Abstract
Two dibenzo-α-pyrones, botrallin (1) and TMC-264 (2) were preparatively separated from crude ethyl acetate extract of the endophytic fungus Hyalodendriella sp. Ponipodef12, which was isolated from the hybrid ‘Neva’ of Populus deltoides Marsh × P. nigra L. using a combination of high-speed counter-current chromatography (HSCCC) and semi-preparative HPLC. Botrallin (1) with 74.73 % of purity and TMC-264 (2) with 82.29 % of purity were obtained through HSCCC by employing a solvent system containing n-hexane–ethyl acetate–methanol–water at a volume ratio of 1.2:1.0:0.9:1.0. It was the first time for TMC-264 (2) to be isolated from this fungus. TMC-264 (2) showed strong antimicrobial and antinematodal activity, and botrallin (1) exhibited moderate inhibitory activity on acetylcholinesterase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Botrallin (1) and TMC-264 (2), belonging to the class of dibenzo-α-pyrones, have been found in some fungal species (Kameda et al. 1974; Sakurai et al. 2003a; Hormazabal et al. 2005; Meng et al. 2012; Mao et al. 2014). Botrallin (1) was firstly isolated from the fungus Botrytis allii (Kameda et al. 1974), and later it was isolated from the endophytic fungi Microsphaeropsis olivacea (Hormazabal et al. 2005) and Hyalodendriella sp. Ponipodef12 (Meng et al. 2012), respectively. Botrallin exhibited a moderate inhibitory activity on acetylcholinesterase (AChE) (Hormazabal et al. 2005), and a strong inhibitory activity on tyrosine kinase (Holler et al. 1999). TMC-264 (2) was first isolated from the fermentation broth of Phoma sp. TC 1674, and was found to selectively inhibit interleukin-4 (IL-4) signaling by interfering phosphorylation of the signal transducer and activator of transcription 6 (STAT6), as well as binding of the phosphorylated STAT6 to the recognition sequence. So it might be an inhibitor of IL-4 signaling for the treatment of allergic disease (Sakurai et al. 2003a, b). These tremendous discoveries about botrallin (1) and TMC-264 (2) have attracted our attention to further investigate their biological activities as well as to efficiently obtain them in large amounts.
High-speed counter-current chromatography (HSCCC), a support-free liquid chromatography method, provides an advantage over the conventional column chromatography by eliminating the use of a solid support where an amount of stationary phase is limited, and the dangers of irreversible adsorption from the support are inevitably present (Chen et al. 2003; Lu et al. 2003; Ito 2005). Compared to other liquid–liquid techniques, HSCCC is advantageous because of its shorter separation time, wider range of selection of solvent systems and quantitative material recovery. It is now accepted as an efficient preparative technique, and widely used for separation and purification of various natural products (Chen et al. 2006; Wang et al. 2010; Feng et al. 2013; Jin et al. 2013; Mudge et al. 2013; Shan et al. 2013).
To the best of our knowledge, there was no information available about the metabolite production from the fungi Hyalodendriella speices except for our previous study about three dibenzo-α-pyrones (i.e., palmariol B, alternariol 9-methyl ether, and botrallin) isolated from the endophytic fungus Hyalodendriella sp. Ponipodef12 associated with the healthy stems of poplar hybrid ‘Neva’ of Populus deltoides Marsh × P. nigra L. (Meng et al. 2012). In this report, we described the isolation and structural identification of another dibenzo-α-pyrone, named TMC-264 from the endophytic fungus Hyalodendriella sp. Ponipodef12, and found that this fungus was a high producer of botrallin and TMC-264. The conventional methods for the separation of dibenzo-α-pyrones including botrallin and TMC-264 from the crude extracts of the fungi were performed by liquid–liquid extraction, followed by multistep silica gel column chromatography (Sakurai et al. 2003a, b; Meng et al. 2012). However, these methods are not suitable for large-scale preparation due to the need of huge amounts of organic solvents and time consuming. The purpose of the study was to establish a method for more efficient separation and purification of botrallin and TMC-264 from the endophytic fungus Hyalodendriella sp. Ponipodef12 by HSCCC to meet the future applications of these two compounds. The separated botrallin and TMC-264 were further purified by semi-preparative HPLC. Their bioactivities, including antibacterial, antifungal, antinematodal and acetylcholinesterase inhibitory properties, were evaluated in order to provide data supporting the development and utilization of Hyalodendriella sp. Ponipodef12.
