Abstract
The predominant fermentable sugar in lean dough is maltose. To improve the leavening ability of baker’s yeast in lean dough, maltose metabolism should be improved. Maltase (alpha-glucosidase, encoded by MAL62) and maltose permease (encoded by MAL61) are the major factors involved in maltose metabolism. The major rate-limiting factor in maltose metabolism and leavening ability of baker’s yeast remains unclear. In this work, MAL61 and/or MAL62 overexpression strains were constructed to investigate the decisive factor for maltose metabolism of industrial baker’s yeast in lean dough. Our results show that elevated maltose permease activity by MAL61 overexpression yielded less improvement in maltose fermentation compared to elevated maltase activity by MAL62 overexpression. Significant increase in maltase activity by MAL62 overexpression could result in a 44 % increase in leavening ability of industrial baker’s yeast in lean dough and a 39 % increase in maltose metabolism in a medium containing glucose and maltose. Thus, maltase was the rate-limiting factor in maltose fermentation of industrial baker’s yeast in lean dough. This study lays a foundation for breeding of industrial baker’s yeast for quick dough leavening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Baker’s yeast (Saccharomyces cerevisiae) is a key leavening and bulking agent that is widely used in food processing (Lin et al. 2014). Baker’s yeast serves as the constituent member of baking ingredients, and is of crucial importance in lean dough leavening (Bell et al. 2001; Sasano et al. 2013). A high leavening ability is the most important characteristic in a baking strain to produce baker’s yeast production of good quality and to save baking time (Hirasawa and Yokoigawa 2001). Starch, which is the main component of lean dough, is hydrolyzed to maltose through amylases. Given that maltose is the most abundant fermentable sugar, the maltose metabolism level is the major factor for the leavening ability in lean dough (Hazell and Attfield 1999). The ability of a baker’s yeast strain to ferment maltose depends on the presence of at least one member of a polygenic family of loci referred to as the MAL loci (MAL1 through MAL4 and MAL6) (Needleman 1991; Srđan et al. 2004), and any of these members can confer the ability of maltose metabolism in yeasts. The typical locus MAL6 is a complex of three genes that are essential in metabolizing maltose; this locus consists of MAL61 (MAL6T) encoding for maltose permease, MAL62 (MAL6S) encoding for maltase, and MAL63 (MAL6R) encoding for a positive regulatory protein, which activates the two enzymes (Cohen et al. 1985; Hu et al. 1999). Maltose is transported across the cell membrane via maltose permease, and cleaved intracellularly into two units of glucose by maltase. The synthesis of both enzymes is induced by maltose and repressed by glucose, and regulation occurs predominantly at the level of transcription (Charron et al. 1986; Naumov et al. 1994).
Glucose is the preferred substrate for baker’s yeast in lean dough, and represses the activities of maltase and maltose permease to negatively affect the leavening ability (Carlson 1999; Dietvorst et al. 2007; Verstrepen et al. 2004). Previous studies regarded the transmembrane transport of maltose as the limiting step for the induction of MAL gene expression and glucose control, and maltose permease has a major function in both maltose sensing and transport (Alves-Araújo et al. 2007; Goldenthal et al. 1987; Klein et al. 1996; Wang et al. 2002). Both the transcription repression of the maltose-permease-encoding gene and the inactivation of maltose permease result in a rapid and irreversible loss of the ability to transport maltose in the presence of glucose (Medintz et al. 1996). Moreover, the distinct functions of maltose permease family members allow the successful adaptation to various environmental conditions to which the yeast cells are exposed (Day et al. 2002). Therefore, many studies intending to boost maltose metabolism have focused on the modification on maltose permease, while disregarding maltase. However, in recent years, several studies revealed that maltase may be more important than maltose permease in improving maltose fermentation (Jiang et al. 2008; Sun et al. 2012). For maltose metabolism and leavening ability of baker’s yeast in lean dough, the major rate-limiting factor remains unclear.
