Abstract
Meteloxetin (1), a new phenolic amino-oxetane, and eight known new source phenols (2–9) have been bioassay-directed isolated from methanolic extract of Datura metel Linn. Their structures were elucidated through modern spectroscopic data. The plant extract showed the significant inhibition potential against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), and its dichloromethane (DCM) fraction exhibited the remarkable inhibition potentials against AChE with IC50 (inhibition concentration) value 1.32 ± 0.02 µg/ml and BChE with IC50 value 1.13 ± 0.01 µg/ml, when compared with the standard drug eserine (AChE, IC50 0.04 ± 0.01 µg/ml) and galanthamine (BChE, IC50 0.92 ± 0.01 µg/ml). The bioactive DCM fraction was subjected to systematic isolation protocol to isolate the 1–9 compounds, and all were subjected to evaluate their AChE and BChE inhibition potentials. From these isolates, compound 1 showed the effective inhibition potential against BChE with IC50 value 0.84 ± 0.03 µg/ml and excellent inhibition potential against AChE with IC50 value 0.07 ± 0.02 µg/ml. This strong inhibition potential of 1 is due to the presence of amino-oxetane groups in it. The in silico studies indicate that oxetane rings contain high-energy oxygen, which makes it a marvelous pharmacophore with diverse biological potentials. The potent nature of compound 1 has also been evaluated by exploring its electronic properties, molecular electrostatic potential and Hirshfeld analysis by density functional theory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nature has provided enormous botanical wealth grown in various regions of the world to serve humanity against various diseases. More than 80% of the world population are dependent on herbal medicines and approximately 33% of drugs are obtained from plants which are being utilized by the people [1]. Medicinal plants possess drug-like phytochemicals which are used for the treatment of various diseases [2]. Due to medicinal values, plants have engrossed the attention of scientists to develop drugs in the pharmaceutical industry against multi-drug resistant bacterial species. Datura metel Linn. (Thorn apple) belongs to the Solanaceae family, which comprises 85 genera and 2800 species [3, 4]. D. metel is an annual herb, distributed in Asia, Mediterranean, Africa and America, growing throughout the year with violet color flowers having fruit diameter 1.25 inches. This herb was used as a local traditional herbal medicine for the remedy of psoriasis, analgesia, skin rashes, jaundices, rheumatism, ulcers and diabetes. The pharmacological analysis confirmed that this plant possesses significant potential of antimicrobial, antioxidant, hypoglycemic and analgesic [5, 6]. D. metel L. is rich in alkaloids, but seeds contain a higher percentage of alkaloids as compared to other parts of the species. Along with alkaloids, this plant also contains steroids, phenols, alkaloids, terpenoids, tannins, flavonoids and coumarins [7,8,9,10].
Cholinesterase (ChE) is a major part of cholinergic synapses, and it hydrolyzes the acetylcholine that is released from the presynaptic nerve terminals. However, scientific investigations described that despite ChE present in cholinergic tissues, it is also present in various non-cholinergic tissues, which include hematopoietic cells [11]. BChE is a non-specific cholinesterase enzyme that hydrolyzes various types of choline-based esters. Therefore, AChE and BChE inhibition belongs to the most effective rational approaches for the remedy of Alzheimer’s disease (AD). AD is a progressive neurodegenerative ailment of the brain, affect the cognitive dysfunction, loss of memory, loss of speech impairment as well as dementia, which is one of the major causes of disability in the elder population in the world [12, 13]. The irreversible impairment of mental processes, primarily the degeneration condition of the brain, leads to neuropathological affected parts of the brain that leads to the formation of amyloid plaques, and damaging of neurons as well. Mental processes as well as cognition ability of the brain declines due to the destruction of the cholinergic system, limbic system and neocortex of the brain. The link of cholinergic impairment as well as cognitive processes, engrossed attention toward the major development of the scientific approach to develop the cognition neurophysiology as well as to discover new therapeutic agents to fight against Alzheimer’s disease. The drugs used in the treatment of AD, mostly target both AChE as well as BChE [14]. ChE inhibition activity is considered as a probable target in the remedy of neurological ailments which include Alzheimer’s disease (AD), dementia, myasthenia gravis, ataxia as well as Parkinsonism disease (PD) [15]. AChE inhibitors were found to be feasible as a therapeutic agent because of the approved effectiveness of inhibition of peripheral, and they are used for the treatment of myasthenia gravis (MG). The classic AChE inhibitors (AChEI): physostigmine and tacrine were utilized for the treatment of AD. Because of the poor tolerability of physostigmine, which means absorption distribution elimination, it was eventually abandoned. New AChE inhibitors are always in search to obtain better therapeutic effects with minimum side effects in the treatment of the above-mentioned damaging diseases [16]. The currently approved drug for the treatment of AD includes rivastigmine, galantamine as well as donepezil. Further, plants have also been reported to serve as potential sources of AChE inhibitors [17].
