Abstract
The effect of sodium chloride (NaCl) on pullulan production by batch culture of Aureobasidium pullulans CCTCC M 2012259 was investigated. NaCl at 3 g/L improved the pullulan titer by 26.7% but reduced the molecular weight of pullulan to only 46.8% of that obtained in the control without NaCl. In order to elucidate the physiological mechanism underlying the effect of NaCl on pullulan production, assays of key enzyme activity, gene expression, energy metabolism, and intracellular uridine diphosphate glucose (UDP-glucose) content were performed. Results indicated that NaCl increased the activities of α-phosphoglucose mutase and glucosyltransferase involved in pullulan biosynthesis, increased the activities of α-amylase being responsible for pullulan degradation, upregulated the transcriptional levels of pgm1, fks, and amy2 genes, enhanced the driving force for ATP supply, and helped to maintain intracellular UDP-glucose at a high level in A. pullulans CCTCC M 2012259. All these results illuminate the reason by which NaCl increases pullulan titer but reduces the molecular weight of pullulan.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pullulan, an important water-soluble microbial exopolysaccharide, is commercially produced by yeast-like fungus Aureobasidium pullulans (Cheng et al. 2011; Li et al. 2015). The structure of pullulan consists of a unique linkage pattern with two α-(1 → 4) and one α-(1 → 6) glycosidic bonds in maltotriose repeating units (Prajapati et al. 2013). Owing to its non-toxic, non-mutagenic, non-carcinogenic, and non-immunogenic properties, pullulan is extensively used in food, cosmetic, and pharmaceutical industries (Farris et al. 2014; Mishra et al. 2011) and has enormous potential for biomedical applications such as drug delivery, gene targeting, tissue engineering, and medical imaging (Bulman et al. 2015; Singh et al. 2015; Singh et al. 2017).
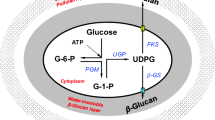
Pullulan is now approved to be produced by A. pullulans from the precursor of uridine diphosphate glucose (UDP-glucose), and α-phosphoglucose mutase (PGM), UDP-glucose pyrophosphorylase (UGP), and glucosyltransferase (FKS) are confirmed as the key enzymes involved in the metabolic pathway for pullulan biosynthesis (Chen et al. 2017; Duan et al. 2008; Sheng et al. 2014). The supply of intracellular UDP-glucose and adenosine triphosphate (ATP) are the vital factors determining the titer, yield, and rate of pullulan biosynthesis (Li et al. 2015). During batch fermentation, efficient pullulan production usually depends on optimal environmental and nutritional conditions (Cheng et al. 2011), among which mineral salts in the fermentation medium are important factors that cannot be ignored (Reeslev and Jensen 1995; Singh et al. 2008). Badr-Eldin et al. (1994) found that a low concentration of inorganic phosphate in the medium was disadvantageous to cell growth, while incorporation of a high concentration of inorganic phosphate suppressed pullulan formation. Reeslev et al. (1990) reported that Fe3+ and Zn2+ ions affected the growth yield, the morphology, and the elaboration of exopolysaccharide, while no effects were observed for Mn2+, Ca2+, or Cu2+ during pullulan fermentation. Gao et al. (2010) optimized mineral salts in the fermentation medium using an orthogonal array method, which enhanced pullulan production by A. pullulans HP-2001. In addition, they also found that potassium phosphate was the most important factor for cell growth, while NaCl was important for pullulan production (Gao et al. 2011).
Increasing attention is being paid to the molecular weight (Mw) of pullulan, which can exert considerable influence on its commercial applications (Lazaridou et al. 2003). The average Mw of pullulan depends on the strain, culture conditions, and culture duration (Lin et al. 2007). Pollock et al. (1992) found that the Mw of pullulan decreased in the late stationary growth phase, and speculated that this may be due to the presence of α-amylase secreted into the medium. Prasongsuk et al. (2007) confirmed this by adding the α-amylase inhibitor acarbose to the medium, showing that pullulan of slightly higher Mw was obtained from late cultures. Manitchotpisit et al. (2011) then cloned the α-amylase gene from A. pullulans NRRL Y-12974 and speculated that α-amylase attacked the minor maltotriose subunits of pullulan and caused the reduction in Mw. Although α-amylase seems to be responsible for the decrease in the Mw of pullulan, few reports have focused on changes in the expression and activity of this enzyme during batch pullulan fermentation.