Materials and methods
General analytical methods
The preparative HSCCC system was a TBE-300B (Tauto Biotech, Shanghai, China) instrument equipped with a polytetrafluoroethylene (PTFE) tube (diameter of tube was 2.6 mm, and total capacity was 280 ml) composed of three coils and a 20-ml sample loop. The HSCCC system was equipped with a TBP-5002 pump and TBD-2000 UV detector (Tauto Biotech, Shanghai, China) operating at 254 nm, and a WH500-USB workstation (Wuhao, Shanghai, China). The experimental temperature was at 25 °C adjusted by HX-1050 constant temperature circulating implement (Boyikang, Beijing, China). The revolution speed of the apparatus can be regulated with a speed controller in the range between 0 and 1,000 rpm.
The semi-preparative HPLC system consisted of one K-501 pump, one K-2501 UV detector (Knauer, Berlin, Germany), one reversed-phase Luna C18 column (250 mm × 10 mm, 5 μm, Phenomenex, Torrance, CA, USA), one 2-ml sample loop and a workstation (Lumtech, Beijing, China). The semi-preparative HPLC column was isocratically eluted with MeOH-H2O (60:40, v/v) in 50 min at a flow rate of 3.0 ml/min and UV detection at 234 nm. The sample injection volume was 1.0 ml.
Analytical reversed-phase HPLC–DAD data were obtained using an HPLC system (Shimadzu, Kyoto, Japan) consisted of two LC-20AT solvent delivery units, a CBM-20Alite system controller, an SIL-20A autosampler, and an SPD-M20A photodiode array detection (DAD) system. Chromatographic separations were performed at 40 °C using a reversed-phase Ultimate TM XB C18 column (250 mm × 4.6 mm, 5 μm, Welch Materials, Inc., Ellicott, MD, USA). The mobile phase composed of methanol–water (60:40, v/v) was eluted at a flow rate of 1.0 ml/min, and the effluent was monitored at 234 nm. The LC solution multi-PDA workstation was employed to acquire and process chromatographic data.
NMR spectra were recorded on a Bruker Avance DRX-400 NMR spectrometer (1H at 400 MHz and 13C at 100 MHz) using tetramethylsilane (TMS) as the internal standard, and chemical shifts were recorded as δ values. HR–ESI–MS spectra were measured on Bruker Apex IV FTMS. The melting points (uncorrected) of the compounds were determined on an XT4-100B microscopic melting-point apparatus (Tianjin Tianguang Optical Instruments Company, Tianjin, China). UV spectra were recorded with a TU-1810 UV–VIS spectrophotometer (Beijing Purkinje General Instrument Company, Beijing, China). A microplate spectrophotometer (PowerWave HT, BioTek Instruments, Winooski, VT, USA) was employed to measure the light absorption value.
Chemicals and reagents
All organic solvents used for HSCCC and preparative HPLC were of analytical grade and purchased from Beijing Chemical Company, Beijing, China. Methanol used for analytical HPLC was of chromatographic grade and purchased from Tianjin Tianhao Chemical Company, Tianjin, China. Water was purified by a Milli-Q system (TTL-30C, Tongtai, Beijing, China). Amphotericin B and 3-(4,5-Dimethylthiazol-2-yl)-2,5-dephenyl tetrazolium bromide (MTT) were purchased from Amresco (Solon, OH, USA). Streptomycin sulfate, carbendazim, acetylcholinesterase (AChE), acetylthiocholine iodide (ATCI), 5,5′-dithio bis-(2-nitrobenzoic acid) (DTNB), and 9-amino-1,2,3,4-tetrahydroacridine hydrochloride hydrate were purchased from Sigma-Aldrich (USA). Avemectin B1, which was kindly provided by Dr. Shankui Yuan at the Institute for the Control of Agrochemicals, Chinese Ministry of Agriculture, was used as the positive control with the purity of 97.2 %. It was a mixture of avermectin B1a and avermectin B1b in the ratio of 9.5–0.5 (w/w). All other chemicals and reagents were of analytical grade.
Preparation of the crude sample
The endophytic fungus Hyalodendriella sp. Ponipodef12 (GenBank accession number HQ731647) was isolated from the healthy stems of the ‘Neva’ hybrid of Populus deltoides Marsh × P. nigra L. in our previous study (Zhong et al. 2011). It was stored both on PDA slants at 4 °C and in 40 % glycerol at −70 °C in the Herbarium of the College of Agronomy and Biotechnology, China Agricultural University (Beijing, China).
The fungus was stored on PDA slants at 4 °C, and grown on PDA plates at 25 °C for 8–10 days before being used. Then, 4–5 plugs of agar medium (0.5 cm × 0.5 cm) with fungal cultures were inoculated in each 250-ml Erlenmeyer flask containing 100 ml potato dextrose broth (PDB) medium, and incubated on a rotary shaker at 150 rpm and 25 °C for 7 days. The endophytic fungus strain Ponipodef12 was cultivated in a 500-ml Erlenmeyer flask, containing 100 g of rice (purchased from the local grocery store) and 100 ml of distilled water. The rice medium was autoclaved at 121 °C for 30 min. After sterilization, the medium was inoculated and mixed with the mycelium pellets, and incubated statically at 25 °C for 45 days.