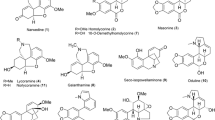
Due to the medicinal importance and presence of different classes of compounds in Datura metel L., a study was planned to evaluate its inhibition potentials against cholinesterase enzymes, bioassay-guided isolation of bioactive constituents from this plant species and in silico studies on the most active isolate. As, inhibiting the activities of these enzymes may prove useful in the treatment of various diseases. In the present work, bioactive dichloromethane-soluble fraction of methanol extract of D. metel was subjected to systematic isolation through bioassay guidelines to isolate the new potent cholinesterase inhibitory compound, meteloxetin (1), along with eight new source known phenols (2-9) as shown in Fig. 1. Their structures were elucidated through modern spectroscopic data. The density functional descriptors in the development of quantitative structure–activity relationship (QSAR) are imperative to explore the nature of interaction, active sites and biological activity of molecules. The molecular descriptors, frontier molecular orbitals, ionization potential, electron affinity, molecular electrostatic potential and Hirshfeld analysis are significant to comprehend the active sites which have been probed in current work. We have also lime lighted the global reactivity descriptors, e.g., electrophilicity index (ω), chemical hardness (η), chemical potential (µ), electronegativity (χ) and softness (S).
2 Experimental
2.1 General
Column chromatography (CC) was performed on 70-230 mesh silica gel (E. Merck), and flash CC was performed on EF-10 Eyela flash chromatography model, using 230-400 mesh silica gel (E. Merck) as absorbent. Precoated silica gel GF-254 preparative plates (PTLC) (E. Merck; 20 × 20, 0.5 mm thick) were used for isolation of mixture. TLC (Thin Layer Chromatography) plates were visualized under UV-lamp at 254 and 366 nm. For optical rotation measurement (JASCO DIP-360) digital polarimeter; for ultraviolet–visible (UV) spectra (Hitachi U-3200) and for IR spectra (JASCO 302-A) were used. Finnigan MAT 311 mass spectrometer was used for the EI-MS (Electron Impact-Mass Spectrum) and HR-EI-MS measurement. The 1D and 2D NMR (Nuclear Magnetic Resonance) spectra were recorded on Bruker spectrometers with chemical shift in ppm (δ) and coupling constants (J) are in Hz.
2.2 Plant material
The fresh plant of Datura metel Linn was collected in June from Dera Ghazi Khan, district of Punjab province of Pakistan and identified by Plant Taxonomist Dr. Muhammad Javid, University of Education Lahore, DG Khan Campus, where a voucher specimen UEBOTDGK001 has been deposited.