Mineral salts (and metal ions) can improve the activity of enzymes involved in the biosynthesis of polysaccharides, and influence the titer and Mw of target products (Miletić et al. 2012; Zhao et al. 2014). In our previous study, the medium for pullulan production was optimized in flask cultures, and NaCl was found to have a significant influence on the titer and Mw of pullulan (Yu et al. 2012). In the present work, we investigated the effect of NaCl on pullulan biosynthesis during batch culture of A. pullulans CCTCC M 2012259. We analyzed the transcriptional levels and activities of key enzymes involved in pullulan biosynthesis and degradation, and evaluated the energy supply and regeneration during batch processes in an attempt to elucidate the physiological mechanism underlying the improved pullulan production and the reduction in Mw of pullulan in the presence of NaCl.
Materials and methods
Microorganism and culture
A. pullulans CCTCC M 2012259, an efficient pullulan-producing strain, was stored at − 70 °C with 20% (v/v) glycerol and used as the starting strain in this study. Seed cultures were prepared in 500-mL Erlenmeyer flasks containing 50 mL of seed medium (20% [w/v] potato juice and 20 g/L glucose, natural pH) by inoculating 1 mL of frozen glycerol stock. Seeds were incubated on a rotary shaker at 200 rpm and 30 °C for 24 h, then inoculated into fermentation medium (50 g/L glucose, 3.0 g/L yeast extract, 0.6 g/L (NH4)2SO4, 2.0 g/L K2HPO4, 1.0 g/L NaCl, 0.2 g/L MgSO4·7H2O) at an inoculating ratio of 10% (v/v).
Batch pullulan production was carried out in a 5-L stirred fermentor (Minifors, Infors HT, Basel, Switzerland) with a working volume of 3 L. The culture was initiated by inoculating 300 mL of fresh seeds into the fermentation medium. The bioreactor was operated at 30 °C and 350 rpm with an aeration rate of 1 vvm. The concentration of NaCl in the fermentation medium varied according to the experimental scheme. The level of dissolved oxygen (DO) was real-time monitored by a DO probe (Mettler-Toledo International, Inc., Swiss). The pH value of the fermentation broth was automatically maintained at 3.8 by feeding with either 3 mol/L H2SO4 or NaOH (Wang et al. 2013).
Glucose was separately autoclaved at 121 °C for 15 min and mixed with other nutrients at room temperature before inoculation. Reagents used were purchased from Sangon Biotech Co. (Shanghai, China). All chemicals were of analytical reagent grade, and the yeast extract was of biological reagent grade.
Cell-free extract preparation
A 5-mL sample of fermentation broth was quickly frozen over 10 min using liquid nitrogen to stop intracellular reactions before harvesting wet cells by centrifugation at 8000×g and 4 °C for 20 min. Cells were resuspended in 5 mL of 0.2 mol/L ice-cold phosphate buffer (pH 7.0) and lysed in an ice bath using a VCX 750 ultrasonic processor (Sonics & Materials Inc., Newton, CT, USA) at 20 kHz. Ultrasonication was conducted for 10 min with 10-s active and passive intervals. Debris was removed by centrifugation at 10,000×g and 4 °C for 20 min. The supernatant served as the cell-free extract and was used for the determination of UDP-glucose, co-factors, and enzyme activities. The protein in the cell-free extract was measured with bovine serum albumin as the standard (Bradford 1976).
Enzyme activity assays
Wet cells collected by centrifugation were resuspended in 50 mL of autoclaved sugar solution containing 50 g/L glucose and 0.2 g/L MgSO4·7H2O at an initial pH value of 6.8. The bioconversion of glucose to pullulan was conducted at 30 °C and 200 rpm on a rotary shaker. The cell capability for pullulan biosynthesis was defined as the amount of pullulan generated by 1 g of cells per hour at 30 °C (Ju et al. 2015). The pullulan-degrading activity was assayed using pure pullulan standard as the substrate according to the method described by Prasongsuk et al. (2007). One unit of pullulan-degrading activity was defined as 1 μmol glucose equivalents liberated per minute at 50 °C.
The FKS activity was assayed using a spectrophotometric method based on the rate of increase in the absence at 410 nm (Wang et al. 2015). A 0.2-mL sample of p-nitrophenyl-α-d-glucopyranoside (10 mmol/L) in sodium acetate buffer (0.1 mol/L, pH 4.0) was mixed with 0.2 mL of cell-free extract and incubated at 40 °C for 5 min. The reaction was terminated by adding 3 mL of glycine-NaOH buffer (0.4 mol/L, pH 10.5). One unit of FKS activity was defined as the release of 1 μmol p-nitrophenol per minute at 40 °C. The activities of PGM and UGP were assayed using commercial ELISA kits (ShhcBio, Shanghai, China) according to the manufacturer’s instructions.
