tritirachium album was the most effective for PLA degradation. Several proteases such as trypsin, elastase, and subtilisin also hydrolyzed l-PLA (Lim et al. 2005). Proteinase K and other serine proteases are capable of degrading l-PLA and dl-PLA, but not D-PLA. It was reported that lipase could hydrolyze low molecular weight l-PLA and random copolymers of PLA such as: dl-PLA, poly(l-lactide-co-glycolide) and poly(d-lactide-co-glycolide) but not D-PLA, PGA and high molecular weight l-PLA (Tokiwa and Calabia 2006). Lipases constitute an important group of esterases for enzymatic degradation of aliphatic polyesters. A lipase cleaves the ester bond randomly along the main chain of the polymer substrate, e.g. PCL, PBS or other polyesters having a relatively large number of methylene groups in their molecules (Tokiwa and Suzuki 1977; Nakayama et al. 1997, 1998). For example, PlaA is a type of lipase from Paenibacillus amylolyticus strain TB-13 that exhibited degradation activities toward dl-PLA (Akutsu-Shigeno et al. 2003). PCL can be degraded by lipases and esterases (Tokiwa et al. 2009). Moreover, cutinase from Aspergillus oryzae has been reported to degrade PBS and PBSA (Maeda et al. 2005).
Enzymes play a significant role in the degradation of polymers. The microbial degradation of polymers is attributed to the biosynthesis of lipases, esterases and proteases. In the case of PLA degradations, the enzymatic degradation of aliphatic polyesters by hydrolysis is a two-step process. The first step is adsorption of the enzyme on the surface of the substrate through a surface-binding domain and the second step is hydrolysis of the ester bond (Tokiwa and Calabia 2006).
In this study, we report the isolation of microorganisms capable of degrading either bioplastics or biopolymers in particular PLA, PCL and PBS. Investigation of the types of enzymes responsible for bioplastics degradation by the microbial isolates is also described.
Materials and methods
Chemicals and materials
PLA pellets (4043D grade) with the number-average molecular weight (\(\overline{M} n\)) of 1.30 × 105 gmol−1 and the weight-average molecular weight (\(\overline{M} w\)) of 1.50 × 105 gmol−1 were supplied by NatureWorks LLC (Minnesota, USA). PBS [grade GS-Pla AZ91TN: \(\overline{M} n\) = 57,200 gmol−1, PDI = 3.0, melt flow rate = 4.5 g (10 min−1)] was purchased from Mitsubishi Chemical Corporation (Toyoto, Japan) and polycaprolactone (PCL 440744) with \(\overline{M} n\) 80,000 gmol−1 and \(\overline{M} w\)/\(\overline{M} n\) < 2 was purchased from Sigma-Aldrich (Brunei, Singapore). PLA film (thickness ≈ 50 µm) was prepared via the blown film technique at the Department of Chemistry, Faculty of Science, Chiang Mai University (Girdthep et al. 2015). T4 DNA ligase and DNA polymerase were purchased from Promega (Madison, WI, USA). Isoproply-β-d-thiogalactopyranoside (IPTG), 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside and ampicillin were purchased from Sigma-Aldrich (Munich, Germany). All other chemicals were of the highest reagent grade commercially available. Commercial proteinase K was purchased from Vivantis (Selangor Darul Ehsan, Malaysia) and lipase from Candida rugosa was purchased from Sigma-Aldrich Japan K.K. (Tokyo, Japan).
Bacterial strains, plasmids and media
Escherichia coli JM109 and pGEM-T Easy (Promega, Madison, USA) were used as the host and plasmid, respectively; E. coli strains carrying plasmids were grown at 37 °C in Luria–Bertani (LB) medium (Sambrook and Russell 2001) containing 0.1 mg/mL of ampicillin. The basal medium was as follows (g/L): (NH4)2SO4—4; K2HPO4—2; KH2PO4—1; MgSO4 ·7H2O—0.5 and yeast extract—1; the final pH was 7.0 (Tomita et al. 2003). For a solid medium, agar (20 g/mL) was added to the basal medium. Emulsified biopolymers were prepared as follows: 1 g of each biopolymer (PLA, PCL and PBS) pellets was dissolved in 20 mL dichloromethane and emulsified using an ultrasonicator (VC 505-VC 750; Sonics & Materials Inc., Newtown, USA) with 50 % power for 5 min in autoclaved, distilled water or 10 mM potassium phosphate buffer (pH 7.0) (modified from Sukkhum et al. 2009). International Streptomyces Project Medium 2 (ISP2) agar (g/L): malt extract 10, yeast extract 40, glucose 40 and agar 20, was used to culture actinomycetes. Bacteria were re-cultured on nutrient agar (Difco™ Nutrient Broth, Becton, Dickinson and Company, France). Fungi were grown on Potato Dextrose agar (Difco™ Potato Dextrose Broth, Becton, Dickinson and Company, France) and Yeast Mold medium (Difco™ Yeast Mold Broth, Becton–Dickinson and Company, France) was used for testing substrate uptake.