At the end of the incubation period, the cultures were harvested, dried, and ground. The dry materials (136 g) were extracted with MeOH (3 × 300 ml). After filtration, the filtrate was concentrated under vacuum at 50 °C, the brown residue was suspended in water (100 ml) and fractionated successively with petroleum ether (3 × 100 ml), ethyl acetate (3 × 100 ml) and n-butanol (3 × 100 ml), to give their corresponding fractions. A total of 400 mg concentrated ethyl acetate crude fraction with the target compounds was obtained.
Solvent system for HSCCC
The two-phase solvent systems containing different ratios of n-hexane, ethyl acetate, methanol and water were prepared (Table 1). The partition coefficients (K values) of the pure compounds (botrallin and TMC-264) were determined by HPLC and calculated according to the ratio of the compound concentrations in the stationary phase and mobile phase according to the method described by Ito (2005). K = C stationary phase/C mobile phase, where C stationary phase is the compound concentration in the stationary phase (upper phase), and C mobile phase is the compound concentration in the mobile phase (lower phase). As the volumes of the upper and lower phases of the pre-equilibrated two-phase solvent system are equal, the K values are then determined according the ratio of the peak area, K = A stationary phase/A mobile phase, where A stationary phase is the peak area of the target compound in the stationary phase (upper phase), and A mobile phase is the peak area of the target compound in the mobile phase (lower phase). Each K value was an average of three replicates. The separation factor (α = K 2/K 1, K 2 > K 1) for two compounds was also obtained (Wen et al. 2009). The HPLC analysis was performed using a reversed-phase C18 column (250 mm × 4.6 mm, 5 μm) at 40 °C. The elution system consisted of solvent A (MeOH) and solvent B (H2O) at the volume ratio of 60:40 (v/v), elution was done at 1.0 ml/min. UV detection was at 234 nm.
Preparation of the two-phase solvent system and sample solution
The two-phase solvent system composed of n-hexane–ethyl acetate–methanol-water (1.2:1.0:0.9:1.0, v/v) was used for HSCCC separation. Each solvent was added to the separatory funnel according to the volume ratios and thoroughly equilibrated at room temperature overnight. The two-phases were separated and degassed by sonication prior for 20 min to use.
The sample solution was prepared by dissolving 300 mg crude extract in a 30 ml of the mixture of upper and lower phase (1:1, v/v) of the solvent system used for HSCCC separation.
HSCCC separation procedure
The upper phase was used as the stationary phase and the lower phase was used as the mobile phase in the tail-to-head elution mode. In each separation, the multilayer-coiled column was first filled entirely with the stationary phase. The mobile phase was then pumped into the tail end of the inlet column at a flow rate of 3.0 ml/min, while the apparatus was rotated at 820 rpm. After the mobile phase front emerged and hydrodynamic equilibrium was established in the column, 10.0 ml of the sample solution containing 100 mg of the crude extract was injected. This process was repeated for three times, and the same fractions were combined and concentrated. During the separation process, the column temperature was controlled at 25 °C, and the effluent of the column was continuously monitored with a UV detector at 254 nm. Each peak fraction was collected according to the chromatogram. The retention ratio (S F) of the stationary phase was calculated from the volume of the stationary phase collected from the column after the separation, and the equation was as follows:
where S F is the retention ratio of stationary phase (upper phase), and V S is the volume (i.e., 97 ml) of stationary phase flowing out. V T is the total volume (i.e., 280 ml) of the coils containing three multilayer coil separation columns (Ito 2005).
Analysis and identification of HSCCC peak fractions
The ethyl acetate crude extract and peak fractions separated by HSCCC were analyzed by HPLC. The analyses were performed with a reversed-phase C18 column at 40 °C. The mobile phase composed of methanol–water (60:40, v/v) was eluted at a flow rate of 1.0 ml/min and the effluent monitored at 234 nm. Then each peak fraction separated by HSCCC was further purified by semi-preparative HPLC with a reversed-phase C18 column eluted at a flow rate of 3.0 ml/min and monitored at 234 nm.
The purified and identified botrallin (1) and TMC-264 (2) were used as the standards. The linear equation of botrallin (1) by HPLC analysis was Y = 5.71711 × 106 X − 85792.5 (R 2 = 0.9997), and that of TMC-264 (2) was Y = 1.62393 × 107 X − 137986 (R 2 = 0.9981), where Y is the peak area, X is quality (μg) of the sample injected for each time, and R is the correlation coefficient. The physicochemical and spectrometric data of two compounds were given as follows.