The activity of α-amylase in the fermentation broth was measured using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. All values determined using enzymatic assays are expressed as the means of at least three independent measurements.
Gene expression assays
The transcription levels of pgm1, pgm2, ugp, fks, amy1, amy2, and amy3 genes (which encode the enzymes PGM, UGP, FKS, and α-amylase, respectively) at 36 h were measured by quantitative real-time PCR (qRT-PCR). The sequences of primers used for the amplification of these genes are listed in Table S1. The qRT-PCR experiments were carried out according to the procedure described in the previous study (Ju et al. 2015). The expression levels of target genes were all normalized against the act1 gene as the reference gene.
Analytical methods
The determination of dry cell biomass, pullulan, glucose, the MW of pullulan, and the calculation of kinetic parameters were performed according to the methods described in our previous study (Yu et al. 2012). Co-factors associated with energy metabolism such as ATP, ADP, NADH, and NAD+ in the cell-free extract were detected using high-performance liquid chromatography (HPLC) with a 4.6 × 250 mm SunFire ODS C18 column (Waters Corp., Milford, MA, USA) and a UV detector at 254 nm (Wang et al. 2013).
Intracellular UDP-glucose was determined at 22 °C using HPLC with a 4.6 × 250 mm Supelcosil LC-18-DB column (Sigma-Aldrich, Bellefonte, PA, USA) following the method described by Ramm et al. (2004). The detection was carried out at 254 nm. The buffer consisting of 40 mmol/L triethylamine-acetic acid (pH 6.0) was used as the mobile phase, and the flow rate was 1 mL/min. Samples were microfiltered using a Sartorious membrane (Sartorius Stedim Biotech S.A., Aubagne, France) with a pore size of 0.20 μm before detection.
Statistical analysis
All experiments were performed in triplicate, and data were expressed as means ± standard deviation (SD). The Student’s t test was employed to evaluate statistical differences, and samples with p ≤ 0.05 were considered to be statistically different.
Results
Batch pullulan production under different concentrations of NaCl
Batch culture of A. pullulans CCTCC M 2012259 for pullulan production with different concentrations of NaCl in the fermentation medium was carried out in a 5-L stirred fermentor. The cell growth and pullulan production during batch fermentation clearly varied with NaCl concentration (from 0 to 5 g/L). As shown in Fig. 1a, cells grew well within the given NaCl concentration range. A biomass of 10.80 g/L was obtained without NaCl (control), which was higher than that obtained in the presence of NaCl, showing that NaCl was not conducive to increasing the biomass of A. pullulans CCTCC M 2012259. By contrast, NaCl markedly increased pullulan production, and resulted in a higher titer of pullulan than that achieved in the control. The maximum pullulan titer of 28.88 g/L was obtained at 3 g/L NaCl, representing an increase of 26.7% relative to the control (Fig. 1b). Regarding the Mw of pullulan produced by the strain, the maximum Mw of pullulan appeared at 12 h with NaCl but at 18 h in the control (Fig. 1c). Additionally, it should be noted that the presence of NaCl reduced the Mw during pullulan biosynthesis, and the final Mw of pullulan at 72 h with NaCl was much lower than that of the control. The final Mw of 0.73 × 106 Da was obtained at 3 g/L NaCl, which was only 46.8% of that obtained in the control.
The kinetic parameters involved in batch pullulan fermentation were calculated and are listed in Table 1. NaCl increased the average specific glucose consumption rate and average specific pullulan production rate, resulting in higher yield and productivity of pullulan in the presence of NaCl. Specifically, the highest yield of pullulan on biomass was obtained at 3 g/L NaCl, which was 59.5% higher than that of the control. In addition, biomass and pullulan could not be enhanced simultaneously at a certain NaCl concentration, and in other words, higher pullulan production was always accompanied by a lower biomass, and vice versa. These results are in accord with the findings presented in our previous study (Wang et al. 2013).