2.3 Extraction and isolation
The whole plants of D. metel Linn (10 kg) were collected, shade dried, ground and extracted with CH3OH (3 × 15 L). The combined filtrate was evaporated under reduced pressure to obtain a crude greenish extract and subjected to measure its inhibition potentials. Extract was suspended in H2O and successively partitioned into n-hexane, CH2Cl2, AcOEt and n-butanol sub-portions, and measured their inhibition potentials. The most bioactive CH2Cl2 portion was subjected to CC using flash silica gel, eluting with n-hexane, CH2Cl2, and MeOH mixture to obtain six major fractions IA–IF. The fraction IA which was obtained with n-hexane-CH2Cl2 (8.3:1.7) was rechromatographed over silica gel and eluted with n-hexane-CH2Cl2 (8.1:1.9) to afford compound 2,6-dimethoxy-4-methylphenol (7) and 4-hydroxybenzoic acid (2), respectively. The binary mixture of fraction IB (7.8:2.2) was subjected to PTLC over silica gel using n-hexane-CH2Cl2 (7.1:2.9) as eluent to obtain two aromatic acids 3-hydroxy-4-methoxybenzoic acid (3) and 3,5-dihydroxybenzoic acid (4). The fraction IC was further eluted with n-hexane-CH2Cl2 (7.0:3.0) as well as using PTLC to give new compounds meteloxetin (1). The fraction ID obtained from n-hexane-CH2Cl2 (6.6:3.4) was a mixture of two components and was separated by CC using the same solvent system to afford compounds 2,6-dimethoxy-4-[(1R)-1-methoxyethyl]-phenol and 2,6-dimethoxy-4-[(1R)-1-methoxypropyl]-phenol (6), respectively. The fraction IE n-hexane-CH2Cl2 (6.0:4.0) was further CC to afford compound 4-hydroxy-3-methoxy benzylacetone (8) and coniferyl methyl ether (9), respectively.
2.4 Meteloxetin (1)
Colorless amorphous powder; λmax (H2O) nm, 275; IR (KBr) υ: 3420 (OH), 3350 (NH2), 1613, 1540, 1410 (aromatic); 1H NMR (400 MHz, CD3OD) δ: 6.66 (2H, s, H-5, -9), 4.71 (1H, d, J = 4.23, H-1), 4.26 (1H, dd, J = 6.81, 9.00, Hα-3), 3.88 (1H, dd, J = 5.00, 9.00, Hβ-3), 3.84 (6H, s, 2 × OMe), 3.14 (1H, m, H-2); 13C NMR (100 MHz, CD3OD) δ: 87.6 (C-1), 55.5 (C-2), 72.8 (C-3), 133.2 (C-4), 104.7 (C-5, -9), 149.4 (C-6, -8), 136.4 (C-7), 56.9 (2 × OCH3); HR-EI-MS (70 eV) m/z: 225.0999 (calcd. 225.1001 for C11H15NO4).
2.5 Cholinesterase inhibition assay
The cholinesterase (BChE and AChE) inhibition was done by modified methods of Ellman et al. 1961 [15, 18]. The total volume of the reaction mixture was 100 µL, which includes 60 µL of buffer Na2H PO4 with 50 mM concentration having 7.7 pH. The tested sample was 10 µL with 0.5 mM concentration per well. Then added 10 µL enzymes having 0.5 unit of BChE or 0.005 unit of AChE per well. After the addition of reaction contents, readings were taken at 405 nm by a spectrophotometer. All ingredients were preincubated for about 10 min at 37 °C. Then, 10 µL of substrate (butyrylthiocholine chloride or acetylthiocholine iodide) was added to each well having a concentration of 0.5 mM per well leads to start the reaction. Then 10 µL of DTNB Ellman’s reagent (5,5′-dithiobis-2-nitrobenzoic acid) with 0.5 mM concentration was added to each well. After incubation for 15 min at 37 °C, the absorbance was measured at 405 nm by using a spectrophotometer. The positive control used was galantamine (for BChE) and eserine (for AChE) (0.5 mM well−1). The experiments were performed independently in triplicates with their controls. Percentage inhibition was calculated by Eq. 1
IC50 values (AChE and BChE) of samples were determined with EZ-Fit Enzyme kinetics software (Perella Scientific Inc. Amherst, USA).
