Abstract
The effect of Tween 80 on the fermentative production of polymalic acid (PMA) and pullulan using Aureobasidium pullulans CCTCC M2012223 was investigated. Tween 80 is beneficial for the biosynthesis of PMA and pullulan, and can regulate the ratio of PMA to pullulan in a dose-dependent manner. After adding 0.05 % Tween 80 to the media, the maximal PMA and pullulan production was 46.45 and 28.8 g/L at 60 h in a 5 L fermenter, with an increase of 75.08 and 27.21 % when compared to the control. Tween 80 could regulate and enhance oxygen uptake rate and carbon dioxide evolution rate in the early phase of fermentation, and change the cell morphology. The transcription levels of mitochondrial dicarboxylate transporter and transmembrane transporter were also dramatically upregulated. The present work will be helpful in deeply understanding the mechanism of Tween 80 on the effect of PMA and pullulan production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polymalic acid (PMA) is a water-soluble polyester with many attractive properties, including biocompatibility, degradability, and water solubility. Its monomer, l-malic acid, is widely used in the food industry and is also a potential C4 platform chemical. PMA has been increasingly used as a drug carrier in the past few years, which can be applied to make various compression-molded pellets, films, microparticles, and nanoparticles for drug delivery (Ding et al. 2011; Lanz-Landazuri et al. 2011, 2012). Pullulan is a linear water-soluble extracellular glucose homopolysaccharide. Owing to its unique linkage pattern with a-1,6-linked maltotriose(Chi et al. 2009), pullulan exhibits distinct physical properties, such as adhesive ability, thin biodegradable films, and the capacity to form fibers. To date, pullulan has long been widely used in various fields such as food, adhesives, and cosmetic additives as well as in flocculants (Cheng et al. 2011; Dionisio et al. 2013; Thomsen et al. 2011).
Recently, the production of PMA and pullulan has attracted wide attention because of their expanding application in the pharmaceutical and food industry (Prajapati et al. 2013; Zou et al. 2013). Aureobasidium pullulans, a cosmopolitan yeast-like fungus, is the mainly used microorganism for PMA and pullulan production. Most existing literatures on the production of PMA and pullulan have focused exclusively on only one of the products (Manitchotpisit et al. 2012; Yu et al. 2012; Zan and Zou 2013). However, PMA and pullulan can be produced simultaneously by A.pullulans fermentation due to their tightly interrelated metabolic pathway (Chi et al. 2009; Zhang et al. 2011). In our previous work, a high PMA yield strain, A.pullulans CCTCC 2012223, was isolated and employed to produce PMA, which was also accompanied by the accumulation of pullulan (Zan and Zou 2013).
Tween 80 is a non-ionic surfactant and is known as polyethylene glycol sorbitan monooleate. It has been shown to be an effective stimulatory agent in the production of useful metabolites, including polysaccharides and enzymes, in some bacteria, fungi, and medicinal mushrooms (Liu and Wu 2012; Silva et al. 2007; Zhang and Cheung 2011). It was reported that Tween 80 could enhance pullulan production in A. pullulans (Sheng et al. 2013), however, the effect of Tween 80 on the biosynthesis of PMA was unclear, and the regulation of Tween 80 on the metabolic flux between PMA and pullulan was also no known. In this study, we performed detailed research on the role of Tween 80 in the co-production of PMA and pullulan with A. pullulans CCTCC M2012223. The underlying mechanism of action of Tween 80 on PMA and pullulan production was discussed with regard to the cell physiological response and gene transcription level analyses.
Materials and methods
Microorganism and culture conditions
The strain A. pullulans CCTCC M2012223 was isolated by our laboratory and can be obtained from the China Center for Type Culture Collection (Wuhan, China). PDA agar slants were inoculated with cells and incubated at 25 °C for 2 days, and then used for seed culture inoculation. For seed culture, the medium composition included (g/L): glucose 60, NH4NO3 2, KH2PO4 0.1, MgSO4 0.1, ZnSO4 0.1, KCl 0.5, CaCO3 20 and corn steep liquor 1. The seed culture was grown in 500 mL shake flask containing 50 mL of liquid medium and incubated at 25 °C on a rotary shaker (220 rpm) for 2 days. The fermentation medium composition included (g/L): glucose 90, NH4NO3 2, KH2PO4 0.1, MgSO4 0.1, ZnSO4 0.1, KCl 0.5, citric acid 5, and CaCO3 30. The fermentation cultivation was inoculated with 10 % (v/v) of the above seed culture medium and kept at 25 °C and 220 rpm for 4 days.