Sample collection
Forty soil samples were collected for PLA-degrading microbe isolation from various sites in Chiang Mai and Lampang provinces, northern Thailand during January–April 2011. Sampling sites (Table 1) included: Muang, Chiang Mai (lat. 18°48′N and long. 98°57′E); Sansai, Chiang Mai (lat. 18°54′N and long. 99°04′E); Mae Rim, Chiang Mai (lat. 18°52′N and long. 98°57′E); Muang, Lampang (lat. 18°30′N and long. 99°38′E) and Hang Chat, Lampang (lat. 18°19′N and long. 99°17′E). A composite sample of 100 g soil was placed in plastic bags, sealed, put in an icebox and transferred by car to the laboratory at Chiang Mai University. All samples were kept at 4 °C in a cold room. Ten grams of soil were analyzed for the presence of fungi, actinomycetes and bacteria within 24 h of collection.
Isolation of PLA-degrading microorganisms
A serial tenfold dilution of 10 g soil samples was carried out. One hundred microliters from 10−4, 10−5, 10−6 dilution of each sample were spread in three replicates on basal medium agar containing 0.1 % (w/v) emulsified PLA. The plates were incubated at 30 °C for 30 days and observed for a clear zone daily. Isolates showing a clear zone around their colonies were collected and stored in 20 % (v/v) glycerol at −20 °C.
Screening of emulsified PLA, PCL and PBS-degrading microorganisms on agar plates
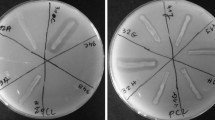
The isolated microorganisms grown on agar plates containing 0.1 % (w/v) PLA for 30 days, commercial proteinase K (1 mg/mL) and lipase (1 mg/mL) were added to basal medium agar plates containing 0.1 % (w/v) emulsified PLA, PCL and PBS. Degradability was determined by the clear zone formation around the colony or the enzyme on the opaque plates after 14 days of incubation at 30 °C (Jarerat et al. 2002). Biopolymer Degradation Index (BDI), the ratio between clear zone diameter and the colony diameter, was calculated.
Screening of emulsified PLA, PCL and PBS-degrading microorganisms in liquid culture
Twenty-five mL of the basal medium containing [0.1 % (w/v)] PLA, PCL and PBS emulsion were added to 125 mL Erlenmeyer flask as a carbon source and inoculated with the isolated microorganisms. The flasks were incubated on a reciprocal shaker for 14 days at 150 rpm and 30 °C. Samples were withdrawn at regular intervals and analyzed for PLA, PCL and PBS-degrading enzyme activity.
Assay for biopolymer-degrading enzyme activity
The turbidity method was used for measuring biopolymer-degrading enzyme activity. Each emulsified PLA, PCL and PBS [0.1 % (w/v)] in 10 mM potassium phosphate buffer (pH 7.0) was used as the substrates. Mixtures of enzyme solutions (0.1 mL) and each substrate emulsion (PLA, PCL and PBS) (1.9 mL) were put into glass test tubes and incubated at 37 °C for 30 min. The decrease in turbidity of the PLA, PCL and PBS emulsions was measured at wavelengths of 630, 650 and 650 nm, respectively, using a spectrophotometer (Spectronic GENESYS 20; Thermo Electron Scientific Instrument Corp., Madison, WI, USA). One unit (U) of PLA-degrading activity was defined as a 1-U decrease in absorbance at 630 nm per min under the assay conditions described (Nakamura et al. 2001). One unit of the PCL-degrading activity was defined as the amount of enzyme required to decrease the OD650 per min by 1.0 under assay condition (Oda et al. 1997). One unit of the PBS-degrading enzyme activity was defined as 0.001 OD decrease in absorbance at 650 nm per min under the described conditions (Li et al. 2011).
Degradation of PLA films in liquid culture
Twenty-five millilitre of the basal medium [containing 0.5 % (w/v) sterile PLA film] was added to a 125 mL flask and inoculated with 2.5 mL of each selected isolate. The flask was incubated on a reciprocal shaker for 7 days at 150 rpm and 30 °C. For microscopic observation (Jarerat et al. 2003), the film samples were washed with distilled water to remove attached cells and air-dried overnight. The films were coated with gold using a SPI Mudule™ Sputter/Carbon Coater (SPI® supplies, Pennsylvania, USA) and observed using a JEOL scanning electron microscope (SEM), Model JSM-5910LV, operating at 15 kv acceleration (JEOL Ltd., Tokyo, Japan).
Identification of selected microorganisms
For actinomycetes, isolates were assigned to the genus Streptomyces or non-Streptomyces according to Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994) after direct microscopic observation of their vegetative and aerial mycelia. Cell wall diaminopimelic acid (A2pm) was analyzed for chemotaxonomy as described by Hasegawa et al. (1983). The morphology and cultural characteristics of actinomycetes were examined according to the guidelines of the International Streptomyces Project (ISP) (Shirling and Gottlieb 1966). Cultures of the pure isolates were observed on various ISP media after incubation at 30 °C for 14 days. The colors of the aerial and substrate mycelia were determined and recorded using the National Bureau of Standards (NBS) color name charts (Kelly 1964). Purified bacteria were identified based on morphological characteristics on nutrient agar, and Gram staining was carried out. Macroscopic and microscopic morphological characterization of purified fungal isolates were carried out and described, e.g. fungal colony growth pattern, pigment production, spore formation, conidia formation, septate or non-septate hyphae for identification to genus (Barnett and Hunter 1987).