DO levels during batch pullulan fermentation under different NaCl concentrations were real-time monitored. As shown in Fig. 1d, no significant differences in the DO level (pO2) were found among batch processes under diverse concentrations of NaCl in the medium. Thus, changes in the titer and Mw of pullulan cannot be attributed to the response of cells to differences in DO levels. The key enzymes and energy supply were then assayed during pullulan production to investigate the physiological mechanism underlying improved pullulan production but reduced Mw of pullulan in the presence of NaCl.
Cell capability for pullulan biosynthesis and pullulan-degrading activity
To clarify the effect of NaCl on changes in pullulan production and Mw of pullulan during batch fermentation, the cell capability for pullulan biosynthesis and pullulan-degrading activity were determined. As shown in Fig. 2a, the cell capability for pullulan biosynthesis increased from 12 to 24 h, and then decreased from 24 to 48 h. The highest cell capability at different culture stages (12, 24, 36, and 48 h) was achieved at 3 g/L NaCl and was increased on average by 42.6% compared with that of the control. These results indicated that 3 g/L was the optimum NaCl concentration to obtain cells with high capability, resulting in a much higher titer of pullulan produced by A. pullulans CCTCC M 2012259.
Cell capability for pullulan biosynthesis (a) and pullulan-degrading activity (b) at different culture stages during batch pullulan production by A. pullulans CCTCC M 2012259 under diverse concentrations of NaCl. Asterisks indicate the level of statistical significance (*p < 0.05) in comparison to the control (0 g/L NaCl) by Student’s t test
As the Mw of pullulan was reduced in the presence of NaCl (Fig. 1c), the pullulan-degrading activities during the middle and late culture stages (from 24 to 60 h) were determined. As shown in Fig. 2b, the pullulan-degrading activities at 24 h were maintained at relatively low levels of ~ 2 mU/mL under different NaCl concentrations. However, the activities increased dramatically along with batch processes until the late culture stage (48 and 60 h). The maximal pullulan-degrading activities were obtained at 3 g/L NaCl, while much lower activities were found in the absence of NaCl in the medium. NaCl increased pullulan-degrading activities and reduced the Mw of pullulan, indicating that NaCl also favored the degradation of pullulan during batch fermentation.
Activities of key enzymes involved in pullulan biosynthesis and degradation
The activities of PGM, UGP, FKS, and α-amylase at different culture stages (from 12 to 60 h) under diverse NaCl concentrations were assayed. As shown in Fig. 3, the activities of PGM, FKS, and α-amylase were all maintained at relatively low levels in the control without NaCl, while the presence of NaCl in the medium increased the activities of these enzymes, and the highest activities were obtained at 3 g/L NaCl. However, no obvious differences in UGP activities were found at the same culture stage in the presence or absence of NaCl. Moreover, higher activities of PGM, UGP, and FKS were achieved at the stage of pullulan biosynthesis (24 and 36 h), while lower activities were obtained at the cell growth stage (12 h) and the late culture stage (48 h). Unlike the above three key enzymes involved in pullulan biosynthesis, α-amylase activity was not detected during the cell growth stage (data not shown), but was significantly increased to relatively high levels during the late culture stage (48 and 60 h). According to the results illustrated in Fig. 3d, 3 g/L was the optimal NaCl concentration to achieve the highest α-amylase activity, especially at 60 h.
The activity of key enzymes of PGM (a), UGP (b), FKS (c) involved in pullulan biosynthesis, and α-amylase (d) responsible for pullulan degradation during batch culture of A. pullulans CCTCC M 2012259 under different concentrations of NaCl. Asterisks indicate the level of statistical significance (*p < 0.05) in comparison to the control (0 g/L NaCl) by Student’s t test
Transcriptional levels of key enzymes involved in pullulan biosynthesis and degradation
In order to investigate whether the increases in activities of key enzymes in the presence of NaCl were associated with gene expression, the transcriptional levels of genes encoding key enzymes involved in pullulan biosynthesis and degradation were determined at 36 h. As shown in Fig. 4, the transcriptional levels of pgm1, fks, and amy2 at NaCl concentrations that varied from 1 to 5 g/L were all significantly upregulated compared with those obtained in the control (0 g/L NaCl). However, no significant differences in the expression of pgm2, ugp, amy1, or amy3 genes were observed with different NaCl concentrations. The highest transcriptional levels of pgm1 and fks were obtained at 3 g/L NaCl, which were upregulated by 1.67- and 1.72-fold, respectively, compared with those of the control. The expression of amy2 was upregulated by NaCl, but results were generally similar for all NaCl concentrations (from 1 to 5 g/L). In addition, changes in transcriptional levels of pgm1, fks, and amy2 consisted with the activities of PGM, FKS, and α-amylase, respectively, which are key enzymes involved in pullulan biosynthesis and degradation (Fig. 3).