Shake-flask fermentation
To evaluate the effect of Tween 80 on the PMA and pullulan production, the different concentration of Tween 80 from 0.05 to 2 % (v/v) was fed into 500 ml shake flask containing 50 mL of fermentation media, respectively. The above seed culture (10 %, v/v) was inoculated, and the fermentation cultivation was then operated at 25 °C and 220 rpm for 4 days.
Batch fermentation in 5 L stirred-tank fermenter
Batch fermentation kinetics was studied in a 5 L stirred tank fermenter (Shanghai Baoxing Co. Ltd, China) containing 3 L of the medium with addition of 0.05 % and 2 % Tween 80, respectively. Unless otherwise noted, the fermentation was inoculated with 300 mL of seed culture grown in a shake flask for 48 h, and operated at 25 °C with agitation at 400–800 rpm and aeration at 1.3 vvm. The exhaust gas was tested by Hartman PS6000 gas analyzer (PS 6000, Hartman, China) for the calculation of carbon dioxide evolution rate (CER), oxygen uptake rate (OUR) and respiration quotient (RQ) (Zou et al. 2009). At the end of fermentation, the cells were taken out and washed with phosphate buffer saline (PBS) liquor (pH 7.0) for cell morphology assay.
Analytical methods
Cell biomass and residual sugar
The cell density was determined by cell dry weight (DCW) method. Before the measurement, excess CaCO3 in the broth was eliminated with the addition of 1 M HCl. The cell suspension was centrifuged at 4,000×g and then overnight drying at 105 °C. The fermentation broth was centrifuged for residual sugar analyse by the dinitrosalicylic acid assay method (Miller 1959).
Assay of PMA and pullulan production
For analysis of PMA, the fermentation broth was centrifuged and then 1 mL of resulted supernatant was mixed with 1 mL 2 M H2SO4 and incubated at 85 °C for 8 h. After neutralization of the solution, the hydrolyzed sample was analyzed by HPLC (Hitachi L-2000, Japan) for its content of malic acid, using a Spursil C18-EP organic acid column eluted with 5 mM H2SO4 at 40 °C and the flow rate of 0.6 mL/min (Zan and Zou 2013). For analysis of pullulan, pullulan was precipitated from the supernatant by adding one volumes of ethanol and maintaining the supernatant at 4 °C for 12 h. The precipitate was centrifuged at 8,000×g for 20 min and dried at 80 °C overnight and then weighed (Sheng et al. 2013).
Intracellular ATP/ADP and NADH/NAD+ ratio
Intracellular ATP/ADP was measured by ATP/ADP ratio assay kit (ELDT-100, Bioassay Systems, USA), based on ATP reaction with D-luciferin to product light (Kimmich et al. 1975). Intracellular NADH/NAD+ was measured by NADH/NAD+ assay kit (E2ND-100, Bioassay Systems, USA), based on lactate dehydrogenase cycling reaction(Zhao et al. 1987).
Real time PCR
To explore the gene transcription levels, several genes that have been sequenced in our previous work were studied by real time PCR. Annotation and sequence of these genes were listed in supplement file Table S1. Total RNA from A.pullulan was extracted using Trizol Reagent (Ambion, USA), and got cDNA using reverse transcriptase (Vazyme, USA). Primers of five genes are show in Table 1. The qRT-RCR was performed according to Sybr Green method (qPCR Master Mix, TaKaRa, Japan) using fluorescence quantitative PCR (Roche, USA).