About 1 mL of the culture medium was used for DNA extraction by Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) and stored at −20 °C until use. Genomic DNA of these isolates was amplified by polymerase chain reaction (PCR) using GoTaq®Flexi DNA Polymerase (Promega, Madison, WI, USA), with primers; ITS4 and ITS5 for 18S rRNA gene of fungi (White et al. 1990), 27F and 1525R for 16S rRNA gene of actinomycetes and bacteria (Edwards et al. 1989) in 25 µL reactions. The PCR products of 16S rRNA and 18S rRNA gene were purified using a Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA) and were cloned into the pGEM-T Easy cloning vector (Promega, Madison, WI, USA), transferred into E. coli JM109-competent cells, and plated on LB agar plates containing ampicillin, isopropyl-β-d-thiogalactopyranoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside as recommended by the manufacturer. Cloned 16S rRNA and 18S rRNA gene were sequenced using an ABI Prism 310 DNA sequencer (Perkin-Elmer Applied Biosystems, Foster City, CA, USA), using the ABI Prism dye terminator cycle-sequencing ready-reaction kit (Perkin-Elmer Applied Biosystems). All sequence chromatograms were edited using Sequence Scanner Software version 2 (Applied Biosystems, Foster City, CA, USA) and were compared with 16S rRNA and 18S rRNA gene sequences available in the GenBank databases by BLAST Search. DNA Data Bank of Japan (DDBJ) accession numbers for the resultant sequences are listed in Table 3. Then the identification of phylogenetic neighbours and the calculation of pairwise 16S rRNA and 18S rRNA gene sequence identities were achieved using the EzTaxon-e database (http://eztaxon-e.ezbiocloud.net/; Kim et al. 2012). Multiple sequence alignments were performed using CLUSTAL W (Thompson et al. 1994). Phylogenetic analyses were carried out using Kimura 2-parameter model (Kimura 1980) using MEGA 6.0 (Tamura et al. 2013) and bootstrap values based on 1000 replications (Felsenstein 1985).
Substrate uptake
Difco™ Yeast Mold agar was amended with 5 % (w/v) skim milk, 1 % (v/v) triolein and 2 % (v/v) tributyrin with 0.4 % (v/v) Tween 20 as a carbon source to test protease, lipase and esterase activity, respectively. The plates were incubated at 30 °C for 7 days. Proteolytic enzyme production and their activity were observed based on the clear zones in opaque milk protein surrounding the isolates, using proteinase K as the positive control. The opaque zone was measured for esterase on tributyrin agar and orange fluorescent halos under UV light (254 nm) was observed for lipase after incubated for 7 days, using lipase from Candida rugosa as the positive control. The halo zone (ratio between the clear zone and colony diameter) was recorded.
Statistical analysis
All experimental data were analyzed by SPSS program version 17.0 (2008 SPSS Inc., Chicago, IL, USA). The data were subjected to analysis of variance (ANOVA) using Duncan’s test (P < 0.05) for mean separation.
Results
Isolation of PLA-degrading microorganisms
Forty soil samples collected from Lampang and Chiang Mai province were separated into three groups; garbage dump, park and agriculture soil. The details of fungi, actinomycetes and bacteria isolated from each type of soil are shown in Table 1. In total 79 isolates of fungi, actinomycetes and bacteria obtained from various soil samples showed clear zones on PLA agar plates when incubated at 30 °C for 30 days. Five isolates were fungi, 67 isolates were actinomycetes and seven isolates were bacteria (Table 1). Actinomycetes (84.8 %) were the predominant microorganism found, and were isolated from 40 soil samples. Fifty isolates (63.3 %) were isolated from Sansai district, Chiang Mai province. Figure 1 shows that soil from an agricultural site in Sansai district, Chiang Mai province gave the highest number of PLA-degrading isolates (31, 39.2 %).
Screening for PLA, PCL and PBS-degrading ability
Seventy-nine isolated microorganisms were screened for PLA, PBS and PCL degrading activity. Clear zones on PLA, PBS and PCL degradation were graded into four levels using the BDI index at P < 0.05 using Duncan’s test (Fig. 2). Four isolates showed a high BDI index on PLA agar, 13 isolates showed a high BDI index on PBS agar and 17 isolates showed a high BDI index on PCL agar, and were rated as level A. For level B, 12 isolates showed moderate clear zones on PLA agar, 14 isolates showed moderate clear zones on PBS agar and 10 isolates showed moderate clear zones on PCL agar. For level C, low BDI indices were shown by 10 isolates on PLA agar, 17 on PBS agar and 31 on PCL agar. The remaining isolates did not form clear zones on PLA, PBS and PCL agar plates, and were categorized as level D. The results in this study showed that microbial degradation of PLA was rarer than PBS and PCL. Seventy-nine isolates could generate clear zone on PLA agar when incubated for 30 days but only 26 isolates showed clear zone within 14 days.