Transcriptional level of genes encoding key enzymes involved in pullulan biosynthesis and degradation at 36 h during batch culture of A. pullulans CCTCC M 2012259 under different concentrations of NaCl. Asterisks indicate the level of statistical significance (*p < 0.05; **p < 0.01) in comparison to the control (0 g/L NaCl) by Student’s t test
Energy supply and regeneration
To investigate the effect of NaCl on energy supply and consumption during pullulan biosynthesis, intracellular levels of co-factors associated with energy metabolism such as NADH, NAD+, ATP, and ADP were determined, and the ratios of NADH/NAD+ and ATP/ADP were also calculated. As shown in Fig. 5, intracellular NADH and ATP were maintained at high levels at 12 h, which would provide sufficient energy for rapid cell growth at this culture stage. The levels of NADH and ATP then decreased along with rapid pullulan biosynthesis, and no significant differences were found among batch processes with or without NaCl in the late culture stage (36 and 48 h). In addition, much higher NADH/NAD+ ratios were obtained with NaCl than those in the control, while no significant differences in ATP/ADP ratios were found at the same culture stage. These results indicated that NaCl improved the transformation from NADH to NAD+ to generate ATP, which would provide more energy for the overproduction of pullulan. Because no significant differences in intracellular ATP levels or ATP/ADP ratios were found at the late stage of batch culture, the effect of NaCl on energy metabolism appeared to be mainly restricted to intracellular NADH levels and the ratio of NADH/NAD+. Thus, the above results indicated that the presence of NaCl increased the driving force for energy supply and regeneration, which undoubtedly contributed to the increase in pullulan production relative to the control.
Intracellular levels of NADH (a) and ATP (b), as well as the ratios of NADH/NAD+ (c) and ATP/ADP (d) at different culture stages during batch pullulan production under diverse concentrations of NaCl. Asterisks indicate the level of statistical significance (*p < 0.05) in comparison to the control (0 g/L NaCl) by Student’s t test
Intracellular UDP-glucose levels
UDP-glucose is the direct precursor in pullulan biosynthesis (Duan et al. 2008). In order to investigate whether the intracellular level of this precursor was affected by NaCl, UDP-glucose content was determined at different culture stages of batch fermentation. As shown in Fig. 6, UDP-glucose remained at relatively high levels at the cell growth stage (12 and 24 h) but gradually decreased during pullulan formation, indicating that the consumption of UDP-glucose was closely related to pullulan biosynthesis. In addition, the presence of NaCl in the medium increased intracellular UDP-glucose levels at the early culture stage compared with the control without NaCl, and the maximal UDP-glucose levels were obtained at 3 g/L NaCl. However, no significant differences in UDP-glucose level were found at the late culture stage (48 h), when the rate of cell respiration was decreased and pO2 levels were greatly increased (Fig. 1d). These results indicated that NaCl helped to maintain UDP-glucose at high levels during vigorous metabolism, which in turn supplied sufficient UDP-glucose for increased pullulan production.
Discussion
Mineral salts are indispensable nutrients for most microorganisms, and usually act as cellular components together with co-factors for certain enzymes. NaCl is one of the most commonly used and affordable salts for influencing cell growth and product formation. Addition of high concentration of NaCl in the fermentation medium was reported to induce lipid accumulation in the freshwater microalgae Desmodesmus abundans (Xia et al. 2014), improve polyhydroxyalkanoate production by Cupriavidus necator (Passanha et al. 2014), and even increase the activity of a recombinant halophilic esterase produced by Escherichia coli BL21 (Rao et al. 2009). These benefits achieved by NaCl addition mainly resulted from the osmotic stress caused by NaCl. However, NaCl at lower concentrations was found to increase pullulan production but decrease the Mw of pullulan in shaking flasks (Yu et al. 2012) and 5-L fermentor in the present study. The physiological mechanisms underlying the differences in pullulan biosynthesis by A. pullulans CCTCC M 2012259 under diverse NaCl concentrations should be explored.
