Abstract
Present study deals with the isolation of rhizobacteria and selection of plant growth promoting bacteria from Crocus sativus (Saffron) rhizosphere during its flowering period (October–November). Bacterial load was compared between rhizosphere and bulk soil by counting CFU/gm of roots and soil respectively, and was found to be ~40 times more in rhizosphere. In total 100 bacterial isolates were selected randomly from rhizosphere and bulk soil (50 each) and screened for in-vitro and in vivo plant growth promoting properties. The randomly isolated bacteria were identified by microscopy, biochemical tests and sequence homology of V1–V3 region of 16S rRNA gene. Polyphasic identification categorized Saffron rhizobacteria and bulk soil bacteria into sixteen different bacterial species with Bacillus aryabhattai (WRF5-rhizosphere; WBF3, WBF4A and WBF4B-bulk soil) common to both rhizosphere as well as bulk soil. Pseudomonas sp. in rhizosphere and Bacillus and Brevibacterium sp. in the bulk soil were the predominant genera respectively. The isolated rhizobacteria were screened for plant growth promotion activity like phosphate solubilization, siderophore and indole acetic acid production. 50 % produced siderophore and 33 % were able to solubilize phosphate whereas all the rhizobacterial isolates produced indole acetic acid. The six potential PGPR showing in vitro activities were used in pot trial to check their efficacy in vivo. These bacteria consortia demonstrated in vivo PGP activity and can be used as PGPR in Saffron as biofertilizers.This is the first report on the isolation of rhizobacteria from the Saffron rhizosphere, screening for plant growth promoting bacteria and their effect on the growth of Saffron plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhizosphere, first described by Hiltner (1904), represents the most dynamic habitat on the earth (Hinsinger et al. 2009). The rhizosphere zone is different from bulk soil, as it is under the influence of root exudates. Sloughing off of root cells, root death and the exudation of carbon compounds select a specific rhizosphere community (Hartmann et al. 2009). Hence, a rather small subset of the whole soil bacterial diversity, majority of which are Gram negative, finally colonize roots successfully (Soderberg et al. 2004; Johansen and Olsson 2005). In the rhizosphere, diverse and complex interaction occurs between plant roots, soil microbiota and the soil which has evolved due to mutual benefits, between plants and microbes. The plant partner provides substrate and energy flow into the rhizosphere and in return gets nutrients and minerals, essential for its development and growth (Hartmann et al. 2009). Nannipieri et al. (2007) have concluded in their review that, the number of microorganisms is higher in rhizosphere than bulk soil as assessed by the “Most Probable Number analysis”. Rhizosphere has been the focus of agricultural research for many years, due to its importance in crop productivity, soil health and sustainable agriculture (Li et al. 2007; Ryan et al. 2009; Ordookhani et al. 2011). Rhizosphere of various plants like rice, cucumber, apple and soyabean has been extensively studied (Johansen and Olsson 2005; Ashrafuzzaman et al. 2009; Joshi and Bhatt 2011; Mahaffee and Kloepper 1997; Mehta et al. 2010; Wahyudi et al. 2011).
Plant growth-promoting rhizobacteria (PGPR) exert plant growth promotion and/or biocontrol effects and are found in the rhizosphere, root surface as well as inside the root tissues. These PGP rhizobacteria can improve the extent or quality of plant growth directly by increasing nutrient cycling such as, biological nitrogen fixation (Ahmad et al. 2008), siderophore production, solubilization of phosphorus, synthesis of phytohormones or indirectly by synthesis of biocontrol compounds to inhibit phytopathogens (Lucy et al. 2004; Cummings 2009).The plant growth promoting bacteria isolated so far, mainly belongs to two divisions namely Firmicutes and Proteobacteria. The use of PGPR is steadily increasing in agriculture as nutrient supplements to soil and as biocontrol agents. They offer an alternative to chemical fertilizers, antibiotics, herbicides and pesticides (Tilak et al. 2005; Ordookhani et al. 2011).