Sixteen isolates were selected to study PLA, PBS and PCL-degrading enzyme production in liquid media using PLA, PBS and PCL as substrates. The selected isolates exhibited the highest clear zones (BDI index) on both PLA, PBS as well as PCL agar. We found that 16 isolates could produce PLA-degrading enzymes in the culture medium at a higher level than PBS-degrading enzymes. Only four isolates (MRCM_Horse-1, SCM_MK2-4, SCM_MKF-5 and SCM_MKF-8) were found to have PCL-degrading activity. Isolate SCM_MK2-4 demonstrated low degradative ability on the PLA agar plate, but showed the highest biopolymer activity when PLA and PCL were used as substrates in liquid culture broth. The positive control strain A. thailandensis CMUPLA07, showed higher PLA-degrading activity (0.052 U/mL) than Amycolatopsis sp. SCM_MK2-4 (0.046 U/mL) and SCM_MK3-3 (0.031 U/mL) in our study. However, A. thailandensis CMUPLA07 and isolate SCM_MK3-3 showed PLA and PBS-degrading activity, but not PCL-degrading activity (Fig. 3).
All 16 isolates significantly produced PLA-degrading enzymes. Therefore, they were tested in the PLA film degrading study. Isolates SCM_MK3-3 and SCM_MK2-4 degraded and showed 79 and 58 % PLA film weight loss, respectively (data not show). About 36.67 % of the PLA film was degraded after 7 days and 58 % of PLA film were degraded after 14 days at 30 °C in the liquid media by Amycolatopsis sp. SCM_MK2-4. SEM was used to observe film characteristic after incubation with Amycolatopsis sp. SCM_MK2-4 in liquid culture. After 7 days, numerous small–large holes were observed throughout the film surface, and the holes became deeper over time (Fig. 4). When we compared the clear zone method and turbidity method, the majority of the 16 isolates formed halo zones on all three biopolymers agar plates but did not decrease the turbidity of all three biopolymers in liquid solution and could not cause disintegration of the PLA film. Only two isolates (SCM_MK2-4 and SCM_MK3-3) had a high enzymatic ability to degrade PLA film.
Identification of selected microorganisms
For actinomycetes, they were classified as Streptomyces or non–Streptomyces based on their morphological characteristics and type of amino acids in cell walls (Table 2). The number of Streptomyces (59.5 %) was higher than non-Streptomyces (22.8 %). However, two isolates could not be identified because both LL and meso-DAP were found in their whole cell hydrolysates. They also did not form spores on ISP2 agar plate. Of the seven bacterial isolates, three were Gram positive and four Gram negative. Five fungal isolates were classified into three genera. Aspergillus was the most commonly isolated fungus (three isolates) (Fig. 5). Fourteen microbial isolates formed marked clear zones on PLA, PBS and PCL agar in 14 days including one bacterial isolate, two fungi and 13 actinomycete isolates. The results described above were supported by phylogenetic analysis based on 16S rRNA gene sequences. Eleven isolates exhibited 98–99 % 16S rRNA gene sequences similarity with Streptomyces. A few of these, may represent a novel species within the genus Streptomyces. For example, isolates SCM_MKF-3 and SCM_MKF-8 which shared only 98.16 and 98.09 % similarity, respectively, with S. atriruber. Similary, isolate SCM_MK2-3 shared 98.29 % similarity with S. cinnamoneus. Many type strains of Streptomyces species, for example Streptomyces thermocarboxydus, Streptomyces thermodiastaticus and Streptomyces thermoviolaceus subsp. thermoviolaceus share high 16S rDNA sequence similarity values with one another (98.7–99 %) but can be separated on the basis of DNA:DNA relatedness data (Kim et al. 1998). Currently, a 16S rRNA gene sequence similarity of <98.7–99 % was proposed for description of a novel species (Stackebrandt and Ebers 2006). The formal description of these strains based on polyphasic taxonomic study will be published elsewhere. The sequences of isolates SCM_MK2-4 and SCM_MK3-3 showed 99 % similarity with members of the genus Amycolatopsis. In addition, the isolate SCM_MKPN-3 is closely related to Paenibacillus barcinonensis with 98.92 % homology. For fungi, the 18S rRNA gene sequence of isolate MCM_Drom4-1 and MCM_HumanF8-1 indicated that they are closely related to Cladosporium (100 %) and Purpureocillium (99.82 %), respectively. The neighbor-joining phylogenetic tree is shown in Fig. 6. Sequence similarity of 16 PLA-degrading microorganisms and their closest neighbours are shown in Table 3, along with their accession number. The largest group of polyester-degrading microorganisms consisted of 11 Streptomyces isolates. However, two fungal isolates (Cladosporium sp. and Purpureocillium sp.) showed the largest clear zone on the three polyester agar media at P < 0.05. The Paenibacillus isolate formed clear zones on PLA, PCL and PBS agar.