In this study, MAL61 and/or MAL62 overexpression strains were constructed to investigate the decisive factor for maltose metabolism of industrial baker’s yeast in lean dough. To evaluate the effects of MAL61 and MAL62 overexpression on maltose metabolism and dough leavening of industrial baker’s yeast, the transformants were characterized in terms of mRNA levels, maltase and maltose permease activities, maltose utilization, growth and fermentation characteristics.
Materials and methods
Strains and plasmids
The industrial baker’s yeast strain BY14 and the strain Escherichia coli DH5α (Φ80 lacZΔM15 ΔlacU169 recA1 endA1 hsdR17 supE44 thi-1 gyrA relA1) were obtained from the Yeast Collection Center of the Tianjin Key Laboratory of Industrial Microbiology.
The plasmid pPGK1 was gifted from the professor Bauer F (Stellenbosch University, South Africa) (Lilly et al. 2000). The plasmids Yep352 and pUG6 used in this study were purchased from Invitrogen (Carlsbad, CA, USA).
Growth, cultivation, and fermentation conditions
Recombinant DNA was amplified in E. coli DH5α, which was grown at 37 °C in Luria–Bertani medium (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) supplemented with 100 μg/mL ampicillin. The plasmid was obtained using a Plasmid Mini Kit II (D6945, Omega, USA).
Yeast cells were statically cultured in yeast extract peptone dextrose (YEPD) medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose) to the stationary phase. 20 mL of the cell culture was inoculated into 200 mL of cane molasses medium at the initial OD600 = 0.4 and cultivated for 24 h at 30 °C with 180 rpm rotary shaking to the final OD600 = 1.8. Cells were harvested through centrifugation (4 °C, 1500×g, 5 min) and were washed twice with sterile water at 4 °C for the succeeding fermentation experiments. To investigate the relationship between maltase and maltose permease, we used the low sugar model liquid dough (LSMLD) medium, which was modified according to Panadero et al. (2005), contains 2.5 g/L (NH4)2 SO4, 5 g/L urea, 16 g/L KH2PO4, 5 g/L Na2HPO4, 0.6 g/L MgSO4, 0.0225 g/L nicotinic acid, 0.005 g/L Ca-pantothenate, 0.0025 g/L thiamine, 0.00125 g/L pyridoxine, 0.001 g/L riboflavin, and 0.0005 g/L folic acid and one of the two specified carbon sources (38 g/L maltose and 33.25 g/L maltose mixed with 5 g/L glucose).
Construction of plasmid and yeast transformants
Yeast genomic DNA was prepared from industrial baker’s yeast strain BY14 using a yeast DNA kit (D3370-01, Omega, USA). The PCR primers used in this study are listed in Table 1. A BamHI KanMX fragment, the dominant selection marker during yeast conversion, was amplified by PCR using pUG6 as template and the primers Kan-F and Kan-R. The KanMX fragment was cloned to vector Yep352 after being digested, thereby generating Yep-K. A BglII/XhoI fragment of MAL61, which was amplified with primers MAL61-F and MAL61-R from genomes of the parental strain BY14 was cloned to vector pPGK1 and resulted in plasmid pPGKM1. The same method was applied to construct pPGKM2. The SphI fragment PM1/PM2 from pPGKM1/pPGKM2 containing the integrated PGK1 and the entire MAL61/MAL62 was cloned to Yep-K and resulted in Yep-KPM1/Yep-KPM2. Yep-KPM1-KPM2 was constructed by inserting PM2 into the preceding plasmid Yep-KPM1.
The respective transformation plasmids Yep-K, Yep-KPM1, Yep-KPM2 and Yep-KPM1-KPM2 were transformed using the lithium acetate/PEG procedure (Gietz and Woods 2002). The YEPD plates were supplemented with 800 mg/L G418 to select the geneticin (G418)-resistant overexpression strains BY14+K (BYK), BY14+MAL61 (BYKPM1), BY14+MAL62 (BYKPM2) and BY14+MAL61+MAL62 (BYKPM1+M2) after transformation. The transformants were then verified by PCR using the primers PGK-F/PGK-R listed in Table 1.