Botrallin (1). Yellow needle-like crystals (MeOH); m.p. 223–227 °C; UV (MeOH) λmax: 234, 266 nm; The molecular formula C16H14O7 was assigned by HR–ESI–MS m/z 319.0814 [M + H]+ (calcd. for C16H15O7, 319.0813); 1H NMR (DMSO-d 6) δ (ppm): 6.25 (1H, s, OH-1), 3.67 (3H, s, OCH3-3), 6.17 (1H, s, H-4), 1.69 (3H, s, CH3-4a), 11.35 (1H, s, OH-7), 6.84 (1H, d, J = 2.36 Hz, H-8), 3.92 (3H, s, OCH3-9), 7.64 (1H, d, J = 2.36 Hz, H-10); 13C NMR (DMSO-d 6) δ (ppm): 141.6 (C-1), 172.1 (C-2), 146.8 (C-3), 55.1 (OMe-3), 125.1 (C-4), 68.5 (C-4a), 29.2 (CH3-4a), 163.6 (C-6), 101.1 (C-6a), 163.5 (C-7), 102.5 (C-8), 165.9 (C-9), 56.2 (OCH3-9), 106.9 (C-10), 135.1 (C-10a), 130.0 (C-10b). After comparing the data with those reported in the literature (Kameda et al. 1974; Hormazabal et al. 2005), the compound was identified as 1,7-dihydroxy-3,9-dimethoxy-4a-methyl-6H-dibenzo[b,d]pyran-2,6-(4aH)-dione, named botrallin.
TMC-264 (2). Yellow amorphous powder (CHCl3); m.p. 164–166 °C; UV (MeOH) λmax: 237, 267 nm; The molecular formula C16H13Cl O7 was assigned by HR-ESI–MS m/z 353.04291 [M + H]+ (calcd. for C16H14ClO7, 353.04226); 1H NMR (400 MHz, CDCl3) δ (ppm): 1.89 (3H, s, CH3-1), 3.35 (1H, br s, OH-1), 3.95 (3H, s, OCH3-3), 11.15 (1H, s, OH-7), 6.58 (1H, d, J = 2.16 Hz, H-8), 3.92 (3H, s, OCH3-9), 7.60 (1H, d, J = 2.16 Hz, H-10); 13C NMR (100 MHz, CDCl3) δ (ppm): 72.3 (C-1), 29.2 (CH3-1), 146.0 (C-2), 146.7 (C-3), 60.7 (OCH3-3), 172.0 (C-4), 141.5 (C-4a), 163.7 (C-6), 101.1 (C-6a), 164.5 (C-7), 103.4 (C-8), 166.8 (C-9), 56.1 (OCH3-9), 107.1 (C-10), 134.1 (C-10a), 128.9 (C-10b). After comparing the data with those reported in the literature (Sakurai et al. 2003a, b), the compound was determined to be 2-chloro-4,6-dihydro-1,7-dihydroxy-3,9-dimethoxy-1-methyl-1H-dibenzo[b,d]pyran-4,6-dione, named TMC-264. The stereochemistry of C-1 remains to be determined.
Biological activity
Antibacterial activity assay
Four Gram-negative (Xanthomonas vesicatoria ATCC 11633, Ralstonia solanacearum ATCC 11696, Pseudomonas lachrymans ATCC 11921, and Agrobacterium tumefaciens ATCC 11158), and two Gram-positive (Bacillus subtilis ATCC 11562 and Staphylococcus haemolyticus ATCC 29970) bacteria were selected for the antibacterial activity assay. DMSO was used as the negative control, and streptomycin sulfate was used as the positive control. A modified broth dilution-colorimetric assay using the chromogenic reagent 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was used to detect the antibacterial activity of the compounds (Langfied et al. 2004). This assay was described in detail in our previous report (Meng et al. 2012). The median inhibitory concentration (IC50) was obtained using the linear relation between the inhibitory probability and concentration logarithm (Sakuma 1998). The IC50 values were calculated from three individual determinations. The percentage (%) of bacterial growth inhibition was determined by the formula:
where A c was an average of three replicates of the light absorption at 510 nm of the negative controls, and A t was an average of three replicates of the light absorption at 510 nm of the samples. The median inhibitory concentration (IC50) was calculated using the linear relation between the inhibitory probability and concentration logarithm.
