Abstract
Leavening ability in sweet dough is required for the commercial applications of baker’s yeast. This property depends on many factors, such as glycolytic activity, sucrase activity, and osmotolerance. This study explored the importance of sucrase level on the leavening ability of baker’s yeast in sweet dough. Furthermore, the baker’s yeast strains with varying sucrase activities were constructed by deleting SUC2, which encodes sucrase or replacing the SUC2 promoter with the VPS8/TEF1 promoter. The results verify that the sucrase activity negatively affects the leavening ability of baker’s yeast strains under high-sucrose conditions. Based on a certain level of osmotolerance, sucrase level plays a significant role in the fermentation performance of baker’s yeast, and appropriate sucrase activity is an important determinant for the leavening property of baker’s yeast in sweet dough. Therefore, modification on sucrase activity is an effective method for improving the leavening properties of baker’s yeast in sweet dough. This finding provides guidance for the breeding of industrial baker’s yeast strains for sweet dough leavening. The transformants BS1 with deleted SUC2 genetic background provided decreased sucrase activity (a decrease of 39.3 %) and exhibited enhanced leavening property (an increase of 12.4 %). Such a strain could be useful for industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Baker’s yeast (Saccharomyces cerevisiae) is a baking ingredient that is significant to leaven dough (Bell et al. 2001; Lin et al. 2014). Baker’s yeast rapidly transforms sugars into CO2, which causes the dough to rise during baking (Lin et al. 2015; Randez-Gil et al. 2013). However, many baker’s yeasts are usually exposed to various environmental stresses such as air-drying, high temperature, and high osmotic pressure, during bread making (Sasano et al. 2012a; Takagi 2008). Therefore, breeding of baker’s yeast strains that can withstand these environmental stresses and possess excellent leavening properties is critical to obtain high-quality baked products.
Sweet (high-sugar) dough provides consumers with fresh and proper-textured bread. Sweet dough generally contains up to approximately 30 % sucrose per weight of flour. High osmotic pressure caused by such a high sucrose concentration can inhibit the leavening ability of baker’s yeast (Sasano et al. 2013; Verstrepen et al. 2004). Many studies intending to improve the leavening ability of baker’s yeast are focused on enhancing the high-sucrose stress tolerance of the yeast by inducing stress proteins, accumulating stress protectants, and altering membrane composition (Randez-Gil et al. 2013; Sasano et al. 2012b; Shima and Takagi 2009). Moreover, there is report that indicated inverse relation between the activity of sucrase and leavening ability of Saccharomyces cerevisiae in high-sucrose concentration (Evans 1990; Hernandez-Lopez et al. 2003). Other studies indicated that some factors like glycerol level other than invertase levels are more influential in determining fermentation activity in high-sucrose media (Myers et al. 1997; Oda and Ouchi 1990). Hence, the importance of sucrase activity for leavening ability of baker’s yeast in sweet dough remains to be further explored.
Sucrase (EC 3.2.1.26, L-D-fructofuranosidase, invertase) is a hydrolytic enzyme that catalyzes the detachment reaction of the terminal non-reducing L-D-fructofuranoside residue in L-D-fructofuranosides. The preferred substrate of sucrase is sucrose, and it is able to hydrolyze sucrose into glucose and fructose. Sucrase is encoded by one or several SUC genes (SUC1 to SUC5 and SUC7). SUC2 is the most common locus found in almost all S. cerevisiae strains and other closely related yeast species (Carlson et al. 1985; Korshunova et al. 2005). In sweet dough, sucrose acts as the principal source of fermentable carbon during dough leavening and is hydrolyzed into glucose and fructose serving as the nutrient substance during fermentation, which are transported into the cell by hexose transporters and are metabolized through glycolysis (Badotti et al. 2008). Sucrose hydrolysis leading to the accumulation of glucose and fructose immensely intensifies the osmotic pressure around the cell (Hernandez-Lopez et al. 2003). Hence, baker’s yeast strains with lower sucrase activities are desirable for sweet dough leavening. Nevertheless, it is neglected to yield yeast strains with high leavening abilities in sweet dough via decreasing sucrase activity.
In the current study, to reveal the importance of sucrase activity on the leavening property of baker’s yeast in high-sucrose dough, the sucrase activity, high-sucrose fermentation ability, and glycerol and trehalose levels of different industrial baker’s yeasts were compared. Furthermore, baker’s yeast strains with different sucrase levels were constructed by deleting SUC2 or replacing the SUC2 promoter with the VPS8 or TEF1 promoter. Our results strongly suggest that low sucrase level is a necessary character of baker’s yeast strains for sweet dough leavening. Importantly, an improved strain BS1 that exhibits lower sucrase activity and increased leavening ability was developed. These findings lay a foundation for the optimization of industrial baker’s yeast strains for sweet dough leavening.