Determination of specific growth rate and biomass yield
After incubating for 24 h, the mixtures of cell culture and medium were mixed in a deep well plate in appropriate proportions, and the growth curve was detected using bioscreen automated growth curves (Type Bioscreen C, Finland). Nitrocellulose filters with a pore size of 0.45 mm (Gelman Sciences, Ann Arbor, MI, USA) were pre-dried in a microwave oven at 150 W for 10 min and were subsequently weighed. To measure the cell dry weight, 10 mL of cell culture in the exponential phase was filtered, washed two times with 10 mL of distilled water, and dried at 105 °C for 24 h. The specific growth rate was determined with the change in the cell dry weight logarithm versus the time during exponential growth. Yeast biomass yield was obtained from cane molasses medium using a YEPD inoculum supplemented with 800 mg/L G418. The biomass yield was determined from the slopes of the plots of biomass dry weight versus the amount of consumed sugar during exponential growth. Results were expressed in gram (dry weight) of yeast cells per litre molasses. Experiments were conducted thrice.
Determination of leavening ability
The leavening ability of yeast cells in lean dough was based on the Chinese National Standards for yeast used in food processing. Results were expressed in milliliter of increased volume per hour per gram (dry weight) of yeast cells. Lean dough was composed of 280 g of flour, 150 mL of water, 4 g of salt, and 8 g of fresh yeast. The dough was evenly stirred for 5 min at 30 ± 0.2 °C, and placed inside a fermentograph box (Type JM451, Sweden). CO2 production was recorded for 80 min at 30 °C. Experiments were conducted thrice.
Test of exogenous addition of alpha-glucosidase to dough before leavening
Different levels (0.000, 0.006, 0.010, 0.030, 0.100 g) of exogenous alpha-glucosidase (G8820, Solarbio, China) were added to lean dough before leavening. The dough was evenly stirred for 5 min at 30 ± 0.2 °C, and placed inside a fermentograph box (Type JM451, Sweden). CO2 production was recorded for 80 min at 30 °C. Leavening ability was expressed in milliliter of increased volume per hour per gram (dry weight) of yeast cells. Experiments were conducted thrice.
Analysis of sugar consumption
For extracellular sugar measurements, high-performance liquid chromatography (HPLC) with a refractive index detector and Aminex® HPX-87H column (Bio-Rad, Hercules, CA, USA) was utilized at 65 °C with 5 mM H2SO4 as the mobile phase at a flow rate of 0.6 mL/min (Hauf et al. 2000) to analyze the sugars filtered through 0.45 μm pore size cellulose acetate filters (Millipore Corp, Danvers, MA, USA). The maltose utilization efficiency in maltose LSMLD medium was determined by the ratio of the consumed maltose in 240 min with the total maltose. The maltose utilization efficiency in glucose-maltose LSMLD medium was determined by the ratio of consumed maltose when glucose was exhausted with the total maltose. Experiments were conducted thrice.
qRT-PCR
Total cellular RNA was extracted using a yeast RNA kit (Omega, Madison, United States). Using mRNA as a template, cDNA was constructed using a Reverse Transcription System Kit (Takara, China). The abundance of mRNAs coding for MAL genes was measured by amplifying the genes using the corresponding cDNAs as PCR templates. The primers used for amplification are listed in Table 1. The expression levels of MAL61 and MAL62 were assessed by real-time quantitative PCR (qRT-PCR) using an Ultra SYBR Two-Step qRT-PCR kit with ROX (reference dye for real-time PCR; TIANGEN, China) in two LSMLD media. The expression level of Actin1 (ACT1) was used as a loading control. The primers used for amplifying target genes and reference gene ACT1 are listed in Table 1. The expression level of the targets gene in glucose-maltose LSMLD medium was normalized with respect to the expression level of ACT1. Experiments were conducted thrice.