Neighbor-joining phylogenetic tree, based on 18S rRNA (a) and 16S rRNA (b) gene sequences, showing the taxonomic positions of sixteen isolates among members of their neighbor genera. Numbers at the nodes are the bootstrap values based on 1000 resamplings. Bootstrap values of above 50 % are shown at branch points. Out group sequences used for analysis were from Penicillium spp. (a) and Pseudomonas spp. (b). Bar 0.05 (a) and 0.02 (b) substitutions per nucleotide position
Substrate uptake
Screening for protease, esterase and lipase activities involved 16 isolated microbes, two commercial enzymes and Amycolatopsis thailandensis PLA07. As indicated in Table 4, Streptomyces sp. ML_R7 showed the largest halo zones on skim milk agar. Streptomyces sp. SCM_MK2-4 and MRCM_Horse-1 showed large halo zone on tributyrin agar. Streptomyces sp. strain ML_R7, SCM_MKF-3, SCM_MKF-8, SCM_MKPN-8 and SCM_TT-5 showed large halo zones on triolein agar. In this study, since all of the isolates except isolates MCM_Horse-1, SCM_MJ-1, SCM_MKPN-3 and SCM_MKPN-8 could use skim milk as a carbon source, these isolates may produce protease to degrade PLA polymers. Some isolates could produce a little esterase or/and lipase. Amycolatopsis sp. SCM_MK2-4 could use all three carbon sources (skim milk, tributyrin and triolein) tested, so this isolate is capable of producing protease, esterase and lipase. A. thailandensis produced protease and esterase, but not lipase.
Discussion
From 40 soil samples, we found that agricultural soil (mostly rice fields) from Sansai district, Chiang Mai province gave the highest number of PLA-degrading microorganism. Rice field samples were cultivated soil with relatively higher population of microorganisms than the fallow land. The soils rich in organic matter generally contain more microbial population than sandy and eroded soils (Kennedy and Smith 1995). Moreover, actinomycetes were a dominant microorganism isolated from 40 soil samples. Many previous studies reported that Amycolatopsis was a major genus that can degrade PLA bioplastic (Pranamuda and Tokiwa 1999; Jarerat and Tokiwa 2003; Sukkhum et al. 2009; Chomchoei et al. 2011). We found that most of the three polyester-degrading microorganisms were species of Streptomyces (11 isolates). Tokiwa and Jarerat (2004) reported that the distribution of the genus Streptomyces in nature is higher than other actinomycetes with 95 % of the isolated soil actinomycetes are classified as Streptomyces strains. Previous studies also showed that Streptomyces strain MG caused clearly detectable hydrolysis on PCL, PHB and PBS plates (Tokiwa and Calabia 2004). Moreover, a PCL-degrading Streptomyces thermoviolaceus subsp. thermoviolaceus isolate 76T-2 was isolated from soil in Taiwan (Chua et al. 2013). Hoang et al. (2007) reported that out of 305 actinomycetes strains, 12 actinomycetes isolates could degrade the three polyesters (PHB, PES and PCL), and that this ability could be identified based on the clear zone appearance of isolates. The majority of isolates belonged to the genus Streptomyces (91.9 %). In addition, Chomchoei et al. (2011) isolated A. thailandensis, a novel actinomycete, from soil in northern Thailand that was capable of degrading poly(l-lactic acid). Sukkhum et al. (2011) isolated a novel poly(l-lactide)-degrading thermophilic actinomycete, Actinomadura keratinilytica strain T16-1. However, there was no report on PCL and PBS degradability of both strains. It is clear that most of the polylactate degrading microorganisms reported were actinomycetes. However, one bioplastic-degrading bacterium was isolated and was preliminarily classified as Paenibacillus sp. Previously, Teeraphatpornchai et al. (2003) reported that Paenibacillus amyloticus strain TB13 could degrade PLA, PBS, PCL and PES but not PHB-co-valerate. This study was the first report that Cladosporium and Purpureocillium fungi could form clear zones on PLA, PBS and PCL agar plates. Zheng et al. (2005) reported that Cladosporium sp. obtained from soil was found to degrade ester-based polyurethane.
The distribution of the polyester-degrading microorganism in soils was found to decrease in the order of PCL = PHB > PBS > PLA (Nishida and Tokiwa 1993; Pranamuda et al. 1997; Tansengeo and Tokiwa 1997; Jarerat et al. 2002). Seventy-nine isolated microorganisms were tested on PLA, PBS and PCL agar plates. Only two isolates (SCM_MK2-4 and SCM_MK3-3) of genus Amycolatopsis could degrade PLA film in culture broth. Comparison between the clear zone method and film degradation method, most of isolated microorganisms could form halo zones on agar plates but could not degrade PLA film into small pieces. Tokiwa et al. (2009) reported that the clear zone method on agar plates was a widely used technique for screening polymer degrading microbes and for assessment of the polymer degradation potential of different microorganisms. The formation of halo zones around the colonies happens when the polymer-degrading microorganisms excrete extracellular enzymes, which diffuse through the agar and degrade the polymer into water soluble materials. Amycolatopsis sp. strain SCM_MK2-4 could form halo zones on skim milk agar, tributyrin agar and triolein agar which indicated that they might produce protease, esterase and lipase enzyme, respectively. Tokiwa and Calabia (2004) reported that lipase can hydrolyze the ester bonds of polyesters having a relatively large number of methylene groups, e.g. PCL, PEC, PES, PBS and PBSA but lipase cannot degrade optically active polymers with high Tm such as PHB, PHV and PLA. As reported by Reeve et al. (1994) proteinase K was not able to cleave the d-stereoisomers of PLA. It seems reasonable to conclude that PLA-degrading enzymes are proteases which recognize the l-lactic acid unit of the PLA such as the l-alanine unit of silk fibroin (protein) but do not degrade PCL, PBS and PHB (Pranamuda et al. 2001; Nakamura et al. 2001; Jarerat and Tokiwa 2001). Further analyses concerning, enzyme production optimization and film degradation by Amycolatopsis sp. SCM_MK2-4 are necessary. Our next aim is to assess bioplastic degradation (PLA, PCL and PBS) and increase production of PLA-degrading enzymes from this strain.