Antifungal activity assay
Antifungal activity assay was similar to that of antibacterial activity which was described in detail in our previous report (Meng et al. 2012). Two fungal species Magnaporthe oryzae P131 and Candida albicans ATCC 2538 were selected for antifungal activity. For M. oryzae P131, the negative control was 5 % acetone, and the positive control was carbendazim. For C. albicans ATCC 2538, the negative control was DMSO, and the positive control was amphotericin B. The IC50 values were calculated from three individual determinations. The percentage of spore germination inhibition was determined by the expression:
where G c is an average of three replicates of germinated spore number in the negative control, and G t is an average of three replicates of germinated numbers in the treated sets. The IC50 value calculation for the spore germination inhibition was the same as that for antibacterial activity assay.
Acetylcholinesterase inhibitory activity assay
The assay was carried out as described by Ellman et al. (1961) using a microplate spectrophotometer with some modifications. In the 96-well plates, 50 μl of pH 7.4 phosphate buffered saline (PBS) solution, 10 μl of 1.0 U/ml enzyme (AChE) solution, 10 μl of a serially diluted solutions of the compounds, and 9-amino-1,2,3,4-tetrahydroacridine hydrochloride hydrate as the positive control were added. After the mixture was maintained at 37 °C for 15 min, 20 μl of acetylthiocholine iodide (ATCI) solution was added. After the mixture was maintained at 37 °C for another 15 min, 30 μl of 1.0 % SDS solution and 30 μl of DTNB solution, the absorbance was measured at 415 nm immediately. pH 7.4 PBS solution was used as the negative control. The percentage (%) of acetylcholinesterase inhibitory activity was determined using the following expression:
where Ac was an average of three replicates of the light absorption at 415 nm of the negative controls, and At was an average of three replicates of the light absorption at 415 nm of the samples. The IC50 value calculation for the acetylcholinesterase activity was the same as that for antibacterial activity assay.
Antinematodal activity assay
This assay was carried out as described by Meng et al. (2012) with some modifications. Three nematode species (i.e., Bursaphelenchus xylophilus, Caenorhabditis elegans, and Panagrellus redivivus) were tested for antinematodal activity of the two compounds. 5 % acetone–water solution was used as the negative control. Avemectin B1 was used as the positive control. Five replicates were carried out for each treatment. The nematodes were considered to be dead when they did not move by treating with a fine needle as the physical stimuli. The mean percentage of mortality was then calculated, and the IC50 value calculation for the antinematodal activity was the same as that for antibacterial activity assay.
Results and discussion
Separation and structural identification of botrallin and TMC-264
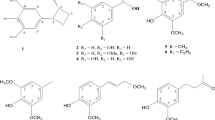
The crude ethyl acetate extract from endophytic fungus Hyalodendriella sp. Ponipodef12 was first analyzed by reversed-phase HPLC (Fig. 1). Two main peaks (i.e., peaks I and II) were satisfactorily separated with methanol–water (60:40, v/v) as the solvent system. Accordingly, two compounds, which correspond to peaks I and II, respectively in Fig. 1, were obtained from the ethyl acetate extract by repeated column chromatography over normal and reverse silica gel. After comparing their physicochemical and spectrometric data with those reported in the literature (Sakurai et al. 2003b; Hormazabal et al. 2005), they were identified as botrallin (1) and TMC-264 (2), whose chemical structures are shown in Fig. 2. Both compounds belong to dibenzo-α-pyrones. This is the first time for TMC-264 (2) to be isolated from the endophytic fungus Hyalodendriella sp. Ponipodef12 as well as being obtained as natural product for the second time. In this work, rice medium was employed instead of potato dextrose broth (PDB) used in our previous study (Meng et al. 2012) to lead to difference of the secondary metabolites. This is an interesting topic about biosynthetic regulation of the dibenzo-α-pyrones in endophyitc fungus Hyalodendriella sp. which needs to be investigated in detail.
Selection of two-phase solvent system for HSCCC
Successful separation by HSCCC largely depends upon the selection of a suitable two-phase solvent system, and the two-phase solvent system was selected according to the partition coefficients (K values) of each target component and an optimum range of K value should be from 0.5 to 2.0 (Han et al. 2012). A smaller K value elutes the solute closer to the solvent front with lower resolution while a larger K value tends to give better resolution but broader peaks due to a longer elution time (Ito 2005). In addition, the value of separation factor (α) between two compounds should be higher than 1.5 (Wen et al. 2009).
In this experiment, the K values for botrallin (1) and TMC-264 (2) in several two-phase solvent systems containing n-hexane–ethyl acetate–methanol-water were measured and shown in Table 1. Among these solvent systems, the most appropriate K values were 0.73 for botrallin (1) and 1.68 for TMC-264 (2) at a ratio of 1.2:1.0:0.9:1.0 (v/v), which was selected to further isolate and purify the two target compounds from the crude extract of the endophytic fungus Hyalodendriella sp. Ponipodef12 by HSCCC in the present study. Under this condition, the separation factor (α) of the two compounds was calculated as 2.30 which should be acceptable.