Materials and methods
Strains and vectors
Table 1 summarizes the genetic properties of all strains and plasmids used in this study. The yeasts ADY1, ADY2, ADY3, ADY4, ADY5, and ADY6 were purchased from the market. The wild-type strains BY14 and BY6 have been deposited in CICC (China Center of Industrial Culture Collection). The strains BY6-a, BY6-α, B-a-S, B-α-S, BS1, B-a-Vp, B-α-Vp, BS2, B-a-Tp, B-α-Tp, and BS3 have been deposited in the Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, Tianjin Industrial Microbiology Key Laboratory.
Growth, cultivation, and fermentation conditions
Recombinant DNA was amplified in Escherichia coli DH5α, which was grown at 37 °C in Luria–Bertani medium (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) supplemented with 100 mg/L ampicillin. The plasmid was obtained using a Plasmid Mini Kit II (D6945, Omega, Norcross, GA, USA).
The yeast strains were cultivated in a yeast extract peptone dextrose (YEPD) medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose) at 30 °C. After 24 h of cultivation, 20 mL of the cell culture was inoculated in 200 mL of cane molasses medium (5 g/L yeast extract, 0.5 g/L (NH4)2SO4, and 12° Brix cane molasses) at the initial OD600 = 0.4. The cells were cultivated for 24 h at 30 °C with 180 rpm rotary shaking to the final OD600 = 1.8. Cells were harvested through centrifugation (4 °C, 1500×g, 5 min) and were washed twice with sterile water at 4 °C for succeeding fermentation experiments. To investigate the glucose accumulation and sucrose consumption of the strains, we used the high-sugar model liquid dough (HSMLD) fermentation medium, which contains 250 g/L sucrose, 2.5 g/L (NH4)2SO4, 5 g/L urea, 16 g/L KH2PO4, 5 g/L Na2HPO4, 0.6 g/L MgSO4, 0.0225 g/L nicotinic acid, 0.005 g/L Ca-pantothenate, 0.0025 g/L thiamine, 0.00125 g/L pyridoxine, 0.001 g/L riboflavin, and 0.0005 g/L folic acid. All of the solid media used in this study contained 2 % agar.
To select Zeocin-resistant yeast strains, 500 mg/L Zeocin (Promega, Madison, USA) was added to the YEPD plates for yeast culture. YEPG medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L galactose) was used to induce the expression of Cre in the yeast transformants.
Plasmid construction and yeast transformation
Genomic yeast DNA was prepared from the industrial baker’s yeast strain BY6-α utilizing a yeast DNA kit (D3370-01, Omega, Norcross, GA, USA). The PCR primers used in the current work are listed in Table S1 in the Supplementary Material.
An upstream homologous fragment of the SUC2 (gene ID: 854644) gene was amplified through PCR using BY6-α genomic DNA as a template with primers As-U and As-D. A downstream homologous fragment was similarly amplified using primers Bs-U and Bs-D. The purified segments As and Bs were digested with EcoRI/KpnI and BamHI/PstI, respectively, and cloned into the pUC19 cloning vector to generate plasmid pUC-AsBs. A KpnI/BamHI KanMX cassette, which was used as the selectable marker, was amplified by PCR using pUG6 as a template with primers K-U and K-D. The cassette was cloned into plasmid pUC-AsBs to obtain the final plasmid pUC-AsKBs.
The pUC-ApKVpBp plasmid, which was used to replace the SUC2 promoter with VPS8 (gene ID: 851261) promoter in the genome, was created similar to the creation of plasmid pUC-AsKBs. The difference between the two creations was the use of fusion PCR with a mixture of VPS8 promoter sequence and the downstream homologous sequence of the SUC2 promoter for the pUC-ApKVpBp plasmid. The VPS8 promoter sequence Vp was amplified by PCR from the genomes of parental strain BY6-α using primers Vp-U and Vp-D (containing overlapping sequences used for fusion PCR). Similarly, the downstream homologous sequence Bp of the SUC2 promoter was amplified with primers Bp-U (containing overlapping sequences for fusion PCR) and Bp-D. Primers Vp-U and Bp-D were used for fusion PCR with a mixture of Vp and Bp sequences as a template. Then, the fusion PCR product was digested with BamHI/PstI and cloned into plasmid pUC19 to create plasmid pUC-VpBp. An upstream homologous sequence of the SUC2 promoter and KanMX marker gene segment were similarly amplified with primers Ap-U/Ap-D and K-U/K-D, respectively. The PCR-generated fragments were digested with EcoRI/KpnI and BamHI/PstI separately and cloned into plasmid pUC-VpBp to produce the final plasmid pUC-ApKVpBp.