PGM and UGP are the key enzymes catalyzing the sequential reactions from glucose-6-phosphate to glucose-1-phosphate and UDP-glucose, and FKS then reacts to UDP-glucose with lipids to form pullulan (Cheng et al. 2011). Thus, the observed increase in the activities of PGM and UGP was consistent with UDP-glucose biosynthesis and accumulation in cells, while higher FKS activity favored an increase in UDP-glucose consumption for pullulan biosynthesis (Chen et al. 2017; Duan et al. 2008). In the present study, although A. pullulans CCTCC M 2012259 could grow and produce pullulan in the absence of NaCl, addition of NaCl in the medium promoted pullulan biosynthesis. Results indicated that NaCl upregulated the transcriptional levels of pgm1 and fks genes (Fig. 4) and increased the enzyme activities of PGM and FKS (Fig. 3) that resulted in higher intracellular UDP-glucose supply (Fig. 6) for improved pullulan production (Fig. 1). However, no significant differences in the transcription of ugp or the activity of UGP were observed at different NaCl concentrations, indicating that UGP was insensitive to NaCl. Based on the above analysis, it can be concluded that PGM and FKS played more important roles in improving pullulan production than UGP. Furthermore, 3 g/L NaCl was found to be optimal for achieving maximal levels of kinetic parameters involved in pullulan production (Table 1). This can also be certified by the cell capability for pullulan biosynthesis shown in Fig. 2a.
In addition to the pullulan titer, the Mw of pullulan is another important factor to be considered during batch pullulan fermentation. The Mw of pullulan depends on the activities of enzymes involved in both its biosynthesis and degradation. Among the enzymes being responsible for the degradation of pullulan in A. pullulans, α-amylase was reported to be the major enzyme (Linardi and Machado 1990; Prasongsuk et al. 2007). In the present study, NaCl was found to upregulate the transcriptional level of the amy2 gene (Fig. 4) and significantly increased the activity of α-amylase in late cultures (Fig. 3d), which contributed to the decrease in the Mw of pullulan (Fig. 1c). According to the literature, A. pullulans strains can also produce other pullulan-degrading enzymes such as glucoamylase and pullulanase when grown on medium containing starch or sucrose (Chi et al. 2009; Moubasher et al. 2013). However, glucose was used as the carbon source in this study, and no activity of glucoamylase or pullulanase was detected. These results presented sufficient evidence for the mechanism underlying the reduced Mw of pullulan by NaCl addition, and can also help us understand the reason why much higher final Mw of pullulan could be obtained in the absence of NaCl.
ATP is indispensable for pullulan biosynthesis, and pullulan was not synthesized in vitro without ATP supply using cell-free enzymes isolated from A. pullulans (Taguchi et al. 1973). The metabolic pathway of pullulan biosynthesis indicated that the supply of ATP could act as a bottleneck for pullulan overproduction (Ma et al. 2014). Our previous studies demonstrated that increasing both intracellular ATP levels and ATP/ADP ratios favored efficient pullulan production (Wang et al. 2013, 2016). Herein, although no significant changes in intracellular ATP levels and ATP/ADP ratios were observed at the same culture stage under different NaCl concentrations, NaCl increased intracellular NADH levels and ratios of NADH/NAD+ (Fig. 5c), which would provide greater driving force for energy regeneration, resulting in more energy supply for increased pullulan biosynthesis than in the control without NaCl (Fig. 5d). Therefore, the increase in energy supply in the presence of NaCl undoubtedly promoted pullulan production.
To the best of our knowledge, this is the first report to elucidate the physiological mechanism underlying the effect of NaCl on pullulan production based on the determination of key enzymes and energy metabolism. The results revealed the importance of choosing the appropriate NaCl concentration during pullulan production for achieving the target Mw. However, only a limited number of genes encoding key enzymes were tested in this study, and we still do not know whether the transcriptional levels of other genes involved in the metabolic pathway of pullulan biosynthesis and degradation were regulated by NaCl. To obtain more molecular evidence that illuminates the reason why NaCl improves the titer of pullulan while reducing the Mw of pullulan, the omics technologies as well as metabolic flux analysis should be applied in future studies.
In conclusion, the presence of NaCl in the medium improved batch pullulan production by A. pullulans CCTCC M 2012259 but decreased the Mw of pullulan. Based on assaying the activities of key enzymes involved in pullulan biosynthesis and degradation, measuring the transcriptional levels of genes encoding the key enzymes, evaluating the energy supply and regeneration, and determining intracellular levels of UDP-glucose under different NaCl concentrations, the physiological mechanism underlying the increased titer but reduced Mw of pullulan by NaCl was revealed. Because NaCl is a cheap and readily available reagent, this study also provided an economical approach for efficient production of other polysaccharides.