Crocus sativus, commonly known as Saffron, is an autumn-flowering perennial plant and is a sterile triploid with chromosome number 3n = 24. Being sterile, reproduces vegetatively by underground, bulb-like, starch-storing organs known as corms and has unique corm–root cycle. Saffron is economically important, as it is world’s highest priced medicinal, aromatic plant and is referred as the ‘Golden Condiment’. Iran, Spain and India (J&K State) are the major Saffron producing countries in the world. In India Saffron is grown in Pulwama district in Kashmir and Kishtwar district in Jammu division so far (Yasmin and Nehvi 2013), though comparable climatic conditions are found in adjoining states. Cultivation of Saffron only in specific belts in world, it’s economic importance and corm-root cycle makes it an interesting candidate for studying it’s rhizosphere. This is first report on the plant growth promoting bacteria from Saffron or indeed any genera of family Iridiaceae to which it belongs.
Materials and methods
Soil sampling
Soil samples were collected from the bulk and rhizosphere of Saffron during the flowering period (October–November 2010). Saffron fields of Wuyan village (74°58′0″E, 34°1′30″N, 5,173 ft) of Pulwama district were selected for composite sampling (Courtesy: State Agriculture Department, J&K, India). The soil sampling was done as per the protocol of Luster et al. (2009). Standard protocol of Hamza et al. (2008) was used for the analysis of pH, electrical conductivity, organic Carbon, Calcium, Magnesium, bulk density, available Nitrogen, Phosphorus and Potassium of the collected soil samples. The soil shed by vigorously shaking of the roots was taken as bulk soil and the soil that remained adhere to the roots was taken as rhizosphere soil. The soil samples were stored at −20 °C for further use.
Bacterial isolation and identification
Isolation of cultivable bacteria from the bulk soil was done by conventional agar plate method (Stotzky et al. 1966) and rhizobacteria were isolated from roots by protocol developed by Luster et al. (2009).The comparison of bacterial load was done by dilution plate technique by comparing the CFU/gm (Stotzky et al. 1966; Joshi and Bhatt 2011). Bacterial isolates were randomly selected and purified by streak plate method which were further stored on LB agar slants at 4 °C. Selected bacterial isolates were identified by microscopy using Gram’s staining kit (Sigma) followed by biochemical characterization by Biochemical test Kits (Himedia). Purified bacterial cultures were preserved in 50 % glycerol at −80 °C.
Identification of bacterial isolates by 16S rRNA amplification
Genomic DNA was isolated using the protocol given by Pitcher et al. (1989). Partial 16S rRNA region, flanking V1–V3 region was amplified (~500 bp) using universal primers Bac8f (5′-AGAGTTTGATCCTGGCTCAG-3′) and Univ529 (5′-ACCGCGGCKGCTGGC-3′). The PCR was performed following the protocol standardized by Fierer et al. (2007) with modifications instead of 0.5 μM, 100 pM primer were used and instead of 25 cycles, 30 cycles PCR were run. The template DNA concentration for PCR reaction was 50 ng and the PCR program as denaturation at 95 °C for 5 min followed by 30 cycles of denaturation at 95 °C for 60 s, annealing at 54 °C for 30 s followed by extension at 72 °C for 90 s and final extension at 72 °C for 10 min.
Sequencing and phylogenetic analyses
16S rDNA amplicons were custom sequenced at CIF, UDSC, New Delhi, India. The resulting nucleotide sequences were assigned bacterial taxonomic affiliations based on the closest match to sequences available at the NCBI database (http://www.ncbi.nlm.nih.gov/) using the EzTaxon version 2.1 (www.eztaxon.org). Sequences of bacteria obtained were deposited in the GenBank nucleotide sequence database under accession no JN084065.1–JN084074.1, JQ713596–JQ713598, JQ751317, JF836006.1 and JX233807. The 16S rRNA gene sequences were aligned using multiple sequence alignment tool ClustalX 2.1 version. Phylogenetic and molecular evolutionary analysis was conducted using Phylip 3.69 (http://evolution.genetics.washington.edu/phylip.html) and MEGA 5.05 software version (Tamura et al. 2011). The phylogenetic tree was constructed by neighbor-joining method using distance matrix from alignment.