Based on the aforementioned strategy, plasmid pUC-ApKTpBp was obtained by inserting the fusion PCR fragment of the TEF1 (gene ID: 856195) promoter sequence and the downstream homologous sequence of the SUC2 promoter, the upstream homologous sequence of the SUC2 promoter, and the KanMX gene into the cloning vector pUC19.
Baker’s yeast transformation was performed through the lithium acetate/PEG method (Gietz and Woods 2002). The deletion cassette As-loxP-KanMX-loxP-Bs was amplified and transformed into the a- and α-type haploids of industrial baker’s yeast BY6. The DNA fragment was integrated into the chromosome at the SUC2 locus of BY6-a and BY6-α through homologous recombination to construct SUC2-deleted strains. SUC2-deleted strains were selected using YEPD medium supplemented with 300 mg/L G418. After selection, recombinant strains were verified with primers YUs-U/YU-K-D and YD-K-U/YDs-D (Table S1 in the Supplementary Material). The replacement cassette Ap-loxP-KanMX-loxP-VPS8p-Bp was amplified and transformed into BY6-a and BY6-α to construct strains in which the expression of the SUC2 gene was controlled by the VPS8 promoter instead of its own promoter. The transformants were verified through PCR with primers YUp-U/YU-K-D and YD-K-U/YDp-D. The same procedure was used to construct strains in which the SUC2 promoter was replaced with the TEF1 promoter. The KanMX marker gene was removed from the transformants using the Cre/loxP recombination system (Güldener et al. 1996).
Construction of the recombinant diploid yeast strains
Recombinant diploid yeast strains were obtained by the hybridization of the purified a- and α-type haploid recombinants. Haploid a- and α-type cells (0.5 mL each) were mixed with 5 mL of YEPD. Yeast strains were hybridized at 30 °C with 120 rpm rotary shaking for 24 h and then transferred to KAC medium (2 g/L potassium acetate and 2 % agar) at 25 °C for 3 days to produce spores. The resulting fusants exhibiting spore formation were verified under a microscope (Olympus, Tokyo, Japan).
Determination of specific growth rate and biomass yield
After 24 h of incubation, the mixtures of cell culture and medium were mixed in a deep well plate in appropriate proportions, and the growth curve was detected using bioscreen automated growth curves (Type FP-1100-C, Oy Growth Curves Ab Ltd., Helsinki, Finland). The specific growth rate was determined with the change in the OD600 napierian logarithm versus the time during exponential growth.
Nitrocellulose filters with a pore size of 0.45 mm (Gelman Sciences, Ann Arbor, MI, USA) were pre-dried in a microwave oven at 150 W for 10 min and subsequently weighed. The cell culture (10 mL) in the exponential phase was filtered, washed twice with 10 mL of distilled water, and then dried at 105 °C for 24 h to measure the cell dry weight. The biomass yield was determined from the slopes of the plots of biomass dry weight versus the amount of consumed sugar during exponential growth. Results were expressed in gram (dry weight) of yeast cells per liter of molasses. Experiments were conducted thrice.
Assay of sucrase activity
The standard reaction mixture (10 mL) contained 2.0 mL of sodium acetate-acetic acid buffer (pH 5.2), 1.0 mL of 1 M sucrose solution, 6.5 mL of distilled water, and 0.5 mL of yeast cell suspension (0.2 g of fresh yeast added to 10 mL of distilled water). The reaction mixture was incubated for 5 min at 30 °C. Then, 1.0 mL of hydrolysate was quickly mixed with 2 mL of DNS (3,5-dinitrosalicylic acid) reagent and immediately immersed in a boiling water bath for 5 min. The reducing sugar concentration was measured at 540 nm with a spectrophotometer when the mixture was cooled to room temperature using the dinitrosalicylic acid method as previously described (Miller 1959; Vitolo and Borzani 1983). One sucrase unit (U) was defined as the amount of enzyme catalyzing the sucrose to form of 1.0 μM reducing sugars per minute under the tested conditions. The specific enzyme activity was expressed in units per gram cell dry weight. Experiments were conducted thrice.