Separation of botrallin and TMC-264 from crude extract by HSCCC
Under the optimized condition with a solvent system containing n-hexane–ethyl acetate–methanol-water at a volume ratio of 1.2:1.0:0.9:1.0, about 100 mg of the crude ethyl acetate extract was separated by HSCCC for each time. Two fractions (i.e., the fractions of peaks I and II shown in Fig. 3) of 25 ml each were collected according to the HSCCC chromatogram over 2 h. This process was repeated for three times, and the same fractions were combined and concentrated. The retention ratio (S F) of the ethyl acetate crude extract in the stationary phase was 65.36 %. The HSCCC chromatogram is shown in Fig. 3.
The collected HSCCC fractions were concentrated and further analyzed by HPLC which gave the chromatograms shown in Fig. 4. The fractions I (28.5 mg) and II (23.5 mg) were analyzed to contain botrallin with 74.73 % of purity and TMC-264 with 82.29 % of purity, respectively. The fractions with the retention time from 24 to 45 min in HSCCC were analyzed by HPLC as other minor compounds in the crude extract. The two main dibenzo-α-pyrones prepared by HSCCC were further purified by semi-preparative HPLC. Finally, 17.0 mg of botrallin (1) and 14.8 mg of TMC-264 (2) were purified from Hyalodendriella sp. Ponipodef12 using a combination of HSCCC and semi-preparative HPLC. Though it was efficient to prepare these two compounds with the method of HSCCC and semi-preparative HPLC, we should admit that the method in this work did not save the organic solvents. In recent years, the elution-extrusion, gradient elution, on-demand solvent preparation modes in HSCCC have been developed and applied to the preparation of natural products (Pereira da Silva et al. 2009; Sethi et al. 2009; Wu et al. 2012). These techniques should be favorable for our subsequent work.
Biological activities
Antimicrobial activity
Both botrallin (1) and TMC-264 (2) were tested with microplate-MTT colorimetric assay for their antifungal and antibacterial activity shown in Table 2. TMC-264 (2) exhibited stronger antimicrobial activity than botrallin (1). In a general way, the halogens favor biological properties of the compounds (Muller et al. 2007; Hagmann 2008). It is possible that chlorine atom in TMC-264 attributes to the strong antimicrobial activity that remains to be confirmed.
Inhibitory activity on acetylcholinesterase
The inhibitory activity of botrallin (1) and TMC-264 (2) on acetylcholinesterase (AChE) is shown in Table 3. The IC50 values were 58.04 μg/ml for botrallin (1) and 78.80 μg/ml for TMC-264 (2), respectively. The inhibitory activity of botrallin (1) on AChE was in agreement with the previous report (Hormazabal et al. 2005). One possible approach for the treatment of Alzheimer’s disease is the restoration of the level of acetylcholine (ACh) through the inhibition of AChE with the inhibitors (Huang et al. 2013). Both botrallin (1) and TMC-264 (2) from the endophyte Hyalodendriella sp. Ponipodef12 might be as the potential candidates for the treatment of Alzheimer’s disease.
Antinematodal activity
Antinematodal activity of botrallin (1) and TMC-264 (2) on the nematodes Bursaphelenchus xylophilus, Caenorhabditis elegans, and Panagrellus redivivus by using the microplate assay expressed as the median inhibitory concentration (IC50) values are depicted in Table 4.
As shown in Table 4, both compounds had moderate antinematodal activity on three nematodes. For each nematode, TMC-264 (2) showed stronger antinematodal activity than botrallin (1). This phenomenon is similar to that of their antimicrobial activity. It is possible that the stronger antinematodal activity of TMC-264 (2) might be due to the presence of chlorine atom in its structure which should be further verified. The mechanisms of inhibitory effects on acetylcholinesterase, bacteria, fungi, and nematodes of these two compounds also need to be further investigated.
The IC50 values of botrallin (1) for biological activity assay in this work were different from those in our previous report (Meng et al. 2012). The phenomena may be attributed to the modified methods as well as the differences of experimental conditions in this study.