Results
Effects of Tween 80 on PMA and pullulan production in shake flask
We investigated the effects of different Tween 80 concentrations on PMA and pullulan production in shake flasks, as shown in Table 2. Compared to the control, the ratio of PMA to pullulan was clearly affected by adding different concentrations of Tween 80. Tween 80 at low concentrations was beneifical for PMA biosynthesis, but at high concentrations, pullulan biosynthesis was favored. After adding 0.05 % Tween 80 in the media, PMA production was 25.69 ± 0.89 g/L, which was an increase of 17.03 % compared to the control (21.95 ± 1.75 g/L). Pullulan production, on the other hand, was 13.99 ± 1.89 g/L, which a decrease of 39.28 % compared to the control (23.04 ± 1.54 g/L). Moreover, after adding 2 % Tween 80 in the media, pullulan production was 28.32 ± 1.61 g/L, which was 22.94 % higher than that of the control, and the PMA production reached 19.83 ± 0.69 g/L, and was slightly reduced compared to the control. These results indicated that Tween 80 was not only acted as a surfactant, but also might play an important role as a chemical stimulatory agent that regulated PMA and pullulan biosynthesis.
Effects of Tween 80 on PMA and pullulan production in 5 L fermenter
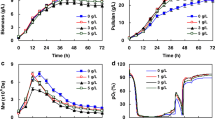
Based on the results from the shake flask, the effect of 0.05 and 2 % Tween 80 on the PMA and pullulan production was further investigated in a 5 L stirred-tank fermenter as shown in Fig. 1. After inoculation, cell growth and the rate of sugar consumption with the addition of Tween 80 was demonstrably faster than that in case of the control. Correspondingly, the rate of biosynthesis of PMA and pullulan was much higher than that of the control. The maximal PMA and pullulan production at 0.05 % Tween 80 was 46.45 and 28.8 g/L at 60 h, respectively, which was an increase of 75.08 and 27.21 % when compared to the control (26.53 and 22.64 g/L at 60 h, respectively). Moreover, at 2 % Tween 80, the highest PMA and pullulan production was 42.58 and 29.8 g/L at 60 h, respectively, which was an increase of 60.5 and 31.63 % when compared to the control. These results are different from those obtained from the shake flask, which may be explained by the better oxygen supplying conditions present in the fermenter. Therefore, these results indicated that Tween 80 was an effective enhancer of PMA and pullulan the biosynthesis.
To further investigate, we looked at on-line physiological parameters, such as OUR, CER and respiratory quotient (RQ). As shown in Fig. 2, adding Tween 80 enhanced the level of OUR and CER, especially in the early phase of fermentation (before 40 h). On the other hand, although the agitation speed under different Tween 80 concentration was gradually enhanced at the later phase of fermentation, the dissolved oxygen (DO) concentration was clearly lower than that of the control because of an increase in the apparent viscosity of the fermentation broth (data no shown). The effect of Tween 80 on cell morphology is shown in Fig. 3. Compared to the control, the cells appeared to be looser and were bigger after adding Tween 80, which might contribute to cell surface increases in substrate-uptake efficiency and metabolite excretion rates.
Figure 4 shows the variation in the ratios of ATP/ADP and NADH/NAD+ under 0.05 and 2 % Tween 80 concentrations. It was clear that the ratio of NADH/NAD+ at 0.05 % Tween 80 remained at relatively high levels compared to the control, which should generate more ATP necessary for cell metabolism through oxidative phosphorylation. However, the ratio of NADH/NAD+ at 2 % Tween 80 concentration was lower than that of the control. The ratio of ATP/ADP was also lower, which might be attributed to the insufficient oxidative phosphorylation because of the low oxygen transfer coefficient.
Tween 80 induced transcription of the PMA and pullulan biosynthetic genes
Based on our previous transcriptome analysis of A. pullulans CCMCC 2012223 (unpublished data), five genes: CL294, CL989, Unigene9513, Unigene38684, and Unigene39091 were analyzed by bioinformatics and identified as citrate synthase, UDP-glucose pyrophosphorylase, transmembrane transporter, fatty acid synthase and dicarboxylate transporter, respectively. These gene sequences and annotations are shown in supplemental file Table S1. Transcription levels of CL294, CL989, Unigene 9513, Unigene 38684, and Unigene 39091 were examined in the control and Tween 80-induced cell cultures at 36 and 48 h as shown in Fig. 5. Citrate synthase is one of the rate-limiting enzymes of the TCA cycle, and catalyzes the first committed step that is the fusion of a carboncarbon bond between oxaloacetate and acetyl CoA, which is regulated by NADH and ATP level. It was shown that CL294 expression level at 2 % Tween 80 increased from 36 to 48 h, but the expression level of CL294 at 0.05 % Tween 80 decreased from 36 to 48 h. In this stage, the DO concentration at 0.05 % Tween 80 was lower than that at 2 % Tween 80. It was indicated that the activity of citrate synthase was regulated by insufficient respiratory metabolism due to the low DO concentration.