Screening of bacteria for PGP traits
Rhizobacteria were analyzed in vitro for plant growth promoting properties such as phosphate solubilization, siderophore and indole acetic acid production. Phosphate-solubilisation was detected by formation of transparent halos around bacterial colonies on the Pikovskaya agar after 72 h incubation, at 25 °C (Sharma et al. 2011). Siderophore production was detected by the formation of orange halos on CAS (chrome azurol S agar) agar plates after 48 h incubation at 25 °C, as described by Alexander and Zuberer (1991). Indole acetic acid production estimated according to the protocol given by Sachdev et al. (2009).
Pot trials
PGPR formulation
Inoculum was prepared by growing each of the selected bacteria with PGP traits in LB broth individually at 28 ± 1 °C with 180 rpm for 48 h. The different bacteria with PGP traits required for consortium were checked for co-inhibition by Kirby beur plate assay (Kirby et al. 1966). The inoculum containing 107–108 CFU/ml of each isolate was prepared by mixing them in equal proportions. Subsequently the consortia were mixed with the sterile talc, Calcium carbonate (autoclaved twice at 121 °C for 15 min) in 1:3 ratios (1 consortium: 3 talc) and dried at 35–37 °C for 4 days. Finally 1 % CMC was mixed to the consortium powder and CFU was calculated by serial dilution method. The PGPR consortium was kept at room temperature prior to seed inoculation. The corms selected for the experiment were of uniform size and shape. Corms were inoculated by mixing with PGPR formulation talc at 10 % w/v. Control consisted of the corms treated with talc having nutrient broth and CMC without the isolates Treated corms were dried under shade for 6–8 h. The soil collected from Saffron fields was air-dried, sieved (2-mm/10-mesh) and filled in the twenty pots. Twenty inoculated and uninoculated corms were sown in soil filled pots maintaining one corm per pot. The pots were arranged randomly with twenty repeats (ten each of treatment and control) at ambient light and 20 °C temperature and were irrigated time to time. The plants were harvested after 5 months and results analyzed. The data collected were statistically analyzed using a completely randomized design in the pot trials. One way ANOVA test was used to test if results were statically significant. All the statistical tests were performed at P < 0.1 (Gupta et al. 2011).
Results
Saffron, C. sativus likes light friable soil that has high nutrient content. It thrives best in deep, well drained clay-calcareous soil that has loose texture and permits easy root penetration. The physiochemical analysis of soil from Saffron fields revealed, that it is neutral in pH (7.35) with 306 kg/ha available Nitrogen, 26 kg/ha Phosphorus, 504 kg/ha Potassium, 3,000 ppm Calcium, 552 ppm Magnesium, 1.36 % organic Carbon, bulk density of 1.198 gm/cc and 0.13 ds/m electric conductivity.
Bacterial isolates from bulk soil and rhizosphere
Bacterial load in the bulk soil and rhizosphere was 1.4 × 106 CFU/gm and 6.4 × 107 CFU/gm respectively, about 40 fold more in rhizosphere than in bulk soil. A total of 100 bacteria were randomly selected (50 each) from the rhizosphere and bulk soil composite samples of Saffron during the flowering period, as roots are fully grown during this period. The bacterial strains isolated from Saffron rhizosphere belonged to 3 phyla namely Bacteroidetes, Firmicutes and Proteobacteria of 4 different genera namely, Acinetobacter, Bacillus, Chryseobacterium and Pseudomonas. Bulk soil bacteria isolates belonged to 3 phyla namely Actinobacteria, Firmicutes and Proteobacteria of 4 different genera namely, Arthobacter, Bacillus, Brevibacterium and Pseudomonads (Tables 1, 2). To ascertain their taxonomic positions, gene sequence analysis of hypervariable region (V1–V3 region) of 16S rRNA was done in addition to microscopy and biochemical characterization. Rhizosphere bacterial isolates were identified into six different bacterial species namely Acetinobacteria calcoaceticus WRF1, Pseudomonas tremae WRF2, Pseudomonas kilonensis WRF3, Chryseobacterium elymi WRF4, Bacillus aryabhattai WRF5 and Pseudomonas koreensis WRF6. Bulk soil isolates were identified as ten different species namely Arthrobacter sp WBF1, Bacillus methylotrophicus WBF2, B. aryabhattai WBF3, B. aryabhattai WBF4A, B. aryabhattai WBF4B, Brevibacterium halotolerans WBF5A, B. halotolerans WBF5B, Brevibacterium frigoritolerans WBF6, B. halotolerans WBF8 and Pseudomonas parafulva WBF7. Percentage sequence similarity of 16S rRNA genes of these bacteria and GenBank accession numbers are given in Table 2. Phylogenetic tree based on 16S rRNA gene sequence (V1–V3 region) cluster the Saffron rhizobacteria and bulk soil bacteria into separate clads, except for B. aryabhattai WRF5 from rhizosphere and P. parafulva WBF7 from Bulk soil (Fig. 1).