References
Akutsu-Shigeno Y, Teeraphatpornchai T, Teamtisong K, Nomura N, Uchiyama H, Nakahara T, Nakajima-Kambe T (2003) Cloning and sequencing of a poly(dl-lactic acid) depolymerase gene from Paenibacillus amylolyticus strain TB-13 and its functional expression in Escherichia coli. Appl Environ Microbiol 69:2498–2504. doi:10.1128/AEM.69.5.2498-2504.2003
Barnett HL, Hunter BB (1987) Illustrated genera of imperfect fungi, 4th edn. Macmillan, New York
Benedict CV, Cook WJ, Jarrett P, Cameron JA, Huang SJ, Bell JP (1983) Fungal degradation of polycaprolactones. J Appl Polym Sci 28:327–334. doi:10.1002/app.1983.070280128
Chomchoei A, Pathom-aree W, Yokota A, Khanongnuch C, Lumyong S (2011) Amycolatopsis thailandensis sp. nov., a poly(l-lactic acid)-degrading actinomycete, isolated from soil. Int J Syst Evol Microbiol 61:839–843. doi:10.1099/ijs.0.023564-0
Chua T, Tseng M, Yang M (2013) Degradation of poly(ε-caprolactone) by thermophilic Streptomyces thermoviolaceus subsp. thermoviolaceus 76T-2. AMB Express 3:8–14. doi:10.1186/2191-0855-3-8
Edwards U, Rogall T, Böcker H, Emdo M, Böttger E (1989) Isolation and direct complete nucleotide determination of entire genes, characterization of a gene coding for 16S ribosomal DNA. Nucl Acids Res 17:7843–7853. doi:10.1093/nar/17.19.7843
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Girdthep S, Worajittiphon P, Molloy R, Leejarkpai T, Punyodom W (2015) Formulation and characterization of compatibilized poly(lactic acid)-based blends and their nanocomposites with silver-loaded kaolinite. Polym Int 64:203–211. doi:10.1002/pi.4775
Hasegawa T, Takisawa M, Tanida S (1983) A rapid analysis for chemical grouping of aerobic actinomycetes. J Gen Appl Microbiol 29:319–322. doi:10.2323/jgam.29.319
Hayase N, Yano H, Kudoh E, Tsutsumi C, Ushio K, Miyahara Y, Tanaka S, Nakagawa K (2004) Isolation and characterization of poly(butylene succinate-co-butylene adipate)-degrading microorganism. J Biosci Bioeng 97:131–133. doi:10.1016/S1389-1723(04)70180-2
Hoang K, Lee C, Tseng M, Chu WS (2007) Polyester-degrading actinomycetes isolated from the Touchien river of Taiwan. World J Microbiol Biotechnol 23:201–205. doi:10.1007/s11274-006-9212-7
Holt JA, Krieg NR, Sneath PHA (1994) Bergey’s manual of determinative bacteriology. Lippincott Williams & Wilkins, Philadelphia
Hsu K, Tseng M, Don T, Yang M (2012) Biodegradation of poly(β-hydroxybutyrate) by a novel isolate of Streptomyces bangladeshensis 77T-4. Bot Stud 53:307–313
Ikura Y, Kudo T (1999) Isolation of a microorganism capable of degrading poly-(l-lactide). J Gen Appl Microbiol 45:247–251. doi:10.2323/jgam.45.247
Jarerat A, Tokiwa Y (2001) Degradation of poly(l-lactide) by a fungus. Macromol Biosci 1:136–140. doi:10.1002/1616-5195(20010601)1:43.0.CO;2-3
Jarerat A, Tokiwa Y (2003) Poly(l-lactide) degradation by Saccharothrix waywayandensis. Biotechnol Lett 25:401–404. doi:10.1023/A:1022450431193
Jarerat A, Pranamuda H, Tokiwa Y (2002) Poly(l-lactide)-degrading activity in various actinomycetes. Macromol Biosci 2:420–428. doi:10.1002/mabi.200290001
Jarerat A, Tokiwa Y, Tanaka H (2003) Poly(l-lactide) degradation by Kibdelosporangium aridum. Biotechnol Lett 25:2035–2038. doi:10.1023/B:BILE.0000004398.38799.29
Kelly KL (1964) Inter-society color council-national bureau of standards color-name charts illustrated with centroid colors. US Government Printing Office, Washington
Kennedy AC, Smith KL (1995) Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 170:75–86. doi:10.1007/BF02183056
Kim M, Park S (2010) Degradation of poly(l-lactide) by a mesophilic bacterium. J Appl Polym Sci 117:67–74. doi:10.1002/app.31950
Kim SB, Falconer C, Williams E, Goodfellow M (1998) Streptomyces thermocarboxydovorans sp. nov. and Streptomyces thermocarboxydus sp. nov., two moderately thermophillic carboxydotrophic species from soil. Int J Syst Bacteriol 48:59–68. doi:10.1099/00207713-48-1-59
Kim O, Cho Y, Lee K, Yoon S, Kim M, Na H, Park S, Jeon YS, Lee J, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. doi:10.1099/ijs.0.038075-0