2.6 Computational details
Density functional theory is an interesting approach to analyze numerous properties of interests in biological sciences. DFT was systematically used to probe the electronic properties of materials [19,20,21,22,23,24,25,26]. The DFT is a consistent approach for the ground state (S0) geometries optimization [27, 28]. The B3LYP is a coherent functional for the S0 geometries of numerous biologically active compounds. In the current study, B3LYP/TZ2P level was adopted to perform S0 geometries optimizations and electronic properties exploration using Amsterdam Density Functional (ADF) modeling suite.
3 Results and discussion
The bioactive dichloromethane subfraction of the methanolic extract of Datura metel Linn was subjected to chromatographic separations techniques to obtain one new meteloetin (1) and eight known 2–9 compounds and their structures were elucidated through 1D-NMR, 2D-NMR, HR-FAB-MS, UV and IR spectroscopic techniques. The isolates were evaluated for their cholinesterase inhibition potential. The structure–activity relationship and in silico studies on new potent cholinesterase inhibitors have also been carried out to check the active sites and other parameters.
Meteloxetin (1) was isolated as a white amorphous powder. Its HR-EI-MS spectrum exhibits the [M+] ion peak at m/z 225.0999, which is consistent with the molecular formula C11H15NO4 (calcd. m/z 225.1001). The UV spectrum of 1 displayed the absorption maxima at 275 nm, indicating the anisolic character. IR spectrum of 1 showed absorptions at 3420, 3350, 1613, 1540 and 1410 cm−1 indicating the presence of hydroxyl, amine and aromatic ring, respectively. The 13C-NMR (100 MHz, CD3OD) and DEPT spectra of 1 showed eight signals (Table 1) which comprised one methyl, one methylene, three methines and three quaternary carbons. From these eight signals, three signals show double intensities indicating two carbons for each. Therefore, compound 1 is an eleven carbon containing compound. The downfield region of the spectrum showed aromatic resonances for one methine at δ 104.7 and three quaternary carbons at δ 133.2, 136.4 and 149.4, indicating the presence of symmetric tetrasubstituted aromatic ring with two oxygen-bearing carbons. The methyl carbon resonated at δ 56.9, indicating its attachment with oxygen atom and has the intensity of two carbons. Among the remaining three carbons, the only methylene carbon showed a signal at δ 72.8 indicating its attachment with an oxygen whereas the methine carbon signals at 87.6 and 55.5 indicating their attachment with oxygen and nitrogen atoms, respectively, and these values were close to the reported values for the oxetane ring [29]. Three pathways have been reported for the biosynthesis of oxetane ring [30]. In the 1H-NMR (400 MHz, CD3OD) spectrum of 1, aromatic protons showed a singlet at δ 6.66 (2H) which revealed the presence of the tetrasubstituted symmetric benzene ring. The oxymethylene protons appeared at δ 4.26 (1H, dd, J = 6.87, 9.00, H-3) and 3.88 (1H, dd, J = 6.28, 11.5, H-3), whereas oxymethine and azomethine protons resonated at δ 4.71 (d, J = 4.22 Hz, H-1) and 3.14 (m, H-2) and two methoxy groups displayed singlet at 3.84 (6H).
In the COSY correlations of 1, oxymethine proton at δ 4.71 (H-1) showed correlation with azomethine proton at δ 3.14 (H-2) whereas oxymethylene proton at δ 4.26 (H-3) exhibited interactions with oxymethylene H at δ 3.88 (H-3) and azomethine proton at 3.14 (H-2) (Fig. 2). In the HMBC experiment of 1, the aromatic proton at δ 6.66 (H-5) displayed 2J correlations at δ 133.2 (C-4) and 149.4 (C-6) whereas 3J correlations at δ 136.4 (C-7) and 87.6 (C-1). The oxymethine H at δ 4.71 (H-1) exhibited 2J and 3J correlations through aromatic carbons at δ 133.2 (C-4) and 104.7 (C-5), confirming its attachment with the aromatic ring, and showed 2J and 3J correlations with azomethine carbon at δ 55.5 (C-2) and oxymethylene carbon at 72.8 (C-3) (Fig. 2). The COSY and HMBC correlations and the shifting of chemical shifts of oxymethine and azomethine to downfield and oxymethylene to upfield as compared to their open chain confirm the presence of oxetane ring. For the stereochemistry of 1, the proton at δ 4.71 (H-1) showed NOESY interactions at δ 3.88 (H-3) and 3.14 (H-2), confirming its cis geometry. On the basis of all spectral evidence, the meteloxetin (1) was confirmed as 4-[(2R,3R)-3-aminooxetanyl]-2,6-dimethoxyphenol, and its structure is shown in Fig. 1. The known phenols 2-9 namely, 4-hydroxybenzoic acid (2), 3-hydroxy-4-methoxybenzoic acid (3), 3,5-dihydroxybenzoic acid (4), 2,6-dimethoxy-4-[(1R)-1-methoxyethyl]-phenol (5), 2,6-dimethoxy-4-[(1R)-1-methoxypropyl]-phenol (6), 2,6- dimethoxy-4-methylphenol (7), 4-hydroxy-3-methoxy benzylacetone, (8), and coniferyl methyl ether (9) were elucidated through their spectroscopic and physical data shown in supporting information.