Bacteria with PGP traits
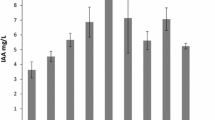
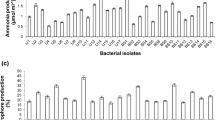
Bacterial isolates were screened for plant growth promotion properties like indole acetic acid production (IAA), phosphate solubilization and siderophore production. 100 % bacterial isolates produced indole acetic acid, 50 % of them produced siderophore and 33 % were able to solubilise phosphate. P. tremae WRF2 and P. kilonensis WRF3 produced indole acetic acid, siderophore and solubilized phosphate whereas P. koreensis WRF6 showed maximum production of the indole acetic acid (28.5 μg/ml) as compared to other isolated Saffron Rhizobacteria (Table 3). Acinetobacteria calcoaceticus WRF1 and C. elymi WRF4 were able to produce only indole acetic acid and B. aryabhattai WRF5 was also able to produce siderophore in addition to IAA (Table 3).
Pot trials
The bacterial isolates showing in vitro PGP traits were subjected to in vivo screening in pot assay. None of the six bacterial isolates selected, showed antagonistic activity against each other. Thus could be used as consortia (1012 CFU/gm) for bacterial formulation. Seed inoculation with bacterial formulation affected the growth of corms positively as compared to control (Table 4). Bacterial consortia increased average number of roots and shoots but the effect on shoot length and root length was insignificant statistically. In addition incidence of corm rot disease occurrence was less (40 %) as compared to uninoculated control (60 %). Significant increase in cormslets/daughter corms production was observed as compared to uninoculated controls (Table 4). The mother corms were shrunken thus giving rise to cormlets in test whereas the control corms remained unaffected.
Discussion
Bacterial load
Fresh roots emerge in October–November at the end of dormant period in corm and grow throughout the flowering season. In the present study, ~40 fold increase in bacterial load was observed in rhizosphere as compared to bulk soil. Higher density of bacteria near roots has been reported in other plants as well (Nannipieri et al. 2007; Joshi and Bhatt 2011; Timmusk et al. 2011). In wild barley ~200 folds increased bacterial load is reported in rhizosphere (0.4 × 106 CFU/gm) than bulk soil (0.2 × 104 CFU/gm) (Timmusk et al. 2011). Joshi and Bhatt (2011) have reported in wheat rhizosphere that the bacterial load increases till 90th day followed by decrease in their number by 120th day, with root decay. It is established that rhizodeposition influences root-microbe interaction in most of the plants which seems to be true for Saffron as well (Soderberg et al. 2004; Johansen and Olsson 2005; Hinsinger et al. 2009).
Bacterial isolates from bulk soil and rhizosphere
To take random samples, 50 bacteria each, were picked from the rhizosphere and bulk soil isolates and all the 100 bacteria were identified by polyphasic method mentioned in the “Materials and methods”.The rhizobacteria were catalogued into four different bacterial genera of six different species, whereas the bulk soil bacterial isolates were grouped into four different genera of ten different bacterial species (Tables 1, 2). Though, sequencing of the entire 1,500-bp sequence is usually required, when describing a new species. However, for most of the bacterial isolates the initial 500-bp sequence (V1–V3 region) provides adequate differentiation for identification, as it has substantial sequence difference between different strains (Clarridge 2004). In total 6 rhizobacteria showing PGP traits were isolated from rhizosphere of Saffron using single culturing media (LB Agar). Isolation of 32, 15, 13 and 10 rhizobacteria have been reported from wheat, sweet potato, apple and rice respectively using similar culturing technique (Mahaffee and Kloepper 1997; Sarode et al. 2009; Yasmin et al. 2009; Ashrafuzzaman et al. 2009). Few types of bacterial isolates retrieved, despite high bacterial load on the same media in the Saffron rhizosphere further substantiates the belief that specific root–microbe interaction occur in Saffron, though it needs to be substantiated with more experiments.