Enzyme activity assays
Maltose permease was determined by measuring uptake of maltose as described by Houghton-Larsen and Brandt (2006).
Crude extracts were prepared according to the Salema-Oom procedure to determine enzyme activities (Salema-Oom et al. 2011). The cells were broken by pulp refiner (Type Precellys 24, Bertin, France) with sterile glass beads (0.5 mm, 1.5 g/mL) at 5500 oscillations min−1 for 16 s. Disrupted samples was centrifuged at 2500×g for 10 min at 4 °C and the supernatants were used as cell extract. Maltase activity was measured using p-nitrophenyl-alpha-D-glucopyranoside as substrate in a spectrophotometric assay. For every strain, three independent biological replicates and technical duplicates were assayed.
Analysis of genetic stability
After 5, 10, 15 and 20 times of passage in YEPD medium, the leavening ability of the transformants was measured in lean dough. Three independent experiments were performed.
Statistical analysis
Data were expressed as mean ± SD and were accompanied by the number of experiments independently performed. The differences of the transformants compared with the parental strain were confirmed by Student’s t test. Differences at P < 0.05 were considered significant differences in statistics.
Results
MAL61 and MAL62 expression levels
To confirm the genes overexpression, the mRNA was quantified. The qRT-PCR results showed that the mRNA levels of MAL61 and MAL62 in the MAL61 and MAL62 overexpression strains, respectively, were higher than the parental strain. The expression levels of the target genes were evidently increased under maltose induction. The MAL61 expression level of BYKPM1 increased by 21 and 15 % in maltose and glucose-maltose LSMLD media, respectively, compared with the parental strain (Fig. 1a). Simultaneously, the MAL62 expression level of BYKPM2 was 29 and 16 % higher than the parental strain in maltose and glucose-maltose LSMLD media, respectively (Fig. 1b). The MAL61 expression level of BYKPM1+M2 was a slight higher than the strain BYKPM1, but the MAL62 expression level of that slightly decreased compared with the strain BYKPM2 in maltose and glucose-maltose LSMLD media (Fig. 1). BYK was used as a blank control to demonstrate any possible effects of an empty vector and displayed no significant differences from the parental strain BY14. These results suggest that the mRNA expression levels of MAL61 and MAL62 were correspondingly enhanced by the control of the promoter PGK1.
Determination of MAL61 and MAL62 expression levels by real-time PCR in LSMLD medium. Fresh yeast cells were inoculated into LSMLD medium and sampled at 1 h. Total RNA was isolated and the expression of a MAL61 and b MAL62 were examined by qRT-PCR. ACT1 was used as a loading control. Data are averages from three independent experiments, and error bars represent ±SD. Significant difference of the transformants (BYKPM1 and BYKPM1+M2 for MAL61 mRNA; BYKPM2 and BYKPM1+M2 for MAL62 mRNA) from the parental strain BY14 was confirmed by Student’s t test (**P < 0.01; *P < 0.05, n = 3)
Enzyme activities
Based on the results regarding gene expression levels, the maltase and maltose permease activities were determined in two LSMLD media. The strain BYK exhibited similar maltase and maltose permease activities to the parental strain BY14. The maltose permease activity of BYKPM1 increased by the maximum of 21 and 32 % in maltose and glucose-maltose LSMLD media, respectively, and no obvious variation in its maltase activity was detected compared with the parental strain (Fig. 2). The similar increasing trend with more notable effect (maximum of 98 and 100 % increases in maltose and glucose-maltose LSMLD media, respectively, compared with the parental strain) of maltase activity was found in BYKPM2 carrying overexpression of MAL62 alone (Fig. 2). Specifically, the maltase activity in BYKPM2 was the highest, whereas its maltose permease activity was similar to that of the parental strain during the whole course. Whether maltase or maltose permease was used, the strain BYKPM1+M2 showed moderate increases of both activities, compared with BYKPM1 and BYKPM2, which demonstrated vigorous maltose permease and maltase activities, respectively. These results reflect that MAL61 and MAL62 overexpression could elevate maltose permease and maltase activities, respectively. However, the enhancement in maltose permease activity was lower than that in maltase activity.