3.1 Acetyl cholinesterase and butyryl cholinesterase inhibition
The whole plants methanolic extract of D. metel and its dichloromethane fraction were evaluated to find its cholinesterase (AChE and BChE) inhibition potential through comparison with standard eserine for AChE and galanthamine for BChE. The result showed that Datura metel extract showed significant inhibition potentials. The DCM fraction showed the remarkable inhibition potentials against AChE and BChE with IC50 values 1.32 ± 0.02 and 1.13 ± 0.01 for AChE and BChE, respectively. Among isolated compounds, shown in Fig. 1, compound 1 showed acetylcholinesterase (AChE) inhibition potential with IC50 values 0.07 ± 0.02 which is just near to the eserine standard (IC50 0.04 ± 0.001) (Table 2) whereas butyrylcholinesterase (BChE) inhibition potential with IC50 values 0.84 ± 0.03 which is better than the galanthamine standard (IC50 00.92 ± 0.01) (Table 3). All the known compounds 2–9 showed moderate inhibition potential. The difference in the huge inhibition potential of compound 1 and known compounds is that compound 1 contains amino, oxetane and phenolic moieties in its structure whereas known compounds contain only phenolic groups in their structure.
Two types of cholinesterases (AChE and BChE) are mainly distributed in the blood as well as present at neural synapses. BChE is the neurotransmitter present in the liver. The major difference between AChE and BChE is of substrates. The drugs used in the treatment of AD mostly target both AChE as well as BChE [14]. The first acetylcholinesterase inhibitor was Tacrine, became available in 1993 and used in the treatment of AD, but a major adverse effect of it is hepatotoxicity [16]. However, these inhibitors are not the ultimate cure, these drugs can only prolong the progression of mental impairment, decrease symptoms of neuropsychics and therefore there is a need for exploration of suitable therapeutic approaches for the treatment of AD. The distribution of the number of hydroxyl or methoxy groups substituted at the phenyl ring on any compound make a major influence for inhibition activities by ChEs (AChE and BChE). Moreover, it has been explored by research that the methyl or ethyl esters of phenolic acids inhibited ChEs (AChE and BchE) more effectively when compared with the free acids. Another evidence [31] proved that the inhibition activity of a compound against AChE was affected by the presence of the OH group in the ortho position at the phenyl ring as well as the existence of the acidic group. It has been explored that oxetanes belong to non-aromatic heterocycles class containing high-energy oxygen make it a marvelous new potential pharmacophores with diverse potential spectrum corresponding to biological activities [32]. It has already been investigated with enhanced synthetic feasibility of O-heterocycles which have led to equally potent lead molecules used for different pathophysiological conditions [33]. It has been proved on the basis of the literature [32] survey as discussed above that because of the presence of oxetane ring compound 1 inhibited strongly AChE and it can be a future possible inhibitor of AChE.