Comparing rhizosphere and bulk soil bacterial isolates at phylum level revealed that the Firmicutes and Proteobacteria are present in both bulk and rhizosphere soils but Bacteroidetes were present only in rhizosphere whereas Actinobacteria were present in bulk soil only. Phylogenetic tree based on 16S rRNA gene sequence (V1–V3 region) clusters the Saffron rhizobacteria and bulk soil bacteria into separate clads, except for B. aryabhattai WRF5 from rhizosphere and P. parafulva WBF7 from Bulk soil (Fig. 1) clearly indicating difference in the microbial types present. However, B. aryabhattai WRF5 from rhizosphere clusters with other Bacillus strains from bulk soil and P. parafulva WBF7 from bulk soil clusters with those from rhizosphere indicating thereby some evolutionary relationship. Phylogenetic clad of Rhizosphere comprises of subclads of various genera like Pseudomonas, Acetinobacteria and Chryseobacterium whereas bulk soil clad comprised of Bacillus, Brevibacteria and Arthrobacter.
The rhizosphere is colonized predominantly by Gram negative microbial community; they are reported to be stimulated by rhizodeposition whereas Gram-positive bacteria are reported to be inhibited (Soderberg et al. 2004; Johansen and Olsson 2005). Similar results were found in Saffron rhizosphere as Pseudomonas genera (consisting of P. tremae, P. kilonensis and P. koreensis), A. calcoaceticus and C. elymi are the Gram negative bacteria, whereas Gram positive bacteria was represented only by single species of B. aryabhattai. However, more Gram positive bacteria were found in bulk soil dominated by different species of Bacillus and Brevibacterium. Saffron though has specific combination of rhizobacteria but it seems to follow the distribution pattern of Gram −ve bacteria near roots and Gram +ve in the bulk as in cucumber and pea plants (Mahaffee and Kloepper 1997; Soderberg et al. 2004).
Saffron rhizobacteria
All the rhizobacteria demonstrated at least one plant growth property with 50 % of isolates producing siderophore, 33 % solubilising phosphate and all of them produced indole acetic acid. Out of 133 isolates from wheat rhizosphere, 29.32 % had ability to produce siderophore, 22.56 % solubilised phosphate and 12.03 % produced indole acetic acid (Joshi and Bhatt 2011). Majority of rhizobacteria are reported from the three subdivisions of Proteobacteria phyla, α-proteobacteria, β-proteobacteria and γ-proteobacteria (Ahmad et al. 2008). Saffron rhizosphere is also dominated by γ-proteobacteria characterized by Pseudomonas and Acinetobacteria genera. Pseudomonas sp. is known to be dominant in rhizosphere of various plants (Tilak et al. 2005; Khakipour et al. 2008) so is true for Saffron rhizosphere as three out of six bacterial strains showing PGP trait were identified as Pseudomonads (P. tremae WRF2, P. koreensis WRF6 and P. kilonensis WRF3). P. koreensis WRF6 and P. kilonensis WRF3 isolated from Saffron rhizosphere have neither been reported from any rhizosphere and nor have their PGP properties assayed, but have been isolated from the agricultural soils (Sikorski et al. 2001; Kwon et al. 2003). P. koreensis WRF6 shows maximum production of the indole acetic acid (28.5 μg/ml) (Table 3) and is comparable to the other common growth promoting Pseudomonads e.g. P. putida (24.08 mg/l) and P. fluorescens (31.6 mg/l) (Khakipour et al. 2008). Not many reports are available on P. tremae with PGP traits except one isolated from healthy wild coffee seedlings. It was able to mobilize mineral phosphate, produce HCN and siderophores, and effectively antagonize deleterious coffee fungal pathogens (Muleta et al. 2009).