Determination of leavening ability
The leavening ability of sweet dough of yeast cells was performed according to the Chinese National Standards (Angel Yeast Corporation 2007) for yeast used in food processing. Leavening ability was determined by milliliter of CO2 production every 2 h/g (dry weight) of yeast cells in high-sugar dough. High-sucrose dough was composed of 280 g of flour, 130 mL of water, 2.8 g of salt, 44.8 g of sucrose, and 9 g of fresh yeast. Low-sucrose dough contained 150 mL of water and 5.6 g of sucrose. The dough was evenly and quickly mixed for 5 min at 30 ± 0.2 °C and placed inside the box of a fermentograph (Type JM451, SJA, Nässjö, Sweden). CO2 production was recorded for 120 min at 30 °C. Experiments were conducted thrice.
Analysis of sugars (glucose, sucrose, and trehalose) and glycerol
Cultures were sampled at appropriate time intervals in HSMLD fermentation medium. Samples were centrifuged at 5000×g for 2 min at 4 °C. The supernatants were used to measure the extracellular glucose, sucrose, and glycerol. For measurements of intracellular glycerol and trehalose, the cells were broken by a pulp refiner (Type Precellys 24, Bertin, Montigny le Bretonneux, France) with sterile ceramic beads (0.5 mm, 3 g/mL) at 5500 oscillations/min for 16 s. Disrupted samples were centrifuged at 2500×g for 10 min at 4 °C, and the supernatants were used as cell extracts. High-performance liquid chromatography (HPLC) with a refractive index detector (Agilent 1100, Santa Clara, CA, USA) and an Ultimate XB-NH2 column (4.6 × 250 mm, 5 μm, Welch Materials, Shanghai, China) was utilized at 35 °C with the mobile phase composed of acetonitrile and water (77:23, V/V) at a flow rate of 1.0 mL/min (Agblevor et al. 2007; Bethke and Busse 2008) to analyze the sugars and glycerol filtered through cellulose acetate filters with a 0.45-μm pore size (Millipore Corp, Danvers, MA, USA).
Statistical analysis
Data were expressed as mean ± SD and were accompanied by the number of experiments independently performed. The differences between the transformants and the parental strain were confirmed by Student’s t-test. Differences at P < 0.05 were considered statistically significant.
Results
Growth and leavening property and sucrase activity of different yeast strains under different conditions
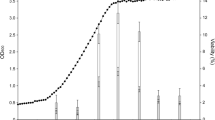
To investigate the relationship between the sucrase activity and leavening ability of baker’s yeast in sweet dough, eight industrial baker’s yeast strains were used and characterized in terms of the specific growth rate in different glucose concentrations (osmotic stress), CO2 production in different sucrose concentrations, and sucrase activity. As shown in Table S2 in the Supplementary Material, no obvious difference in specific growth rate was observed in the strains in glucose hypoosmotic medium. Nevertheless, the strains showed different specific growth rates in glucose hyperosmotic medium. Contrary to strain ADY6, the strain ADY2 displayed the best growth ability under hyperosmotic stress. The strains in low-sucrose dough exhibited strong gassing abilities without a significant difference, whereas those in high-sucrose dough demonstrated a significant difference in gassing ability. The strain BY6, in which the CO2 production could reach 857 cm3, showed the strongest high-sucrose gassing ability among the strains tested. By contrast, the strain ADY2 showed the lowest CO2 production (202 cm3) in high-sucrose dough. Parameters μ 2/μ 1 and V 2/V 1 were used to describe the tolerance and leavening ability of yeast strains under osmotic and high-sucrose conditions, respectively. The order of resistance to hyperosmotic stress was ADY2 → ADY5 → ADY4 (BY6) → ADY3 → ADY1 → BY14 → ADY6 (Fig. 1). The order of leavening ability under high-sucrose conditions was BY6 → ADY5 → ADY4 → ADY3 → ADY6 → ADY1 → BY14 → ADY2 (Fig. 1). For sucrase activity, the eight strains showed a significant difference. Importantly, the order of sucrase activity from strong to weak was contrary to that of leavening ability under high-sucrose conditions (Fig. 1).
Growth and leavening property and sucrase activity of different yeast strains under different conditions. μ 2/μ 1 and V 2/V 1 were calculated from Table S2 in the Supplementary Material. Data are average of three independent experiments and error bars represent ± SD
These results suggest that based on a certain level of osmotolerance, the sucrase activity of industrial baker’s yeast and gassing ability are negatively correlated under high-sucrose conditions.
Glucose accumulation and sucrose consumption of the strains BY6 and ADY2 in HSMLD medium
The strains ADY2 and BY6 were used to test the glucose accumulation and sucrose consumption in HSMLD medium with sucrose as the sole carbon source. When inoculated into the HSMLD medium, the strains ADY2 and BY6 quickly utilized sucrose and converted it into glucose (Fig. 2). The strain ADY2 utilized sucrose and accumulated glucose faster than the strain BY6. The maximum accumulation of glucose in the strain ADY2 was 6.99 g/100 mL, which was 23.3 % higher than that in the strain BY6. When sucrose was exhausted, these two strains fully utilized glucose at similar rates (Fig. 2).