References
Badr-Eldin SM, El-Tayeb OM, El-Masry HG, Mohamad FH, El-Rahman OA (1994) Polysaccharide production by Aureobasidium pullulans: factors affecting polysaccharide formation. World J Microbiol Biotechnol 10:423–426
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bulman SE, Coleman CM, Murphy JM, Medcalf N, Ryan AE, Barry F (2015) Pullulan: a new cytoadhesive for cell-mediated cartilage repair. Stem Cell Res Ther 6:34
Chen X, Wang Q, Liu N, Liu G, Chi Z, Chi Z (2017) A glycosyltransferase gene responsible for pullulan biosynthesis in Aureobasidium melanogenum P16. Int J Biol Macromol 95:539–549
Cheng KC, Demirci A, Catchmark JM (2011) Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol 92:29–44
Chi Z, Wang F, Chi Z, Yue L, Liu G, Zhang T (2009) Bioproducts from Aureobasidium pullulans, a biotechnologically important yeast. Appl Microbiol Biotechnol 82:793–804
Duan X, Chi Z, Wang L, Wang X (2008) Influence of different sugars on pullulan production and activities of α-phosphoglucose mutase, UDPG-pyrophosphorylase and glucosyltransferase involved in pullulan synthesis in Aureobasidium pullulans Y68. Carbohydr Polym 73:587–593
Farris S, Unalan IU, Introzzi L, Fuentes-Alventosa JM, Cozzolino CA (2014) Pullulan-based films and coatings for food packaging: present applications, emerging opportunities, and future challenges. J Appl Polym Sci 131:40539
Gao W, Kim YJ, Chung CH, Li JH, Lee JW (2010) Optimization of mineral salts in medium for enhanced production of pullulan by Aureobasidium pullulans HP-2001 using an orthogonal array method. Biotechnol Bioprocess Eng 15:837–845
Gao W, Chung C, Li J, Lee J (2011) Application of statistical experimental design for optimization of physiological factors and their influences on production of pullulan by Aureobasidium pullulans HP-2001 using an orthogonal array method. Korean J Chem Eng 28:2184–2189
Ju X, Wang D, Zhang G, Cao D, Wei G (2015) Efficient pullulan production by bioconversion using Aureobasidium pullulans as the whole-cell catalyst. Appl Microbiol Biotechnol 99:211–220
Lazaridou A, Biliaderis CG, Kontogiorgos V (2003) Molecular weight effects on solution rheology of pullulan and mechanical properties of its films. Carbohydr Polym 52:151–166
Li Y, Chi Z, Wang GY, Wang ZP, Liu GL, Lee CF, Ma ZC, Chi ZM (2015) Taxonomy of Aureobasidium spp. and biosynthesis and regulation of their extracellular polymers. Crit Rev Microbiol 41:228–237
Lin Y, Zhang Z, Thibault J (2007) Aureobasidium pullulans batch cultivations based on a factorial design for improving the production and molecular weight of exopolysaccharides. Process Biochem 42:820–827
Linardi VR, Machado KMG (1990) Production of amylase by yeasts. Can J Microbiol 36:751–753
Ma ZC, Fu WJ, Liu GL, Wang ZP, Chi ZM (2014) High-level pullulan production by Aureobasidium pullulans var. melanogenium P16 isolated from mangrove system. Appl Microbiol Biotechnol 98:4865–4873
Manitchotpisit P, Skory CD, Leathers TD, Lotrakul P, Eveleigh DE, Prasongsuk S, Punnapayak H (2011) a-Amylase activity during pullulan production and a-amylase gene analyses of Aureobasidium pullulans. J Ind Microbiol Biotechnol 38:1211–1218
Miletić N, Nastasović A, Loos K (2012) Immobilization of biocatalysts for enzymatic polymerizations: possibilities, advantages, applications. Bioresour Technol 115:126–135
Mishra B, Vuppu S, Rath K (2011) The role of microbial pullulan, a biopolymer in pharmaceutical approaches: a review. J Appl Pharm Sci 1(6):45–50
Moubasher H, Wahsh SS, El-Kassem NA (2013) Isolation of Aureobasidium pullulans and the effect of different conditions for pullulanase and pullulan production. Microbiology 82:155–161
Passanha P, Kedia G, Dinsdale RM, Guwy AJ, Esteves SR (2014) The use of NaCl addition for the improvement of polyhydroxyalkanoate production by Cupriavidus necator. Bioresour Technol 163:287–294