Maltose permease and maltase activity for these five strains during cultivation in LSMLD medium. Cells were inoculated into a maltose LSMLD medium, and b glucose-maltose LSMLD medium. Cells were sampled at selected time intervals until a constant trendline was obtained. Data are averages from three independent experiments, and error bars represent ±SD
Sugar consumption in LSMLD medium
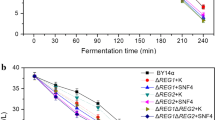
The effects of MAL61 and MAL62 overexpression on maltose metabolism were tested by measuring the trend of sugars consumption in two LSMLD media. Compared with the parental strain BY14, the three transformants (BYKPM1, BYKPM2 and BYKPM1+M2) exhibited rapid maltose utilization. The maltose utilization efficiency of the strain BYKPM1 was 10 and 7 % higher than the parental strain in maltose and glucose-maltose LSMLD media, respectively, while lower than the strain BYKPM2, which carried MAL62 overexpression alone with 16 and 39 % increases compared with the parental strain (Fig. 3). The reduction in maltose level in BYKPM2 was the greatest among all sampling stages (Fig. 3). The maltose utilization in the strain BYKPM1+M2 was slower than the strain BYKPM2, possibly because the decreased maltase and maltose permease activity. Similar results to the parental strain BY14 was found in the strain BYK. These findings demonstrate that both MAL61 and MAL62 overexpression resulted in positive effects in maltose utilization. However, single overexpression of MAL61 and co-overexpression of MAL61 and MAL62 were not as efficient as single overexpression of MAL62 in improving maltose metabolism.
Concentration of residual sugar in these five yeast strains during fermentation in LSMLD medium. Fresh yeast cells were inoculated into a maltose LSMLD medium and b glucose-maltose LSMLD medium, and sampled at selected time intervals for 4 h. Data are averages of three independent experiments and error bars represent ±SD
Growth and fermentation characteristics
To investigate the more crucial effect of MAL62 and MAL61 overexpression on maltose metabolism, we tested the growth and fermentation properties of the five strains. As illustrated in Table 2, the transformants exhibited a decreased specific growth rate compared with the parental strain, but the effects of MAL61 and MAL62 overexpression on the final biomass yield were not significant. For leavening ability, two strains (BYKPM2 and BYKPM1+M2) carrying MAL62 overexpression performed well. Compared with the parental strain BY14, the leavening ability of the strain BYKPM1 only increased from 335.4 mL/h g dw to 360.7 mL/h g dw, whereas 481.3 mL/h g dw and 418.4 mL/h g dw were observed in BYKPM2 and BYKPM1+M2, respectively (Table 2). Moreover, fresh yeast was compounded into lean dough, in which variations in the released CO2 production of the parental strain and transformants were identified. Compared with the parental strain BY14, the amounts of released CO2 of BYKPM2, BYKPM1+M2, and BYKPM1 within 50 min increased by 31, 20, and 13 %, respectively (Fig. 4). Furthermore, compared with the parental strain, the fermentation times of BYKPM2 and BYKPM1+M2 decreased by 18 %, but that for BYKPM1 only slightly changed (Fig. 4). Importantly, the leavening ability of the transformants remained stable after 20 times of passages (data not shown). Similar results of BYK to BY14 eliminated any possible effects of the transformed empty vector. These results were consistent with the results of maltose metabolism and indicate that MAL61 overexpression was less effective than MAL62 overexpression for enhancing the leavening ability of baker’s yeast in lean dough.