These results indicate that higher sucrase activity allows the quicker sucrose consumption and higher glucose accumulation of baker’s yeast under high-sucrose conditions. With more glucose accumulation, the leavening ability of baker’s yeast was significantly repressed in high-sucrose dough.
Glycerol and trehalose levels of different yeast strains in HSMLD medium
Extracellular and intracellular glycerol levels and intracellular trehalose levels produced by the eight industrial baker’s yeasts in HSMLD medium were measured to assess the relative importance of these solutes for leavening ability in the tested yeast strains. As shown in Table 2, the strains exhibited similar levels of glycerol and trehalose under high-sucrose conditions, suggesting native high levels of glycerol and trehalose in the industrial baker’s yeasts.
These results suggest that based on a certain level of osmotolerance, the glycerol and trehalose levels of the tested industrial baker’s yeasts had no correlation with gassing ability under high-sucrose conditions.
Sucrase activity and leavening property of SUC2-deleted and promoter-replaced transformants
In S. cerevisiae strains, SUC2 is the most common locus encoding sucrase. To further verify the importance of sucrase activity for sweet dough leavening, baker’s yeast strains with distinct sucrase activities were constructed by deleting SUC2 or replacing the SUC2 promoter with the VPS8/TEF1 promoter. Sucrase activity was determined in living cells. Sucrase activity varied when SUC2 was deleted or its promoter was replaced (Fig. 3). The sucrase activities in the strains B-a-Vp, B-α-Vp, and BS2, in which the SUC2 promoters were replaced with the VPS8 promoter, decreased from 42.25, 39.23, and 33.12 U/g CDW to 34.25, 32.87, and 25.58 U/g CDW, respectively. These values were 18.9, 16.2, and 22.8 % lower than those of the parental strains BY6-a, BY6-α, and BY6, respectively. A similar effect, but with a higher level of reduction in sucrase activity, was observed in the SUC2-deleted mutants. Compared with the parental strains BY6-a, BY6-α, and BY6, the sucrase activities of the strains B-a-S, B-α-S, and BS1 evidently decreased by 26.8, 24.8, and 39.3 %, respectively. By contrast, the strains with the TEF1 promoter exhibited higher sucrase activities than the parental strains. The sucrase activities of the strains B-a-Tp, B-α-Tp, and BS3 increased to 67.69, 63.74, and 63.02 U/g CDW, respectively, which were 60.2, 62.5, and 81.0 % higher than those of the parental strains, respectively. These results suggest that sucrase activity can be adjusted by SUC2 expression level. Complete deletion of SUC2 could effectively decrease sucrase activity. The VPS8 promoter, which exhibits a weak promoter activity, can downregulate SUC2 expression. However, the TEF1 promoter upregulated SUC2 expression.
To investigate the effect of SUC2 deletion and promoter replacement mutations on dough leavening, we tested the CO2 production of the parental strains and transformants in sweet dough. The variation of leavening ability was contrary to that of sucrase level (Fig. 3). The leavening abilities of the strains B-a-Vp, B-α-Vp, and BS2 increased from 390, 405, and 427 mL/2 h/g CDW to 416, 427, and 462 mL/2 h/g CDW, respectively, which were 6.4, 5.7, and 8.2 % higher than those of the corresponding parental strains. The gassing abilities of the SUC2-deleted strains further increased. Compared with the parental strains, the leavening abilities of the strains B-a-S, B-α-S, and BS1 increased by 10.8, 10.4, and 12.4 %, respectively. Nevertheless, the leavening abilities of the strains with the TEF1 promoter significantly decreased. Compared with the parental strains, the leavening abilities of the strains B-a-Tp, B-α-Tp, and BS3 decreased by 11.0, 13.6, and 10.5 %, respectively. These results were contrary to those of sucrase activity, further indicating the negative correlation between sucrase activity and leavening ability in sweet dough. SUC2 deletion or control by the VPS8 promoter decreased sucrase activity, which could improve the leavening ability of baker’s yeast in sweet dough. However, the SUC2 gene controlled by the TEF1 promoter increased sucrase activity.