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. doi:10.1007/BF01731581
Lee S, Kim M (2010) Isolation of bacteria degrading poly(butylene succinate-co-butylene adipate) and their lip A gene. Int Biodeterior Biodegrad 64:184–190. doi:10.1016/j.ibiod.2010.01.002
Li F, Hu X, Guo Z, Wang Z, Wang Y, Liu D, Xia H, Chen S (2011) Purification and characterization of a novel poly(butylene succinate)-degrading enzyme from Asperillus sp. XH0501-a. World J Microbiol Biotechnol 27:2591–2596. doi:10.1007/s11274-011-0731-5
Lim HA, Raku T, Tokiwa Y (2005) Hydrolysis of polyesters by serine proteases. Biotechnol Lett 27:459–464. doi:10.1007/s10529-005-2217-8
Maeda H, Yamagata Y, Abe K, Hasegawa F, Machida M, Ishioka R, Gomi K, Nakajima T (2005) Purification and characterization of a biodegradable plastic-degrading enzyme from Aspergillus oryzae. Appl Microbiol Biotechnol 67:778–788. doi:10.1007/s00253-004-1853-6
Masaki K, Kamini NR, Ikeda H, Lefuji H (2005) Cutinase-like enzyme from the yeast Cryptococcus sp. strain S-2 hydrolyzes polylactic acid and other biodegradable plastics. Appl Environ Microbiol 71:7548–7550. doi:10.1128/AEM.71.11.7548-7550.2005
Nakamura K, Tomita T, Abe N, Kamio Y (2001) Purification and characterization of an extracellular poly(l-lactic acid) depolymerase from a soil isolate, Amycolatopsis sp. strain K104-1. Appl Environ Microbiol 67:345–353. doi:10.1128/AEM.67.1.345-353.2001
Nakayama A, Kawasaki N, Maeda Y, Arvanitoyannis I, Aiba S, Yamamoto N (1997) Study of biodegradability of poly(δ-valerolactone-co-l-lactide)s. J Appl Polym Sci 66:741–748. doi:10.1002/(SICI)1097-4628(19971024)66:4<741:AID-APP14>3.0.CO;2-U
Nakayama A, Kawasaki N, Aiba S, Maeda Y, Arvanitoyannis I, Yamamoto N (1998) Synthesis and biodegradability of novel copolyesters containing γ-butyrolactone units. Polymer 39:1213–1222. doi:10.1016/S0032-3861(97)00401-1
Nishida H, Tokiwa Y (1993) Distribution of poly(β-hydroxybutyrate) and poly(ɛ-caprolactone) aerobic degrading microorganisms in different environments. J Polym Environ 1:227–233. doi:10.1007/BF01458031
Oda Y, Asari H, Urakami T, Tonomura K (1995) Microbial degradation of poly(3-hydroxybutyrate) and polycaprolactone by filamentous fungi. J Ferment Bioeng 80:265–269. doi:10.1016/0922-338X(95)90827-M
Oda Y, Oida N, Urakami T, Tonomura K (1997) Polycaprolactone depolymerase produced by the bacterium Alcaligenes faecalis. FEMS Microbiol Lett 152:339–343. doi:10.1111/j.1574-6968.1997.tb10449.x
Plastic Europe (2015) An analysis of European plastics production, demand and waste data. Plastics—the facts 2014/2015. http://www.plasticseurope.org. Accessed Jan 2015
Pranamuda H, Tokiwa Y (1999) Degradation of poly(l-lactide) by strains belonging to genus Amycolatopsis. Biotechnol Lett 21:901–905. doi:10.1023/A:1005547326434
Pranamuda H, Tokiwa Y, Tanaka H (1997) Polylactide degradation by an Amycolatopsis sp. Appl Environ Microbiol 63:1637–1640
Pranamuda H, Tsuchii A, Tokiwa Y (2001) Poly(l-lactide)-degrading enzyme produced by Amycolatopsis sp. Macromol Biosci 1:25–29. doi:10.1002/1616-5195(200101)1:1<25:AID-MABI25>3.0.CO;2-3
Reeve MS, McCarthy SP, Downey MJ, Gross RA (1994) Polylactide stereochemistry: effect on enzymatic degradability. Macromol 27:825–831. doi:10.1021/ma00081a030
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, New York
Sanchez J, Tsuchii A, Tokiwa Y (2000) Degradation of polycaprolactone at 50°C by a thermotolarant Aspergillus sp. Biotechnol Lett 22:849–853. doi:10.1023/A:1005603112688
Sekiguchi T, Sato T, Enoki M, Kanehiro H, Uematsu K, Kato C (2010) Isolation and characterization of bidegradable plastic degrading bacteria from deep-sea environments. JAMSTEC Rep Res Dev 11:33–41. doi:10.5918/jamstecr.11.33
Shinozaki Y, Kikkawa Y, Sato S, Fukuoka T, Watanabe T, Yoshida S, Nakajima-kambe T, Kitamoto HK (2013) Enzymatic degradation of polyester films by a cutinase-like enzyme from Pseudozyma antarctica: surface plasmon resonance and atomic force microscopy study. Appl Microbiol Biotechnol 97:8591–8598. doi:10.1007/s00253-012-4673-0