As shown in Fig. 5, CL989 (UDP-glucose pyrophosphorylase) expression levels at 0.05 and 2 % Tween 80 increased by 6- and 3.04-fold compared to the control at 36 h, respectively, and increased by 2.72-fold at 0.05 % Tween 80 at 48 h. This results indicated that the flux of pullulan biosynthesis was enhanced. The Unigene 38684 expression level at the 0.05 and 2 % Tween 80 concentration decreased by 0.3- and 0.81-fold at 36 h, and increased by 2.55-fold with 2 % Tween 80 at 48 h. These results showed that Tween 80 had a different effect on cell membrane structure.
The expression level of mitochondrial dicarboxylate transporter (Unigene 39091) and transmembrane transporter (Unigene 9513) were studied as shown in Fig. 5. The expression level of Unigene 39091 at 0.05 % Tween 80 was much higher than that of 2 % Tween 80 at 36 and 48 h, which was 13.96- and 14.07-fold increase compared to the control at 36 and 48 h, respectively. These results showed that malic acid might accumulate easily in the cytoplasm due to transporter gene upregulation, which would contribute to PMA biosynthesis. Similarly, the expression level of Unigene 9513 at 0.05 and 2 % Tween 80 increased significantly at 36 and 48 h compared to the control. These results suggested that the transmembrane transporter, Unigene 9513, might be favorable for the extraction of PMA and pullulan intracellularly to the culture broth.
Discussion
Although A. pullulans produces and secretes PMA and pullulan, these two metabolites have different molecular weight distributions (Cheng et al. 2011; Leathers and Manitchotpisit 2013). Tween 80 can affect fungal metabolism that is associated with cell structure integrity and transport activity across the mycelia membrane (Zhang and Cheung 2011). In this study, we showed that Tween 80 acted as an effective stimulatory agent for PMA and pullulan biosynthesis. In a 5 L fermenter, the maximal PMA and pullulan production at 0.05 % Tween 80 was 46.45 and 28.8 g/L at 60 h, respectively, which was an increase of 75.08 and 27.21 % when compared to the control (26.53 and 22.64 g/L at 60 h, respectively).
As shown in Fig. 2, OUR and CER characterized the activity of the microbial metabolism, which was enhanced by Tween 80 in the early phase of fermentation (before 40 h). Moreover, Compared to the control, the cells appeared to be looser and were bigger after adding Tween 80, which might contribute to single cell surface increases in substrate-uptake efficiency and metabolite excretion rates. It was reported that larger cells increased cell-surface contact with substrate, thus enhancing nutrition ingestion, which would lead to pullulan production (Li et al. 2009). In the cultivation of medicinal fungus Cordyceps sinensis Cs-HK1, the effects of Tween 80 on mycelia morphology might be attributed in part to its surface-active properties, lowering the mycelium-liquid interfacial tension and thus the potential or tendency of mycelia to form aggregates (Liu and Wu 2012).
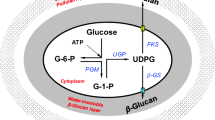
It is known that the PMA, which is biosynthesized from malic acid through the TCA cycle, and that pullulan, which originates from glucose units, need the presence of three enzymes: α-phosphoglucose mutase, UDPG-pyrophosphorylase, and glucosyltransferase (Cheng et al. 2011; Ruodong et al. 2012). CL989 (UDP-glucose pyrophosphorylase), reported to be the key enzyme in pullulan biosynthesis, could catalyze glucose-1-phosphate from the glycolysis pathway to form UDP-glucose (Duan et al. 2008). CL989 expression levels at 0.05 and 2 % Tween 80 increased by 6- and 3.04-fold compared to the control at 36 h, respectively, and increased by 2.72-fold at 0.05 % Tween 80 at 48 h. The results indicated that the flux of pullulan biosynthesis pathway was strengthened by Tween 80 induction.