Glucose accumulation and sucrose consumption of the strains BY6 and BS1 in HSMLD medium
The glucose accumulation and sucrose consumption of the strain BS1, in which the sucrase activity was the lowest and the leavening ability was the strongest, were tested and compared with those of the parental strain BY6 in HSMLD medium. Compared with the parental strain BY6, the strain BS1 exhibited slower sucrose consumption and glucose accumulation (Fig. S1 in the Supplementary Material). The maximum accumulation of glucose in the parental strain BY6 was 5.67 g/100 mL, whereas that in the strain BS1 was 5.00 g/100 mL. These two strains exhibited similar utilization capacities for glucose after sucrose was exhausted (Fig. S1 in the Supplementary Material).
These results indicate that the lower sucrase activity brought about less sucrose consumption and glucose accumulation of baker’s yeast under high-sucrose conditions. Less glucose accumulation can moderately affect the leavening ability of baker’s yeast in high-sucrose dough.
Growth characteristics of SUC2-deleted and promoter-replaced VPS8 transformants
Growth performance is important for industrial strains applied in fermentation (Randez-Gil et al. 1999). High dough leavening ability and stable growth property are required for baker’s yeast used in the baking industry. We investigated the basic growth characteristics (specific growth rate and biomass yield) of transformants with modified SUC2 level and enhanced sweet dough leavening ability. Table 3 shows that the specific growth rate comparatively remained stable (small difference with no statistical significance). The biomass yield was affected by the matched type, but no significant difference was observed between the transformants and the parental strains of identical matched type.
These results indicate that SUC2 deletion or promoter replacement exerts no influence on the growth properties of baker’s yeast under the tested condition.
Discussion
Leavening ability in sweet dough is one of the properties required for baker’s yeast for commercial applications (Attfield 1997). This property depends on many factors, such as glycolytic activity, sucrase activity, and osmotolerance (Tokashiki et al. 2011). In this study, we explored the importance of sucrase level for the leavening ability of baker’s yeast in sweet dough. The results verify that sucrase activity negatively affects the leavening ability of baker’s yeast strain under high-sucrose conditions, suggesting the importance of appropriate sucrase activity for sweet dough leavening.
The industrial baker’s yeast strains used in this work exhibited a certain level of osmotolerance. The sucrase activities of the baker’s yeasts negatively correlated with the gassing ability under high-sucrose conditions. The strains with higher sucrase activities showed a low efficiency in sweet dough fermentation. Conversely, the strains with low sucrase activity displayed a strong leavening ability. The relationship between sucrase activity and leavening ability provided here supports the reported inverse relationship between the sucrase activity and sweet dough leavening ability of baker’s yeast (Evans 1990). Industrial baker’s yeast strains with osmotolerance and low sucrase activities are necessary to enhance leavening ability in sweet dough. Osmotic stress in high-sucrose dough is mainly caused by glucose accumulation via sucrase hydrolysis. Low sucrase activity could result in the poor accumulation of glucose and high leavening ability in sweet dough. Thus, sucrase level is important for the leavening ability of baker’s yeast in sweet dough. Myers et al. (1997) showed that factors other than invertase levels, most likely glycerol production and retention, are more influential in determining fermentation activity in high-sucrose condition. To confirm the importance of sucrase level, the glycerol and trehalose levels of the eight industrial baker’s yeasts were assayed in HSMLD medium. The results shown in Table 2 suggest that the industrial baker’s yeasts used in this work naturally possess high levels of glycerol and trehalose under high-sucrose conditions. These features are the main causes of the inconformity of the present findings with the results of Myers et al. (1997). Thus, based on a certain level of osmotolerance, sucrase level is an influential determinant of baker’s yeast in sweet dough fermentation. Furthermore, modification on sucrase activity might be an effective method to further improve the leavening characteristics of baker’s yeast in sweet dough.
Promoter replacement can moderately control gene expression compared with gene knock out and strong overexpression of the target gene in the genome (Nevoigt et al. 2006). Replacing the promoter of a gene in the genome with another promoter whose expression can be controlled easily can overcome problems associated with the expression of the same gene from a promoter on a plasmid (Verstrepen and Thevelein 2004). Therefore, the SUC2 promoter was replaced by the VPS8 and TEF1 promoters in the genome to achieve controlled (weak and strong) expression of the SUC2 gene, which could enable various sucrase levels to investigate the importance of sucrase activity and obtain transformants with excellent fermentation performance in sweet dough. Complete deletion of SUC2 and replacement of the SUC2 promoter with VPS8 effectively decreased the sucrase activity and enhanced the leavening ability of baker’s yeast in sweet dough. These results provide ideas for breeding industrial baker’s yeast strains for sweet dough leavening. Deletion of SUC2 owning the lowest sucrase activity could acquire the maximum of sweet dough leavening ability, which corresponded with the view described by Hernandez-Lopez et al. (2003), who believed that baker’s yeast strains with low sucrase activity has an advantage in high-sugar dough fermentation. S. cerevisiae has six unlinked loci for invertase structural genes: SUC1-SUC5 and SUC7. These genes are similar in structure and expression but not identical (Hohmann and Zimmermann 1986). The detected sucrase activity in the SUC2-deleted mutants could originate from other SUC genes encoding sucrase other than SUC2 (Carlson et al. 1981; Naumov et al. 1996). Therefore, the leavening ability of industrial baker’s yeast may be enhanced by deletion of other SUC genes under high-sugar conditions. Deletion of SUC2 and replacement of the SUC2 promoter did not affect the growth characteristics of baker’s yeast in the tested condition (Table 3). These characteristics are consistent with the required properties of baker’s yeast strains for sweet dough leavening (Evans 1990).