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Evol Microbiol 16:313–340. doi:10.1099/00207713-16-3-313
Stackebrandt E, Ebers J (2006) Taxonomic parameters revisited: tarnished gold standards. Microbiol Today 33:152–155
Sukkhum S, Tokuyama S, Tamura T, Kitpreechavanich V (2009) A novel poly(l-lactide) degrading actinomycetes isolated from Thai forest soil, phylogenic relationship and the enzyme characterization. J Gen Appl Microbiol 55:459–467. doi:10.2323/jgam.55.459
Sukkhum S, Tokuyama S, Kongsaeree P, Tamura T, Ishida Y, Kitpreechavanich V (2011) A novel poly(l-lactide) degrading thermophilic actinomycetes, Actinomadura keratinilytica strain T16-1 and pla sequencing. Afr J Microbiol Res 5:2575–2582. doi:10.5897/AJMR10.722
Szumigaj J, Żakowaka Z, Klimek L, Rosicka-Kaczmarek J, Bartkowiak A (2008) Assessment of polylactide foil degradation as a result of filamentous fungi activity. Pol J Environ Stud 17:335–341
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Tansengeo ML, Tokiwa Y (1997) Thermophilic microbial degradation of polyethylene succinate. World J Microbiol Biotechnol 14:133–138. doi:10.1023/A:1008897121993
Teeraphatpornchai T, Nakajima-Kambe T, Shigeno-Akutsu Y, Nakayama M, Nomura N, Nakahara T, Uchiyama H (2003) Isolation and characterization of a bacterium that degrades various polyester-based biodegradable plastics. Biotechnol Lett 25:23–28. doi:10.1023/A:1021713711160
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680. doi:10.1093/nar/22.22.4673
Tokiwa Y, Calabia BP (2004) Degradation of microbial polyesters. Biotechnol Lett 26:1181–1189. doi:10.1023/B:BILE.0000036599.15302.e5
Tokiwa Y, Calabia BP (2006) Biodegradability and biodegradation of poly(lactide). Appl Microbiol Biotechnol 72:244–251. doi:10.1007/s00253-006-0488-1
Tokiwa Y, Jarerat A (2004) Biodegradation of poly(l-lactide). Biotechnol Lett 26:771–777. doi:10.1023/B:BILE.0000025927.31028.e3
Tokiwa Y, Suzuki T (1977) Hydrolysis of polyesters by lipases. Nature 270:76–78. doi:10.1038/270076a0
Tokiwa Y, Calabia BP, Ugwu C, Aiba S (2009) Biodegradability of plastics. Int J Mol Sci 10:3722–3742. doi:10.3390/ijms10093722
Tomita K, Kuroki Y, Nagai K (1999) Isolation of thermophiles degrading poly(l-lactic acid). J Biosci Bioeng 87:752–755. doi:10.1016/S1389-1723(99)80148-0
Tomita K, Tsuji H, Nakajima T, Kikuchi Y, Ikarashi K, Ikeda N (2003) Degradation of poly(d-lactic acid) by a thermophile. Polym Degrad Stab 81:167–171. doi:10.1016/S0141-3910(03)00086-7
Torres A, Li SM, Roussos S, Vert M (1996) Screening of microorganisms for biodegradation of poly(lactic acid) and lactic acid-containing polymers. Appl Environ Microbiol 62:2393–2397
Uchida H, Nakajima-Kambe T, Shigeno-Akutsu Y, Nomura N, Tokiwa Y, Nakahara T (2000) Properties of a bacterium which degrades solid poly(tetramethylene succinate)-co-adipate, a biodegradable plastic. FEMS Microbiol Lett 189:25–29. doi:10.1111/j.1574-6968.2000.tb09201.x
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Shinsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York
Williams DF (1981) Enzymic hydrolysis of polylactic acid. Eng Med 10:5–7. doi:10.1243/EMED_JOUR_1981_010_004_02
Zheng Y, Yanful EK, Bassi AS (2005) A review of plastic waste biodegradation. Crit Rev Biotechnol 25:243–250. doi:10.1080/07388550500346359
Acknowledgments
This research was supported by The Royal Golden Jubilee Ph.D. Program (PHD/0142/2553). The National Research University Project under Thailand’s Office of the Higher Education Commission and The Graduate School, Chiang Mai University are thankfully acknowledged. The authors wish to thank Dr. Robert J. McGovern (The Graduate School, Chiang Mai University, Chiang Mai, Thailand) for valuable comments and improving the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Penkhrue, W., Khanongnuch, C., Masaki, K. et al. Isolation and screening of biopolymer-degrading microorganisms from northern Thailand. World J Microbiol Biotechnol 31, 1431–1442 (2015). https://doi.org/10.1007/s11274-015-1895-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1895-1