Tween 80 is a non-ionic surfactant which can upset the cytomembrane, and it was reported that the fatty acid synthase subunit alpha protein could be upregulated in P. tuber-regium by Tween 80 to promote the synthesis of long-chain fatty acids and their incorporation into the mycelial cell membranes, increasing the membrane permeability (Zhang et al. 2012). Zhang and Cheung (2011) also reported that Tween 80 could affect fungal metabolism, which is associated with the cell structure stability and transport activity across the mycelia membrane. The expression level of Unigene 9513 (transmembrane transporter) at 0.05 and 2 % Tween 80 increased significantly at 36 and 48 h compared to the control. These results suggested that Unigene 9513 might be favorable for the extraction of PMA and pullulan intracellularly to the culture broth.
Malic acid in mitochondria can be transported to cytoplasm (Huypens et al. 2011; Sousa et al. 1992; Valentini et al. 2011). Transcription levels of Unigene 39091 (mitochondrial dicarboxylate transporter) was upregulated dramatically by Tween 80 induction. These results showed that malic acid might accumulate easily in the cytoplasm due to transporter gene upregulation, which would contribute to PMA biosynthesis. The stimulating effect of Tween 80 on PMA and pullulan was based on its putative function as a chemical stimulatory agent, which could affect the cell growth, cell membrane structure and cell morphology, and further induced the level of energy supply. Moreover, Tween 80 could interact with the biosynthesis pathway and transmembrane transport at the gene transcription level, thereby facilitating the release of PMA and pullulan into the extracellular medium. The presented work will be helpful for further understanding of the mechanism by which Tween 80 enhances PMA and pullulan production.
References
Cheng KC, Demirci A, Catchmark JM (2011) Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol 92(1):29–44
Chi Z, Wang F, Chi Z, Yue L, Liu G, Zhang T (2009) Bioproducts from Aureobasidium pullulans, a biotechnologically important yeast. Appl Microbiol Biotechnol 82(5):793–804
Ding H, Portilla-Arias J, Patil R, Black KL, Ljubimova JY, Holler E (2011) The optimization of polymalic acid peptide copolymers for endosomolytic drug delivery. Biomaterials 32(22):5269–5278
Dionisio M, Cordeiro C, Remunan-Lopez C, Seijo B, Rosa da Costa AM, Grenha A (2013) Pullulan-based nanoparticles as carriers for transmucosal protein delivery. Eur J Pharm Sci 50(1):102–113
Duan X, Chi Z, Wang L, Wang X (2008) Influence of different sugars on pullulan production and activities of α-phosphoglucose mutase, UDPG-pyrophosphorylase and glucosyltransferase involved in pullulan synthesis in Aureobasidium pullulans Y68. Carbohydr Polym 73(4):587–593
Huypens P, Pillai R, Sheinin T, Schaefer S, Huang M, Odegaard ML, Ronnebaum SM, Wettig SD, Joseph JW (2011) The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia 54(1):135–145
Kimmich GA, Randles J, Brand JS (1975) Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem 69(1):187–206
Lanz-Landazuri A, Garcia-Alvarez M, Portilla-Arias J, de Ilarduya AM, Patil R, Holler E, Ljubimova JY, Munoz-Guerra S (2011) Poly(methyl malate) nanoparticles: formation, degradation, and encapsulation of anticancer drugs. Macromol Biosci 11(10):1370–1377
Lanz-Landazuri A, Garcia-Alvarez M, Portilla-Arias J, De Ilarduya AM, Holler E, Ljubimova J, Munoz-Guerra S (2012) Modification of microbial polymalic acid with hydrophobic amino acids for drug-releasing nanoparticles. Macromol Chem Phys 213(15):1623–1631
Leathers TD, Manitchotpisit P (2013) Production of poly(beta-l-malic acid) (PMA) from agricultural biomass substrates by Aureobasidium pullulans. Biotechnol Lett 35(1):83–89
Li BX, Zhang N, Peng Q, Yin T, Guan FF, Wang GL, Li Y (2009) Production of pigment-free pullulan by swollen cell in Aureobasidium pullulans NG which cell differentiation was affected by pH and nutrition. Appl Microbiol Biotechnol 84(2):293–300