Based on a certain level of osmotolerance, sucrase level plays a relative significant role in the fermentation performance of baker’s yeast, and appropriate sucrase activity is an important determinant for the leavening property of baker’s yeast in sweet dough. Modification on sucrase activty is an effective method for improving the leavening properties of baker’s yeast in sweet dough, which thus provides guidance for the breeding of industrial baker’s yeast strains for high sweet dough leavening. Importantly, baker’s yeast BS1 obtained in this study featuring a deleted SUC2 genetic background that provides lower sucrase activity is sufficient to enable the strain to ferment sucrose and enhance its leavening property. Such a strain could be useful for industrial applications.
References
Agblevor FA, Hames BR, Schell D, Chum HL (2007) Analysis of biomass sugars using a novel HPLC method. Appl Biochem Biotechnol 136:309–326
Angel Yeast Corporation (2007) Yeast used for food processing. Chinese National Standard GB/T 20886–2007
Attfield PV (1997) Stress tolerance: the key to effective strains of industrial baker’s yeast. Nat Biotechnol 15:1351–1357
Badotti F, Dário MG, Alves SL Jr, Cordioli ML, Miletti LC, de Araujo PS, Stambuk BU (2008) Switching the mode of sucrose utilization by Saccharomyces cerevisiae. Microb Cell Factories 7:4. doi:10.1186/1475-2859-7-4
Bell PJ, Higgins VJ, Attfield PV (2001) Comparison of fermentative capacities of industrial baking and wild-type yeasts of the species Saccharomyces cerevisiae in different sugar media. Lett Appl Microbiol 32:224–229
Bethke PC, Busse JC (2008) Validation of a simple, colorimetric, microplate assay using amplex red for the determination of glucose and sucrose in potato tubers and other vegetables. Am J Pot Res 85:414–421
Carlson M, Osmond BC, Botstein D (1981) Mutants of yeast defective in sucrose utilization. Genetics 98:25–40
Carlson M, Celenza JL, Eng FJ (1985) Evolution of the dispersed SUC gene family of Saccharomyces by rearrangements of chromosome telomeres. Mol Cell Biol 5:2894–2902
Evans IH (1990) Yeast strains for baking: recent developments. In: Spencer JFT, Spencer DM (eds) Yeast technology. Springer, Germany, pp. 13–54
Gietz RD, Woods RA (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350:87–96
Güldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH (1996) A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res 24:2519–2524
Güldener U, Heinisch J, Köehler GJ, Voss D, Hegemann JH (2002) A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res 30:e23
Hernandez-Lopez MJ, Prieto JA, Randez-Gil F (2003) Osmotolerance and leavening ability in sweet and frozen sweet dough. Comparative Analysis between Torulaspora delbrueckii and Saccharomyces cerevisiae baker’s yeast strains. Antonie Van Leeuwenhoek 84:125–134
Hohmann S, Zimmermann FK (1986) Cloning and expression on a multicopy vector of five invertase genes of Saccharomyces cerevisiae. Curr Genet 11:217–225
Jiang TX, Xiao DG, Gao Q (2008) Characterisation of maltose metabolism in lean dough by lagging and non-lagging baker’s yeast strains. Ann Microbiol 58:655–660
Korshunova IV, Naumova ES, Naumov GI (2005) Comparative molecular-genetic analysis of the beta-fructosidases in yeast Saccharomyces. Mol Biol 39:413–419
Lin X, Zhang CY, Bai XW, Song HY, Xiao DG (2014) Effects of MIG1, TUP1 and SSN6 deletion on maltose metabolism and leavening ability of baker’s yeast in lean dough. Microb Cell Factories 13:93. doi:10.1186/s12934-014-0093-4
Lin X, Zhang CY, Bai XW, Xiao DG (2015) Enhanced leavening ability of baker’s yeast by overexpression of SNR84 with PGM2 deletion. J Ind Microbiol Biotechnol 42:939–948. doi:10.1007/s10295-015-1618-5
Lu J, Dong J, Wu D, Chen Y, Guo X, Shi Y, Sun X, Xiao D (2012) Construction of recombinant industrial brewer’s yeast with lower diacetyl production and proteinase A activity. Eur Food Res Technol 235:951–961