Chryseobacterium elymi WRF4 and A. calcoaceticus WRF1 isolated from Saffron rhizosphere showed only Indole acetic acid production (Table 3). C. elymi RHA3-1 reported from the rhizosphere of wild rye produced indole acetic acid (Cho et al., 2010). A. calcoaceticus SCW1 isolated from wheat rhizosphere had various PGP traits like phosphate solubilization, siderophore and indole acetic acid production (Sarode et al. 2009) and A. calcoaceticus P23 isolated from duckweed rhizosphere has phosphate solubilizing property (Yamaga et al. 2010). B. aryabhattai, isolated from halophytic plants rhizosphere was able to fix nitrogen but could not produce IAA (Siddikee et al. 2010) which was in contrast to our observation. B. aryabhattai is the common Gram positive bacteria between Saffron rhizosphere and bulk soil (rhizosphere: B. aryabhattai WRF5 and bulk soil; B.aryabhattai WBF3, WBF4A and WB4B). 16S rRNA gene sequences of all the strains were compared and were found to be 98 % similar. The biochemical tests like catalase, oxidase, nitrate reduction, carbohydrate fermentation were similar for these strains but they varied in solubilisation of phosphate. All the three bulk soil strains solubilized phosphate, but surprisingly the strain isolated from rhizosphere did not. Moreover, three bulk soil B.aryabhattai strains differed in their colony morphology and microscopy too. The results indicated that B. aryabhattai WRF5 isolated from rhizosphere is specific to Saffron rhizosphere and has not migrated from the bulk soil.
Pot trials
The bacterial formulation of the bacterial isolates showing PGP traits was prepared and subjected to Pot trails by following the method developed by Gupta and coworker (2011). Bacterial formulation prepared significantly promoted growth of Saffron as is evident by the statistical tool ANOVA in all the traits tested except for shoot length and root length. In general, inoculation resulted in increased shoot and root number, increased production of daughter cormlets and decreased occurrence of the corm rot disease incidence in pots. This is in concordance with the findings of Sharaf-eldin et al. (2008) where the authors have observed positive effect of commercially available PGPR Bacillus subtilis FZB24 strain on the Saffron. In another study, Aytekin and Acikgoz (2008) have reported the effect of commercially available synthetic growth hormone, biohumus and Effective Microorganisms™ (EM) on the production of Saffron. Synthetic hormone consists of Polystimulins A6 and K and two different microorganism based materials consists of biohumus/vermicompost and Effective Microorganisms™ (EM). Saffron corms were treated in four different ways—hormone alone, biohumus alone, EM alone and EM + biohumus to determine whether these treatments have any statistically meaningful effects on corm numbers and dry and wet stigma weights. It has been shown that EM + biohumus were the most effective choice for improved Saffron cultivation.
In the both the mentioned reports effect of commercially available Bacillus subtilis or hormones and Effective microorganism was observed on the growth and production of Saffron but bacteria mentioned have not been isolated from Saffron rhizosphere (Sharaf-eldin et al. 2008; Aytekin and Acikgoz 2008). The synthetic application of bacteria to any plant possesses the risk of inoculum colonization and sustainability which is not the case if PGPR used are indigenous to plant. We therefore propose that use of the bacterial formulation prepared in present study for Saffron growth and production will be a good alternative to chemical treatments.
Conclusion
Many factors are responsible for restricted cultivation of C. sativus in specific belts of particular geographical regions. Soil is one of the important factors and so are the microbes residing in the soil. Present communication for the first time, reports the cultivable PGPR present in rhizosphere of Saffron (grown in Pulwama, J&K, India), their phylogenetic and biochemical characterization along with potential plant growth promoting functions. 40 fold enhancement of bacterial load in rhizosphere in comparison to bulk soil establish specific root–microbe interaction. The three species of Pseudomonas isolated from the Saffron rhizosphere are specific to Saffron rhizosphere, as P. koreensis WRF6 and P. kilonensis WRF3 have not been reported from any other rhizosphere and P. tremae have been isolated only from coffee seedlings. Pseudomonas pudita and P. flouresence, the common PGPR of most of the plants, are absent in Saffron rhizosphere though A. calcoaceticus was present. Bacterial formulation of all the six isolates showing PGPR, showed the positive effect on the growth of Saffron.