Liu YS, Wu JY (2012) Effects of Tween 80 and pH on mycelial pellets and exopolysaccharide production in liquid culture of a medicinal fungus. J Ind Microbiol Biotechnol 39(4):623–628
Manitchotpisit P, Skory CD, Peterson SW, Price NP, Vermillion KE, Leathers TD (2012) Poly(beta-l-malic acid) production by diverse phylogenetic clades of Aureobasidium pullulans. J Ind Microbiol Biotechnol 39(1):125–132
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Prajapati VD, Jani GK, Khanda SM (2013) Pullulan: an exopolysaccharide and its various applications. Carbohydr Polym 95(1):540–549
Ruodong C, Hao W, Hua Z, Yuanyuan C, Ruoping H, Ping W (2012) Investigation of poly(β-malic acid) synthesis pathways and regulation by strains of Aureobasidium pulluans. CIESC J 63(11):3639–3644
Sheng L, Zhu G, Tong Q (2013) Mechanism study of Tween 80 enhancing the pullulan production by Aureobasidium pullulans. Carbohydr Polym 97(1):121–123
Silva CC, Dekker RFH, Silva RSSF, da Silva MDLC, Barbosa AM (2007) Effect of soybean oil and Tween 80 on the production of botryosphaeran by Botryosphaeria rhodina MAMB-05. Process Biochem 42(8):1254–1258
Sousa MJ, Mota M, Leao C (1992) Transport of malic acid in the yeast Schizosaccharomyces pombe: evidence for proton-dicarboxylate symport. Yeast 8(12):1025–1031
Thomsen LB, Lichota J, Kim KS, Moos T (2011) Gene delivery by pullulan derivatives in brain capillary endothelial cells for protein secretion. J Control Release 151(1):45–50
Valentini M, Storelli N, Lapouge K (2011) Identification of C-4-dicarboxylate transport systems in Pseudomonas aeruginosa PAO1. J Bacteriol 193(17):4307–4316
Yu X, Wang Y, Wei G, Dong Y (2012) Media optimization for elevated molecular weight and mass production of pigment-free pullulan. Carbohydr Polym 89(3):928–934
Zan Z, Zou X (2013) Efficient production of polymalic acid from raw sweet potato hydrolysate with immobilized cells of Aureobasidium pullulans CCTCC M2012223 in aerobic fibrous bed bioreactor. J Chem Technol Biotechnol 88(10):1822–1827
Zhang B-B, Cheung PCK (2011) A mechanistic study of the enhancing effect of Tween 80 on the mycelial growth and exopolysaccharide production by Pleurotus tuber-regium. Bioresour Technol 102(17):8323–8326
Zhang H, Cai J, Dong J, Zhang D, Huang L, Xu Z, Cen P (2011) High-level production of poly (beta-l-malic acid) with a new isolated Aureobasidium pullulans strain. Appl Microbiol Biotechnol 92(2):295–303
Zhang B-B, Chen L, Cheung PCK (2012) Two-dimensional gel electrophoresis analysis of mycelial cells treated with Tween 80: differentially expressed protein related to enhanced metabolite production. J Agric Food Chem 60(42):10585–10591
Zhao Z, Hu X, Ross CW (1987) Comparison of tissue preparation methods for assay of nicotinamide coenzymes. Plant Physiol 84(4):987–988
Zou X, Hang HF, Chu J, Zhuang YP, Zhang SL (2009) Oxygen uptake rate optimization with nitrogen regulation for erythromycin production and scale-up from 50 L to 372 m3 scale. Bioresour Technol 100(3):1406–1412
Zou X, Zhou Y, Yang ST (2013) Production of polymalic acid and malic acid by Aureobasidium pullulans fermentation and acid hydrolysis. Biotechnol Bioeng 110(8):2105–2113
Acknowledgments
This work was supported in part by grants from the National High Technology Research and Development Program of China (863 Program) (No. 2014AA021205), National Transformation Fund for Agricultural Science and Technology (2012F1003006), and Fundamental Research Funds for the Central Universities (XDJK2013B039 and 2362014XK07).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11274_2014_1779_MOESM1_ESM.xlsx
Table S1: The sequence annotation of different expression gene with addition of different Tween 80 concentration in 5L stirred-tank fermenter (XLSX 12 kb)
Rights and permissions
About this article
Cite this article
Tu, G., Wang, Y., Ji, Y. et al. The effect of Tween 80 on the polymalic acid and pullulan production by Aureobasidium pullulans CCTCC M2012223. World J Microbiol Biotechnol 31, 219–226 (2015). https://doi.org/10.1007/s11274-014-1779-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-014-1779-9