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Myers DK, Lawlor DT, Attfield PV (1997) Influence of invertase activity and glycerol synthesis and retention on fermentation of media with a high sugar concentration by Saccharomyces cerevisiae. Appl Environ Microbiol 63:145–150
Naumov GI, Naumova ES, Sancho ED, Korhola MP (1996) Polymeric SUC genes in natural populations of Saccharomyces cerevisiae. FEMS Microbiol Lett 135:31–35
Nevoigt E, Kohnke J, Fischer CR, Alper H, Stahl U, Stephanopoulos G (2006) Engineering of promoter replacement cassettes for fine-tuning of gene expression in Saccharomyces cerevisiae. Appl Environ Microbiol 72:5266–5273
Oda Y, Ouchi K (1990) Effect of invertase activity on the leavening ability of yeast in sweet dough. Food Microbiol 7:241–248
Randez-Gil F, Sanz P, Prieto JA (1999) Engineering baker’s yeast: room for improvement. Trends Biotechnol 17:237–244
Randez-Gil F, Córcoles-Sáez I, Prieto JA (2013) Genetic and phenotypic characteristics of baker’s yeast: relevance to baking. Annu Rev Food Sci Technol 4:191–214. doi:10.1146/annurev-food-030212-182609
Sasano Y, Haitani Y, Hashida K, Ohtsu I, Shima J, Takagi H (2012a) Enhancement of the proline and nitric oxide synthetic pathway improves fermentation ability under multiple baking-associated stress conditions in industrial baker’s yeast. Microb Cell Factories 11:40. doi:10.1186/1475-2859-11-40
Sasano Y, Haitani Y, Ohtsu I, Shima J, Takagi H (2012b) Proline accumulation in baker’s yeast enhances high-sucrose stress tolerance and fermentation ability in sweet dough. Int J Food Microbiol 152:40–43. doi:10.1016/j.ijfoodmicro.2011.10.004
Sasano Y, Haitani Y, Hashida K, Oshiro S, Shima J, Takagi H (2013) Improvement of fermentation ability under baking-associated stress conditions by altering the POG1 gene expression in baker’s yeast. Int J Food Microbiol 165:241–245. doi:10.1016/j.ijfoodmicro.05.015
Shima J, Takagi H (2009) Stress-tolerance of baker’s-yeast (Saccharomyces cerevisiae) cells: stress-protective molecules and genes involved in stress tolerance. Biotechnol Appl Biochem 53:155–164. doi:10.1042/BA20090029
Takagi H (2008) Proline as a stress protectant in yeast: physiological functions, metabolic regulations, and biotechnological applications. Appl Microbiol Biotechnol 81:211–213. doi:10.1007/s00253-008-1698-5
Tokashiki T, Yamamoto H, Watanabe H, Nakajima R, Shima J (2011) A functional compound contained in sugar cane molasses enhances the fermentation ability of baker’s yeast in high-sugar dough. J Gen Appl Microbiol 57:303–307
Verstrepen KJ, Thevelein JM (2004) Controlled expression of homologous genes by genomic promoter replacement in the yeast Saccharomyces cerevisiae. Methods Mol Biol 267:259–266
Verstrepen KJ, Iserentant D, Malcorps P, Derdelinckx G, Van Dijck P, Winderickx J, Pretorius IS, Thevelein JM, Delvaux FR (2004) Glucose and sucrose: hazardous fast-food for industrial yeast? Trends Biotechnol 22:531–537
Vitolo M, Borzani W (1983) Measurement of invertase activity of cells of Saccharomyces cerevisiae. Anal Biochem 130:469–470
Acknowledgments
The current study was financially supported by the National Natural Science Foundation of China (31571809) and the National High Technology Research and Development Program of China (2013AA102106).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical statement
This manuscript is in compliance with ethical standards. This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Cui-Ying Zhang and Xue Lin are the co-first authors in this study.
Electronic Supplementary Material
ESM 1
(PDF 301 kb)
Rights and permissions
About this article
Cite this article
Zhang, CY., Lin, X., Feng, B. et al. Enhanced leavening properties of baker’s yeast by reducing sucrase activity in sweet dough. Appl Microbiol Biotechnol 100, 6375–6383 (2016). https://doi.org/10.1007/s00253-016-7449-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7449-0