Conclusions
Endophytic fungi are potential sources of bioactive natural compounds for their application in medicine, agriculture, and food industry (Strobel 2003; Guo et al. 2008; Zhou et al. 2010; Kharwar et al. 2011; Zhao et al. 2011; Gutierrez et al. 2012; Kumar and Kaushik 2012). The preparative separation of natural compounds from endophytic fungi by classical methods is tedious, time consuming, and requires multiple chromatographic steps (Shan et al. 2013). In this work, two dibenzo-α-pyrones, botrallin (1) and TMC-264 (2) were successfully obtained from crude ethyl acetate extract of the endophytic fungus Hyalodendriella sp. Ponipodef12 using a combination of HSCCC and semi-preparative HPLC. This is the first report on the application of HSCCC for the preparative separation of dibenzo-α-pyrones from the cultures of endophytic fungus Hyalodendriella sp. Ponipodef12. The present study will provide a basis for a large preparation of dibenzo-α-pyrones, and also demonstrates that HSCCC is an efficient technique in preparatively separating bioactive compounds from fungi.
TMC-264 (2) was isolated from the endophytic fungus Hyalodendriella sp. Ponipodef12 for the first time as well as being obtained as the natural product for the second time. TMC-264 (2) showed strong antimicrobial and antinematodal activity, and botrallin (1) exhibited moderate inhibitory activity on acetylcholinesterase, which indicates these two compounds have their potential applications. To the best of our knowledge, this is the first report on the antimicrobial, antinematodal and acetylcholinesterase inhibitory activities of TMC-264 (2). The results indicate the potential of the endophytic fungus Hyalodendriella sp. Ponipodef12 as a source of active dibenzo-α-pyrones. Some issues such as the mechanisms of action of botrallin (1) and TMC-264 (2), isolation of the other active compounds from this fungus, and efficient strategies for increasing content and yield of botrallin and TMC-264 in fermentation culture of Hyalodendriella sp. Ponipodef12 need to be further investigated.
References
Chen L-J, Games DE, Jones J (2003) Isolation and identification of four flavonoid constituents from the seeds of Oroxylum indicum by high-speed counter-current chromatography. J Chromatogr A 988:95–105
Chen J, Wang F, Lee F, Wang X, Xie M (2006) Separation and identification of water-soluble salvianolic acids from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography and ESI-MS analysis. Talanta 69:172–179
Ellman G, Courtney K, Andres V, Featherstone R (1961) A new and rapid colorimetric determination of acetylcholinestrase activity. Biochem Pharmacol 7:88–90
Feng Z, Chen X, Zhang J, Di D (2013) Activity-screening-guided isolation and purification for vasodilative effects compounds from Radix Astragali by high-speed counter-current chromatography using gradient elution. Nat Prod Res 27:1020–1022
Guo B, Wang Y, Sun X, Tang K (2008) Bioactive natural products from endophytes: a review. Appl Biochem Microbiol 44:136–142
Gutierrez RMP, Gonzalez AMN, Ramirez AM (2012) Compounds derived from endophytes: a review of phytochemistry and pharmacology. Curr Med Chem 19:2992–3030
Hagmann WK (2008) The many roles for fluorine in medicinal chemistry. J Med Chem 51:4359–4369
Han L, Ji L, Boakye-Yiadom M, Li W, Song X, Gao X (2012) Preparative isolation and purification of four compounds from Cistanches deserticola Y.C. Ma by high-speed counter-current chromatography. Molecules 17:8276–8284
Holler U, Konig G, Wright A (1999) A new tyrosine kinase inhibitor from a marine isolate of Ulocladium botrytis and new metabolites from the marine fungi Asteromyces cruciatus and Varicosporina ramulosa. Eur J Org Chem 1999:2949–2955
Hormazabal E, Schmeda-Hirschmann G, Astudillo L, Rodriguez J, Theoduloz C (2005) Metabolites from Microsphaeropsis olivacea, an endophytic fungus of Pilgerodendron uviferum. Z Naturforsch C 60:11–21
Huang L, Su T, Li X (2013) Natural products as sources of new lead compounds for the treatment of Alzheimer’s disease. Curr Top Med Chem 13:1864–1878
Ito Y (2005) Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J Chromatogr A 1065:145–168
Jin J, Li Y, Tanui EK, Han L, Jia Y, Zhang L, Wang Y, Zhang X, Zhang Y (2013) Fishing and knockout of bioactive compounds using a combination of high-speed counter-current chromatography (HSCCC) and preparative HPLC for evaluating the holistic efficacy and interaction of the components of Herba Epimedii. J Ethnopharmacol 147:357–365