3.2 Electronic properties
The lowest unoccupied molecular orbitals (LUMOs) and highest occupied molecular orbitals (HOMOs) of compound 1 at B3LYP/TZ2P level are shown in Fig. 3. In compound 1, intramolecular charge transfer (ICT) was found from HOMO to LUMO. The energies of HOMO (EHOMO), HOMO − 1 (EHOMO−1), LUMO + 1 (ELUMO+1), LUMO (ELUMO), and energy gap (Egap∆H–L) are vital to explore the electronic nature. The EHOMO−1, EHOMO, ELUMO, ELUMO+1 and Egap∆H–L of compound 1 at B3LYP/TZ2P level at ground state (S0) are exhibited in Table 4. Global chemical reactivity descriptors (GCRD) are imperative parameters to comprehend the structure stability and reactivity. Here, we assessed numerous GCRD parameters like chemical hardness (η), electronegativity (χ), softness (S), chemical potential (µ), and electrophilicity index (ω) using HOMO and LUMO energy values, see Table 4 (details can be seen in supporting information). The η of phytochemicals is interrelated to aromaticity [34, 35]. The μ expresses the electron trend to rush out from the electronic cloud. The η represents the extent of the impediment of the electronic cloud to deformation and ω means the stabilization energy when electrons from the external environment saturate the compound.
The work functions (ω) values of Au and Al are 5.10 and 4.08 eV, respectively (20). We have probed hole/electron injection energies (HIEs/EIEs) of compound 1 to Al and Au electrodes distinctly. The EIEs for compound 1 were observed (3.81 eV = − 0.27 − (−4.08)) and (4.83 eV = − 0.27 − (−5.10)) while HIEs were perceived (1.60 eV = − 4.08 − (−5.68)), and (0.58 eV = − 5.10 − (−5.68)) by considering Al and Au, respectively. One can see that for better electron injection, Al might be an appropriate electrode whereas for better hole injection Au would be suitable.
The charge analysis and MEP surfaces revealed that in the case of nucleophilic attack, substantial repulsion might be at oxygen and nitrogen atoms while for electrophilic attack significant attraction might be at oxygen and nitrogen atoms. It is also projected that with the proviso electrophilic/nucleophilic attack substantial repulsion/attraction might be at carbon atoms, respectively (See Fig. 4). The Hirshfeld, Mulliken and Badar charges of compound 1 are illustrated in Fig. 5.
4 Conclusion
Through bioassay direction, a new phenolic amino-oxetane, meteloxetin, was isolated from DCM fraction of methanolic extract of Datura metel. The new compound showed outstanding inhibition potential against AChE as compared to standard eserine and potent inhibition potential against BChE as compared to standard galantamine. The frontier molecular orbitals, molecular descriptors, MEP and charge analysis are describing that compound 1 would be an excellent biological active compound which is in correlation to our experimental work. The measurement of other parameters in in silico studies also fully supported the outstanding inhibition potential of the meteloxetin.
References
Ekor, M.: The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharm. 4, 177 (2014)
Newman, D.J.; Cragg, G.M.: Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79(3), 629–661 (2016)
Babalola, S.; Sulaiman, M.; Hassan, A.; Adawa, D.: Evaluation of the crude methanolic seed extract of datura metel l. As a potential oral anaesthetic in dogs. Vet. Res. 6, 115–119 (2013)
Jamdhade, M.; Survase, S.; Kare, M.; Bhuktar, A.: Phytochemical studies on datura metel linn. In marathwada region, maharashtra. J. Phytol. 2(12), 46–48 (2010)