Pollock TJ, Thorne L, Armentrout RW (1992) Isolation of new Aureobasidium strains that produce high molecular-weight pullulan with reduced pigmentation. Appl Environ Microbiol 58:877–883
Prajapati VD, Jani GK, Khanda SM (2013) Pullulan: an exopolysaccharide and its various applications. Carbohydr Polym 95:540–549
Prasongsuk S, Berhow MA, Dunlap CA, Weisleder D, Leathers TD, Eveleigh DE, Punnapayak H (2007) Pullulan production by tropical isolates of Aureobasidium pullulans. J Ind Microbiol Biotechnol 34:55–61
Ramm M, Wolfender JL, Queiroz EF, Hostettmann K, Hamburger M (2004) Rapid analysis of nucleotide-activated sugars by high-performance liquid chromatography coupled with diode-array detection, electrospray ionization mass spectrometry and nuclear magnetic resonance. J Chromatogr A 1034:139–148
Rao L, Zhao X, Pan F, Li Y, Xue Y, Ma Y, Lu JR (2009) Solution behavior and activity of a halophilic esterase under high salt concentration. PLoS One 4:e6980
Reeslev M, Jensen B (1995) Influence of Zn2+ and Fe3+ on polysaccharide production and mycelium/yeast dimorphism of Aureobasidium pullulans in batch cultivations. Appl Microbiol Biotechnol 42:910–915
Reeslev M, Nielsen JC, Jørgensen BB (1990) Nutritional dependent dimorphism in the exopolysaccharide producing deuteromycete Aureobasidium pullulans. In: Christiansen C, Munck L, Villadsen J (eds) Proceedings of the 5th European Congress on Biotechnology. Munksgaard International Publisher, Copenhagen, pp 1053–1056
Sheng L, Zhu G, Tong Q (2014) Effect of uracil on pullulan production by Aureobasidium pullulans CGMCC1234. Carbohydr Polym 101:435–437
Singh RS, Saini GK, Kennedy JF (2008) Pullulan: microbial sources, production and applications. Carbohydr Polym 73:515–531
Singh RS, Kaur N, Kennedy JF (2015) Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydr Polym 123:190–207
Singh RS, Kaur N, Rana V, Kennedy JF (2017) Pullulan: a novel molecule for biomedical applications. Carbohydr Polym 171:102–121
Taguchi R, Sakano Y, Kikuchi Y, Sakuma M, Kobayashi T (1973) Synthesis of pullulan by acetone-dried cells and cell-free enzyme from Pullularia pullulans, and the participation of lipid intermediate. Agric Biol Chem 37:1635–1641
Wang D, Yu X, Wei G (2013) Pullulan production and physiological characteristics of Aureobasidium pullulans under acid stress. Appl Microbiol Biotechnol 97:8069–8077
Wang D, Chen F, Wei G, Jiang M, Dong M (2015) The mechanism of improved pullulan production by nitrogen limitation in batch culture of Aureobasidium pullulans. Carbohydr Polym 127:325–331
Wang D, Bian J, Wei G, Jiang M, Dong M (2016) Simultaneously enhanced production and molecular weight of pullulan using a two-stage agitation speed control strategy. J Chem Technol Biotechnol 91:467–475
Xia L, Rong J, Yang H, He Q, Zhang D, Hua C (2014) NaCl as an effective inducer for lipid accumulation in freshwater microalgae Desmodesmus abundans. Bioresour Technol 161:402–409
Yu X, Wang Y, Wei G, Dong Y (2012) Media optimization for elevated molecular weight and mass production of pigment-free pullulan. Carbohydr Polym 89:928–934
Zhao W, Chai D, Li H, Chen T, Tang Y (2014) Significance of metal ion supplementation in the fermentation medium on the structure and anti-tumor activity of tuber polysaccharides produced by submerged culture of Tuber melanosporum. Process Biochem 49:2030–2038
Funding
This work was supported by the National Natural Science Foundation of China (21776189) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 63 kb)
Rights and permissions
About this article
Cite this article
Wang, DH., Ni, TF., Ju, XM. et al. Sodium chloride improves pullulan production by Aureobasidium pullulans but reduces the molecular weight of pullulan. Appl Microbiol Biotechnol 102, 8921–8930 (2018). https://doi.org/10.1007/s00253-018-9292-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9292-y