Effect of exogenous addition of alpha-glucosidase to dough before leavening
Exogenous addition of alpha-glucosidase to the dough was used before leavening. As shown in Table 3, the leavening ability of the parental strain was changed by the exogenous addition of alpha-glucosidase at different levels. Addition of 0.006 g of alpha-glucosidase produced better gassing ability. Within 50 min, the amounts of released CO2 by addition of 0.006 g of alpha-glucosidase increased by 13 % compared with no alpha-glucosidase addition (Fig. 5), and the leavening ability increased from 335.4 mL/h g dw to 366.7 mL/h g dw (Table 3). With the increased amount of exogenous addition of alpha-glucosidase, the CO2 production in lean dough decreased. The CO2 production evidently decreased with 0.100 g of alpha-glucosidase added. These results show that appropriate addition of alpha-glucosidase to dough before leavening was an effective method to improve leavening ability of baker’s yeast in lean dough.
Discussion
Based on the approach of plasmid introduction carrying a multi-copy of the regulatory or structural genes to test gene regulation models at the molecular levels, a similar process was performed in our work to investigate the differences between the effects of MAL61 and MAL62 overexpression on yeast maltose metabolism. This study demonstrated that maltase encoded by MAL62 was the rate-limiting factor in maltose fermentation of industrial baker’s yeast in lean dough.
In this study, the single overexpression of MAL61, single overexpression of MAL62 and co-overexpression of two genes were established in industrial baker’s yeast cells, which resulted in positive effects to maltose utilization with stable growth characteristics. Compared with the parental strain, the leavening abilities of all three overexpression strains increased. These results demonstrate that rapid maltose metabolism was significantly correlated with strong leavening ability caused by altered MALS and/or MALT genes dosage, which supported the conclusion that some modification on the MAL genes enhanced maltose utilization (Bell et al. 2001; Goldenthal et al. 1987). However, overexpression of both MAL61 and MAL62 led to different effects including changes in sugar content, gas escape and leavening ability (Figs. 3, 4; Table 2). It is probably because that the regulation of MAL61 and MAL62 mature transcripts was affected by the control of the promoter PGK1. The results were verified by the identification of MAL mRNA (Fig. 1). Regardless of sugar content, gas escape, or leavening ability, MAL62 overexpression was more effective than MAL61 overexpression. MAL62 overexpression enabled yeast cells to convert more maltose to glucose to CO2 (Table 2). Their differences were probably due to the tremendously different enzyme activities of maltase and maltose permease. This finding corresponded with the conclusion described by Goldenthal et al. (1987), who reported that the regulation of the MALS and MALT genes still exhibit some important distinctions despite the coordinated induction by maltose.
In the present study, the enhancement in maltase activity was stronger than that in maltose permease activity (Fig. 2). The significant increase in maltase activity resulted in a great increase of 44 % for the leavening ability. In the MAL62 overexpression strain, maltose permease was sufficient in transporting an adequate amount of maltose to increase maltase, and the sugar was metabolized to increase the leavening ability. However, the increase of maltose permease activity in the MAL61 overexpression strain was lower than that of maltase activity in MAL62 overexpression. Moreover, the leavening ability in the MAL61 overexpression strain only increased by 8 % compared with the parental strain. Although the increase in maltose permease facilitated maltose uptake, maltase could not immediately convert maltose to glucose to CO2, which resulted in a smaller increase in the leavening ability. Thus, maltase may be an important rate-limiting factor in maltose metabolism and leavening ability of the industrial baker’s yeast strain used in this work. This finding was consistent with that of Jiang et al. (2008) and Sun et al. (2012), who insisted that maltase is an essential enzyme in maltose metabolism for high extracellular maltose concentrations. In addition, maltase activity is inhibited by glucose (Görts 1969). With the increase of intracellular maltose and rapid conversion to glucose, the maltase activity could be inhibited by redundant glucose. The decreased maltase activity was negative to maltose utilization. Hence, co-overexpression of MAL61 and MAL62 cannot achieve the maximum of maltose metabolism and leavening ability. Overexpression of MAL62 alone was more effective than co-overexpression of MAL61 and MAL62 in maltose fermentation, which adequately suggests that increasing maltase activity is sufficient to improve maltose fermentation of industrial baker’s yeast in lean dough.