Muthusamy, A.; Punitha, M.; Beslin, L.G.: Phytochemical screening of Datura metel Linn and its antimicrobial activity on selected human pathogens. Int. J. Bioassays 3(11), 3474–3478 (2014)
Sangeetha, S.; Deepa, M.; Sugitha, N.; Mythili, S.; Sathiavelu, A.: Antioxidant activity and phytochemical analysis of Datura metel. Int. J. Drug Dev. Res. 6(4), 46–53 (2014)
Liu, Y.; Guan, W.; Yang, C.-L.; Luo, Y.-M.; Liu, Y.; Zhou, Y.-Y.; Liu, L.-N.; Yang, B.-Y.; Kuang, H.-X.: Steroids with potential anti-inflammatory activity from the roots of datura metel l. Can. J. Chem. 98(2), 74–78 (2020)
Tan, J.-Y.; Liu, Y.; Cheng, Y.-G.; Sun, Y.-P.; Pan, J.; Yang, S.-H.; Kuang, H.-X.; Yang, B.-Y.: Anti-inflammatory sesquiterpenoids from the leaves of Datura metel L. Fitoterapia 142, 104531 (2020)
Yang, B.-Y.; Jiang, H.-B.; Liu, Y.; Chen, J.; Kuang, H.-X.: Steroids from the seeds of datura metel. J. Asian Nat. Prod. Res. 22(3), 257–263 (2018)
Srivastava, N.; Chauhan, A.; Sharma, B.: Isolation and characterization of some phytochemicals from Indian traditional plants. Biotech. Res. Int. 549850, 1–8 (2012)
Srivastava, N.; Sharma, R.; Singh, N.; Sharma, B.: Acetylcholinesterase from human erythrocytes membrane: a screen for evaluating the activity of some traditional plant extracts. Cell. Mol. Biol. 58(1), 160–169 (2012)
Lateef, M.; Azhar, A.; Siddiqui, B.S.; Zarina, S.; Anwar, M.F.; Siddiqui, K.; Azhar, K.F.; Iqbal, L.; Mehmood, R.; Perveen, S.: New anthrarobin acyl derivatives as butyrylcholinesterase inhibitors: synthesis, in vitro and in silico studies. Heliyon 3(7), e00350 (2017)
Lolak, N.; Boga, M.; Tuneg, M.; Karakoc, G.; Akocak, S.; Supuran, C.T.: Sulphonamides incorporating 1, 3, 5-triazine structural motifs show antioxidant, acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibitory profile. J. Enzyme Inhib. Med. Chem. 35(1), 424–431 (2020)
Mehta, M.; Adem, A.; Sabbagh, M.: New acetylcholinesterase inhibitors for alzheimer’s disease. Inter. J. Alzheimer’s Dis (2012). https://doi.org/10.1155/2012/728983
Imran, M.; Irfan, A.; Ibrahim, M.; Assiri, M.A.; Khalid, N.; Ullah, S.; Al-Sehemi, A.G.: Carbonic anhydrase and cholinesterase inhibitory activities of isolated flavonoids from oxalis corniculata l. And their first-principles investigations. Ind. Crops Prod. 148, 112285 (2020)
Ibach, B.; Haen, E.: Acetylcholinesterase inhibition in alzheimer’s disease. Current Pharmaceut. Design 10(3), 231–251 (2004)
Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J.: Acetylcholinesterase inhibitors from plants. Phytomedicine 14(4), 289–300 (2007)
Ellman, G.L.; Courtney, K.D.; Andres Jr., V.; Featherstone, R.M.: A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 7(2), 88–95 (1961)
Demirtaş, G.; Dege, N.; Ağar, E.; Şahin, S.: The crystallographic, spectroscopic and theoretical studies on (e)-2-[((4-fluorophenyl)imino)methyl]-4-nitrophenol and (e)-2-[((3-fluorophenyl)imino)methyl]-4-nitrophenol compounds. Iran. J. Chem. Chem. Engineer. 37(5), 55–65 (2018)
Irfan, A.; Imran, M.; et al.: Exploration of electronic nature and intrinsic mobility of 10-(1,3-dithiol-2-ylidene)anthracene based organic semiconductor materials. Optik 224, 165530 (2020)
Irfan, A.; Al-Zeidaneen, F.K.; Ahmed, I.; Al-Sehemi, A.G.; Assiri, M.A.; Ullah, S.; Abbas, G.: Synthesis, characterization and quantum chemical study of optoelectronic nature of ferrocene derivatives. Bull. Mater. Sci. 43(1), 45 (2020)