References
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizosphereric bacteria for their multiple growth promoting activities. Microbiol Res 163:173–181
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertile Soil 12(1):39–45
Ashrafuzzaman M, Hossen FA, Ismail MR, Hoque MA, Islam MZ, Shahidullah SM, Meon S (2009) Efficiency of plant growth-promoting rhizobacteria (PGPR) for the enhancement of rice growth. Afr J Biotechnol 8(7):1247–1252
Aytekin A, Acikgoz AO (2008) Hormone and microorganism treatment in the cultivation of Saffron (Crocus sativus L.) plants. Molecules 13:1135–1146
Cho SH, Lee KS, Shin DS, Han JH, Park KS, Lee CH, Park KH, Kim SB (2010) Four new species of Chryseobacterium from the rhizosphere of coastal sand dune plants, Chryseobacterium elymi sp. nov., Chryseobacterium hagamense sp. nov., Chryseobacterium lathyri sp. nov. and Chryseobacterium rhizosphaerae sp. nov. Syst. Appl Microbiol 33(3):122–127
Clarridge JE (2004) Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 17(4):840–862
Cummings SP (2009) The application of plant growth promoting rhizobacteria (PGPR) in low input and organic cultivation of graminaceous crops; potential and problems. Environ Biotechnol 5(2):43–50
Fierer N, Breitbart M, Nulton J, Salmon P, Lozupone C, Jones R, Robeson M, Edward RA, Felts B, Rayhawk S, Knigh R, Rohwer F, Jackson RB (2007) Metagenomics and small subunit rRNA analysis reveal the genetic diversity of bacteria, archaea, fungi and virus in soil. Appl Environ Microbiol 73(21):7059–7066
Gupta V, Kalha CS, Razdan VK, Dolly (2011) Etiology and management of corm rot of saffron in Kishtwar district of Jammu and Kashmir. Indian J Mycol Plant Pathol 41(3):361–376
Hamza MA (2008) Understanding soil analysis data. Resource management technical report 327. Western Australian Agriculture Authority
Hartmann A, Schmid M, van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
Hiltner L (1904) ÜberneuereErfahrungen und Probleme auf demGebiete der BodenbakteriologieunterbesondererBerücksichtigung der Gründüngung und Brache. Arbeiten der Deutschen Landwirtschaftlichen Gesellschaft 98:59–78
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152
Johansen A, Olsson S (2005) Using phospholipid fatty acid technique to study short-term effects of the biological control agent Pseudomonas fluorescensDR54 on the microbial microbiota in barley rhizosphere. Microb Ecol 49:272–281
Joshi P, Bhatt AB (2011) Diversity and function of plant growth promoting rhizobacteria associated with wheat rhizosphere in North Himalayan region. Int J Environ Sci 1(6):1135–1143
Khakipour N, Khavazi K, Mojallali H, Pazira E, Asadirahmani H (2008) Production of Auxin Hormone by Fluorescent Pseudomonads. Am Eurasian J Agric Environ Sci 4(6):687–692
Kirby WM, Bauer AW, Sherris JC et al (1966) Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 45:493–496
Kwon SW, Kim JS, Park IC, Yoon SH, Park DH, Lim CK, Go SJ (2003) Pseudomonas koreensis sp. nov., Pseudomonas umsongensis sp. nov. and Pseudomonas jinjuensis sp. nov., novel species from farm soils in Korea. Int J Sys Evol Microbiol 53:21–27
Li L, Li SM, Sun JH, Zhou LL, Bao XG, Zhang HG, Zhang FS (2007) Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. PNAS 104(27):11192–11196
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. A Van Leeuw J Microb 86(1):1–25
Luster J, Göttlein A, Nowack B, Sarret G (2009) Sampling, defining, characterising and modeling the rhizosphere—the soil science tool box. Plant Soil 321:457–482
Mahaffee WF, Kloepper JW (1997) Temporal changes in the bacterial communities of soil, rhizosphere and endorhiza associated with field-grown cucumber (Cucumis sativusL.). Microb Ecol 34:210–223
Mehta P, Chauhan A, Mahajan R, Mahajan PK, Shirkot CK (2010) Strain of Bacillus circulans isolated from apple rhizosphere showing plant growth promoting potential. Curr Sci 98(4):538–542
Muleta D, Assefa F, Hjort K, Roos S, Granhall U (2009) Characterization of Rhizobacteria Isolated from Wild Coffea Arabica L. Eng Life Sci 9(2):100–108