Considering the scruple for genetically engineered strains in food processing, the leavening ability by exogenous addition of alpha-glucosidase to the dough before leavening was tested in the parental strain. The appropriate addition of alpha-glucosidase to dough before leavening improved the leavening ability of baker’s yeast to some extent (Fig. 5; Table 3), which further showed the importance of maltase in dough leavening and provided a technological guidance for baking industry. However, such approach was not as effective as overexpression of MAL62 in improving the leavening ability of industrial baker’s yeast in lean dough.
As a whole, overexpression of MAL62 alone was more effective than overexpression of MAL61 alone and co-overexpression of two genes in yeast maltose fermentation. Thus, maltase was the most crucial factor as well as the rate-limiting factor in maltose fermentation of industrial baker’s yeast in lean dough. In addition, overexpression of MAL62 was better than direct exogenous addition of alpha-glucosidase to the dough before leavening in improving the leavening ability of baker’s yeast in lean dough. This study provides guidance for breeding of industrial baker’s yeast for quick leavening.
References
Alves-Araújo C, Pacheco A, Almeida MJ, Spencer-Martins I, Leão C, Sousa MJ (2007) Sugar utilization patterns and respiro-fermentative metabolism in the baker’s yeast Torulaspora delbrueckii. Microbiology 153:898–904
Bell PJ, Higgins VJ, Attfield PV (2001) Comparison of fermentative capacities of industrial baking and wild-type yeasts of the species Saccharomyces cerevisiae in different sugar media. Lett Appl Microbiol 32:224–229
Carlson M (1999) Glucose repression in yeast. Curr Opin Microbiol 2:202–207
Charron MJ, Dubin RA, Michels CA (1986) Structural and functional analysis of the MAL1 locus of Saccharomyces cerevisiae. Mol Cell Biol 6:3891–3899
Cohen JD, Goldenthal MJ, Chow T, Barbara B, Marmur J (1985) Organization of the MAL loci of Saccharomyces. Physical identification and functional characterization of three genes at the MAL6 locus. Mol Gen Genet 200:1–8
Day RE, Higgins VJ, Rogers PJ, Dawes IW (2002) Characterization of the putative maltose transporters encoded by YDL247w and YJR160c. Yeast 19:1015–1027
Dietvorst J, Blieck L, Brandt R, Van Dijck P, Steensma HY (2007) Attachment of MAL32-encoded maltase on the outside of yeast cells improves maltotriose utilization. Yeast 24:27–38
Gietz RD, Woods RA (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350:87–96
Goldenthal MJ, Vanoni M, Buchferer B, Marmur J (1987) Regulation of MAL gene expression in yeast: gene dosage effects. Mol Gen Genet 209:508–517
Görts CP (1969) Effect of glucose on the activity and the kinetics of the maltose-uptake system and of α-glucosidase in Saccharomy cescerevisiae. Biochim Biophys Acta 184:299–305
Hauf J, Zimmermann FK, Müller S (2000) Simultaneous genomic overexpression of seven glycolytic enzymes in the yeast Saccharomyces cerevisiae. Enzyme Microb Technol 26:688–698
Hazell B, Attfield P (1999) Enhancement of maltose utilisation by Saccharomyces cerevisiae in medium containing fermentable hexoses. J Ind Microbiol Biotechnol 22:627–632
Hirasawa R, Yokoigawa K (2001) Leavening ability of baker’s yeast exposed to hyperosmotic media. FEMS Microbiol Lett 194:159–162
Houghton-Larsen J, Brandt A (2006) Fermentation of high concentrations of maltose by Saccharomyces cerevisiae is limited by the COMPASS methylation complex. Appl Environ Microbiol 72:7176–7182
Hu Z, Gibson AW, Kim JH, Wojciechowicz LA, Zhang B, Michels CA (1999) Functional domain analysis of the Saccharomyces MAL-activator. Curr Genet 36:1–12