Khalil Warad, I.; Al-Nuri, M.; Ali, O.; Abu-Reidah, I.M.; Barakat, A.; Ben Hadda, T.; Zarrouk, A.; Radi, S.; Touzani, R.; Hicham, E.: Synthesis, physico-chemical, hirschfield surface and dft/b3lyp calculation of two new hexahydropyrimidine heterocyclic compounds. Iran. J. Chem. Chem. Eng. 38(4), 59–68 (2019)
Mikulski, D.; Eder, K.; Molski, M.: Quantum-chemical study on relationship between structure and antioxidant properties of hepatoprotective compounds occurring in cynara scolymus and silybum marianum. J. Theor. Comput. Chem. 13(01), 1450004 (2014)
Najafi, M.; Naqvi, S.A.R.: Theoretical study of the substituent effect on the hydrogen atom transfer mechanism of the irigenin derivatives antioxidant action. J. Theor. Comput. Chem. 13(02), 1450010 (2014)
Sadasivam, K.; Jayaprakasam, R.; Kumaresan, R.: A DFT study on the role of different oh groups in the radical scavenging process. J. Theor. Comput. Chem. 11(04), 871–893 (2012)
Seif, N.; Farhadi, A.; Badri, R.; Kiasat, A.R.: An experimental and theoretical study on bicyclo-3,4-dihydropyrimidinone derivative: synthesis and DFT calculation. Iran. J. Chem. Chem. Eng. (2019). https://doi.org/10.30492/ijcce.2019.35673
Irfan, A.; Assiri, M.; Al-Sehemi, A.G.: Exploring the optoelectronic and charge transfer performance of diaza[5]helicenes at molecular and bulk level. Org. Electron. 57, 211–220 (2018)
Al-Sehemi, A.G.; Irfan, A.: Effect of donor and acceptor groups on radical scavenging activity of phenol by density functional theory. Arab. J. Chem. 10(Supplement 2), S1703–S1710 (2017)
Omura, S.; Murata, M.; Imamura, N.; Iwai, Y.; Tanaka, H.; Furusaki, A.; Matsumoto, T.: Oxetin, a new antimetabolite from an actinomycete. J. Antibiot. 37(11), 1324–1332 (1984)
Bull, J.A.; Croft, R.A.; Davis, O.A.; Doran, R.; Morgan, K.F.: Oxetanes: recent advances in synthesis, reactivity, and medicinal chemistry. Chem. Rev. 116(19), 12150–12233 (2016)
Akhtar, M.N.; Lam, K.W.; Abas, F.; Ahmad, S.; Shah, S.A.A.; Choudhary, M.I.; Lajis, N.H.: New class of acetylcholinesterase inhibitors from the stem bark of knema laurina and their structural insights. Bioorg. Med. Chem. Lett. 21(13), 4097–4103 (2011)
Vil, V.; Terent’ev, A.O.; Al Quntar, A.A.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M.: Oxetane-containing metabolites: origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 103(6), 2449–2467 (2019)
Singh, P.K.; Silakari, O.: The current status of o-heterocycles: a synthetic and medicinal overview. Chem. Med. Chem. 13(11), 1071–1087 (2018)
Geerlings, P.; De Proft, F.; Langenaeker, W.: Conceptual density functional theory. Chem. Rev. 103(5), 1793–1874 (2003)
Vektariene, A.; Vektaris, G.; Svoboda, J.: A theoretical approach to the nucleophilic behavior of benzofused thieno [3,2-b] furans using dft and hf based reactivity descriptors. Arkivoc 7, 311–329 (2009)
Acknowledgements
The authors would like to acknowledge the support from the Deanship of Scientific Research at the King Khalid University for funding through the research groups program under Grant Number R.G.P.2/76/41.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Imran, M., Mehmood, R., Hussain, R. et al. Meteloxetin (1) Novel Phenolic Amino-Oxetane Cholinesterase Inhibitors from Datura metel Linn and First-Principle Investigations. Arab J Sci Eng 46, 5681–5690 (2021). https://doi.org/10.1007/s13369-020-05237-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05237-4