Kameda K, Aoki H, Namiki M (1974) An alternative structure for botrallin a metabolite of Botrytis allii. Tetrahedron Lett 1:103–106
Kharwar RN, Mishra A, Gond SK, Stierle A, Stierle D (2011) Anticancer compounds derived from fungal endophytes: their importance and future challenges. Nat Prod Rep 28:1208–1228
Kumar S, Kaushik N (2012) Metabolites of endophytic fungi as novel source of biofungicide: a review. Phytochem Rev 11:507–522
Langfied RD, Scarano FJ, Heitzman ME, Kondo M, Hammond GB, Neto CC (2004) Use of a modified microplate bioassay method to investigate antibacterial activity in the Peruvian medicinal plant Peperomia galioides. J Ethnopharmacol 94:279–281
Lu H-T, Jiang Y, Chen F (2003) Preparative separation and purification of squalene from the microalga Thraustochytrium ATCC 26185 by high-speed counter-current chromatography. J Chromatogr A 994:37–43
Mao Z, Sun W, Fu L, Luo H, Lai D, Zhou L (2014) Natural dibenzo-α-pyrones and their bioactivities. Molecules 19:5088–5108
Meng X, Mao Z, Lou J, Xu L, Zhong L, Peng Y, Zhou L, Wang M (2012) Benzopyranones from the endophytic fungus Hyalodendriella sp. Ponipodef12 and their bioactivities. Molecules 17:11303–11314
Mudge E, Lopes-Lutz D, Brown PN, Schieber A (2013) Purification of phenylalkanoids and monoterpene glycosides from Rhodiola rosea L. roots by high-speed counter-current chromatography. Phytochem Anal 24:129–134
Muller K, Faeh C, Diederich F (2007) Fluorine in pharmaceuticals: looking beyond intuition. Science 317:1881–1886
Pereira da Silva V, Oliveira RR, Figueiredo MR (2009) Isolation of limonoids from seeds of Carapa guianensis Aublet (Meliaceae) by high-speed counter-current chromatography. Phytochem Anal 20:77–81
Sakuma M (1998) Probit analysis of preference data. Appl Entomol Zool 33:339–347
Sakurai M, Nishio M, Yamamoto K, Okuda T, Kawano K, Ohnuki T (2003a) TMC-264, a novel inhibitor of STAT6 activation produced by Phoma sp. TC 1674. J Antibiot 56:513–519
Sakurai M, Nishio M, Yamamoto K, Okuda T, Kawano K, Ohnuki T (2003b) TMC-264, a novel antiallergic heptaketide produced by the fungus Phoma sp. TC 1674. Org Lett 5:1083–1085
Sethi N, Anand A, Sharma A, Chandrul KK, Jain G, Srinivasa KS (2009) High speed counter current charomatogrphy-a support free LC technique. J Pharm Bioallied Sci 1:14–22
Shan T, Lu S, Luo C, Luo R, Mou Y, Wang M, Peng Y, Zhou L (2013) Preparative separation of spirobisnaphthalenes from endophytic fungus Berkleasmium sp. Dzf12 by high-speed counter-current chromatography. Molecules 18:12896–12908
Strobel GA (2003) Endophytes as sources of bioactive products. Mirobes Infect 5:535–544
Wang J, Wen Y, Chen X, Lin Y, Zhou J, Xie Y, Wang H, Jiang H, Zheng W (2010) Preparative separation of six antimycin A components from antimycin fermentation broth by high-speed counter-current chromatography. J Chromatogr A 1217:5687–5692
Wen Y, Wang J, Chen X, Le Z, Chen Y, Zheng W (2009) Application of silver ion in the separation of macrolide antibiotic components by high-speed counter-current chromatography. J Chromatogr A 1216:4668–4672
Wu D, Cao X, Wu S (2012) Overlapping elution-extrusion counter-current chromatography: a novel method for efficient purification of natural cytotoxic andrographolides from Andrographis paniculata. J Chromatogr A 1223:53–63
Zhao J, Shan T, Mou Y, Zhou L (2011) Plant-derived bioactive compounds produced by endophytic fungi. Mini-Rev Med Chem 11:159–168
Zhong L, Zhou Y, Gao S, Xu L, Zhao J, Shan T, He W, Zhou L (2011) Endophytic fungi from the hybrid ‘Neva’ of Populus deltoides Marsh × Populus nigra L. and their antimicrobial activity. Afr J Microbiol Res 5:3924–3929
Zhou L, Zhao J, Shan T, Cai X, Peng Y (2010) Spirobisnaphthalenes from fungi and their biological activities. Mini-Rev Med Chem 10:977–989
Acknowledgments
This work was co-financed by the grants from the National Basic Research Program of China (2010CB126105), Hi-Tech R&D Program of China (2011AA10A202), and Program for Changjiang Scholars and Innovative Research Team in University of China (IRT1042).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, Z., Luo, R., Luo, H. et al. Separation and purification of bioactive botrallin and TMC-264 by a combination of HSCCC and semi-preparative HPLC from endophytic fungus Hyalodendriella sp. Ponipodef12. World J Microbiol Biotechnol 30, 2533–2542 (2014). https://doi.org/10.1007/s11274-014-1678-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-014-1678-0