Jiang TX, Xiao DG, Gao Q (2008) Characterisation of maltose metabolism in lean dough by lagging and non-lagging baker’s yeast strains. Ann Microbiol 58:655–660
Klein CJ, Olsson L, Rønnow B, Mikkelsen JD, Nielsen J (1996) Alleviation of glucose repression of maltose metabolism by MIG1 disruption in Saccharomyces cerevisiae. Appl Environ Microbiol 62:4441–4449
Lilly M, Lambrechts MG, Pretorius IS (2000) Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl Environ Microbiol 66:744–753
Lin X, Zhang CY, Bai XW, Song HY, Xiao DG (2014) Effects of MIG1, TUP1 and SSN6 deletion on maltose metabolism and leavening ability of baker’s yeast in lean dough. Microb Cell Fact 13:93. doi:10.1186/s12934-014-0093-4
Medintz I, Jiang H, Han EK, Cui W, Michels CA (1996) Characterization of the glucose-induced inactivation of maltose permease in Saccharomyces cerevisiae. J Bacteriol 178:2245–2254
Naumov GI, Naumova ES, Michels CA (1994) Genetic variation of the repeated MAL loci in natural populations of Saccharomyces cerevisiae and Saccharomyces paradoxus. Genetics 136:803–812
Needleman R (1991) Control of maltase synthesis in yeast. Mol Microbiol 5:2079–2084
Panadero J, Randez-Gil F, Prieto JA (2005) Validation of a flour-free model dough system for throughput studies of baker’s yeast. Appl Environ Microbiol 71:1142–1147
Salema-Oom M, De Sousa HR, Assunçao M, Gonçalves P, Spencer-Martins I (2011) Derepression of a baker’s yeast strain for maltose utilization is associated with severe deregulation of HXT gene expression. J Appl Microbiol 110:364–374. doi:10.1111/j.1365-2672.2010.04895.x
Sasano Y, Haitani Y, Hashida K, Oshiro S, Shima J, Takagi H (2013) Improvement of fermentation ability under baking-associated stress conditions by altering the POG1 gene expression in baker’s yeast. Int J Food Microbiol 165:241–245. doi:10.1016/j.ijfoodmicro.2013.05.015
Srđan N, Vesna ZK, Vladimir M (2004) Regulation of maltose transport and metabolism in Saccharomyces cerevisiae. Food Technol Biotech 42:213–218
Sun X, Zhang C, Dong J, Wu M, Zhang Y, Xiao D (2012) Enhanced leavening properties of baker’s yeast overexpressing MAL62 with deletion of MIG1 in lean dough. J Ind Microbiol Biotechnol 39:1533–1539
Verstrepen KJ, Iserentant D, Malcorps P, Derdelinckx G, Van Dijck P, Winderickx J, Pretorius IS, Thevelein JM, Delvaux FR (2004) Glucose and sucrose: hazardous fast-food for industrial yeast? Trends Biotechnol 22:531–537
Wang X, Bali M, Medintz I, Michels CA (2002) Intracellular maltose is sufficient to induce MAL gene expression in Saccharomyces cerevisiae. Eukaryot Cell 1:696–703
Acknowledgments
This study was financially supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT1166), the National High Technology Research and Development Program of China (2013AA102106), the National Natural Science Foundation of China (31171730), the Major Project of Research Program on Applied Fundamentals and Advanced Technologies of Tianjin (10JCZDJC16700), and the 2015 outstanding doctoral dissertation innovation fund of Tianjin University of Science and Technology (201503).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Cui-Ying Zhang and Xue Lin have contributed equally to the article.
Rights and permissions
About this article
Cite this article
Zhang, CY., Lin, X., Song, HY. et al. Effects of MAL61 and MAL62 overexpression on maltose fermentation of baker’s yeast in lean dough. World J Microbiol Biotechnol 31, 1241–1249 (2015). https://doi.org/10.1007/s11274-015-1874-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1874-6