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G, Valori F (2007) Microbial diversity and microbial activity in the rhizosphere. Ci. Suelo (ARGENTINA) 25(1):89–97
Ordookhani K, Sharafzadeh S, Zare M (2011) Influence of PGPR on growth, essential oil and nutrients uptake of sweet basil. Adv Environ Biol 5(4):672–677
Pitcher DG, Saunders NA, Owen RJ (1989) Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett App Microbiol 8:151–156
Ryan PR, Dessaux Y, Thomashow LS, Weller DM (2009) Rhizosphere engineering and management for sustainable agriculture. Plant Soil 32:363–383
Sachdev DP, Chaudhari HG, Kasture VM, Dhavale DD, Chopra BA (2009) Isolation and characterization of indole acetic acid (IAA) producing Klebsiella pneumonia strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian J Exp Biol 47(12):993–1000
Sarode PD, Rane MR, Chaudhari BL, Chincholkar SB (2009) Siderophoregenic Acinetobacter calcoaceticus isolated from wheat rhizosphere with strong PGPR activity. Malays J Microbiol 5:6–12
Sharaf-eldin M, Elkholy S, Fernandez J, Junge H, Cheetham R, Guardiola J, Weathers P (2008) Bacillus subtilis FZB24 affects quantity and quality of Saffron (Crocus sativus L.). Planta Med 74:1316–1320
Sharma S, Kumar V, Tripathi RB (2011) Isolation of phosphate solubilizing microorganism (PSMs) From Soil. J Microbiol Biotech Res 1(2):90–95
Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa T (2010) Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halo tolerant bacteria derived from coastal soil. J Microbiol Biotechnol 20(11):1577–1584
Sikorski J, Stackebrandt E, Wackernagel W (2001) Pseudomonas kilonensis sp. nov., a bacterium isolated from agricultural soil. Int J Sys Evol Microbiol 51:1549–1555
Soderberg K, Probanza A, Jumpponen A, Baath E (2004) The microbial community in the rhizosphere determined by community-level physiological profiles (CLPP) and direct soil-and cfu-PLFA technique. Appl Soil Ecol 25:135–145
Stotzky G, Mund R, Tsui R (1966) Rapid serial dilution technique with self-filling syringes. App Microbiol 14(3):472–473
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tilak KVBR, Ranganayaki N, Pal KK, De R, Saxena AK, Nautiyal CS, Mittal S, Tripathi AK, Johri BN (2005) Diversity of plant growth and soil health supporting bacteria. Curr Sci 89(1):136–150
Timmusk S, Paalme V, Pavlicek T, Bergquist J, Vangala A, Danilas T, Nevo E (2011) Bacterial Distribution in the Rhizosphere of Wild Barley under Contrasting Microclimates. PLoS ONE 6(3):1–7
Wahyudi AT, Astuti RP, Widyawati A, Meryandini A, Nawangsih AA (2011) Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting rhizobacteria. J Microbiol Antimicrob 3(2):34–40
Yamaga F, Washio K, Morikawa M (2010) Sustainable Biodegradation of Phenol by Acinetobacter calcoaceticus P23 isolated from the rhizosphere of Duckweed Lemna aoukikusa. Environ Sci Technol 44:6470–6474
Yasmin S, Nehvi FA (2013) Saffron as a valuable spice: a comprehensive review. Afr J Agric Res 8(3):234–242
Yasmin F, Othman R, Sijam K, Saad MS (2009) Characterization of beneficial properties of plant growth-promoting rhizobacteria isolated from sweet potato rhizosphere. Afr J microbiol Res 3(11):815–821
Acknowledgments
Authors are grateful to Prof. Michel Aragno, Honorary professor University of Neuchatel, Switzerland for his scientific advice. We are thankful to Mr Farooq Ahmad and Mr C.L. Bhat and, State agriculture Department J&K, India, for their help in sample collection and information about Saffron cultivation. SA is thankful to CSIR-UGC for Fellowship. We are also thankful to Department of Biotechnology for financial support under DBT funded project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ambardar, S., Vakhlu, J. Plant growth promoting bacteria from Crocus sativus rhizosphere. World J Microbiol Biotechnol 29, 2271–2279 (2013). https://doi.org/10.1007/s11274-013-1393-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1393-2