Abstract
A locally isolated Acinetobacter sp. Strain AQ5NOL 1 was encapsulated in gellan gum and its ability to degrade phenol was compared with the free cells. Optimal phenol degradation was achieved at gellan gum concentration of 0.75% (w/v), bead size of 3 mm diameter (estimated surface area of 28.26 mm2) and bead number of 300 per 100 ml medium. At phenol concentration of 100 mg l−1, both free and immobilized bacteria exhibited similar rates of phenol degradation but at higher phenol concentrations, the immobilized bacteria exhibited a higher rate of degradation of phenol. The immobilized cells completely degrade phenol within 108, 216 and 240 h at 1,100, 1,500 and 1,900 mg l−1 phenol, respectively, whereas free cells took 240 h to completely degrade phenol at 1,100 mg l−1. However, the free cells were unable to completely degrade phenol at higher concentrations. Overall, the rates of phenol degradation by both immobilized and free bacteria decreased gradually as the phenol concentration was increased. The immobilized cells showed no loss in phenol degrading activity after being used repeatedly for 45 cycles of 18 h cycle. However, phenol degrading activity of the immobilized bacteria experienced 10 and 38% losses after the 46 and 47th cycles, respectively. The study has shown an increased efficiency of phenol degradation when the cells are encapsulated in gellan gum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenol or phenolic compounds are widely distributed in the environment partly as a result of natural processes and more importantly, due to human and industrial activities. These compounds originate mainly from industrial processes such as resin manufacturing, oil refineries, petrochemicals, pharmaceuticals, dyes, textiles and plastic industries (Hori et al. 2006; Whitely and Bailey 2000; Kumaran and Paruchuri 1997). Phenols, being persistent compounds and due to their toxic, mutagenic and carcinogenic characteristics, are classified as highly hazardous chemicals (Hooiveld et al. 1998; Buckley et al. 2000).
Degradation of pollutants and toxic compounds in the environment are mainly due microbial activities as shown in numerous reports such as the bioremediation of pesticides (Song et al. 2005; Chatterjee et al. 2010), diesel (Sadouk et al. 2009; Moslemy et al. 2002), azo dyes (Syed et al. 2009; Revankar and Lele 2006; González-Gutiérrez et al. 2009), heavy metals (Shukor et al. 2010; Zhou et al. 2007; Ozdemir et al. 2005) and phenol derivatives (Sejákova et al. 2009; Aguayo et al. 2009; Machado et al. 2005; Čejková et al. 2005). However, the growth of these microorganisms is inhibited at high concentrations of the xenobiotics, thus limiting the efficiency of the biodegradation of phenols (Prieto et al. 2002).
The efficiency of phenol biodegradation can be enhanced by cell immobilization (Chung et al. 2003; Adav et al. 2007; Liu et al. 2009). For example, immobilized Pseudomonas putida CCRC14365 was reported to degrade phenol up to a concentration of 1,000 mg l−1 as compared to 600 mg l−1 by the free cells (Chung et al. 2003).
The most commonly used immobilization matrix for phenol degradation is calcium-alginate mainly because the procedure is simple, relatively mild and is not toxic to the cells (Aksu and Bulbul 1999; Dursun and Tepe 2005). However, the material is susceptible to degradation and has relatively low mechanical stability (Ha et al. 2009). Gellan gum is another commonly used matrix as the gel is stable over a wide pH range of 2–10, non-toxic and recommended in fermentation technology due to its mechanical and thermal stability (Norton and Lacroix 1990; Camelin et al. 1993; Ashtaputre and Shah 1995; Moslemy et al. 2002, 2003).
To date, reports that describe phenol degrading ability of up to 1,500 mg l−1 in immobilized cells by Acinetobacter sp. are few (Adav et al. 2007). To achieve better degradation of elevated concentration of phenol, Acinetobacter sp. strain AQ5NOL 1 was encapsulated in gellan gum and optimization of the beads was carried out to investigate its potential in the bioremediation of phenol in waste waters.
Materials and methods
Chemicals and growth conditions
All chemicals used were of analytical grade and purchased either from Sigma (USA) or Merck (Germany). Phenol degradation was monitored by using 4-aminoantipyrine in the colorimetric assay according to that reported by the American Public Health Association (1998).
Acinetobacter sp. strain AQ5NOL 1, previously isolated from a pesticide-polluted site, was cultured at 30°C in sterilized mineral salt medium (MSM) at pH 7.5 containing (g l−1): K2HPO4, 0.4; KH2PO4, 0.2; NaCl, 0.1; MgSO4, 0.1; MnSO4.H20, 0.01; Fe2(SO4).H2O, 0.01; NaMoO4.2H2O, 0.01; (NH4)2SO4, 0.4; in a 250 ml conical flask. The medium was supplemented with filter-sterilized phenol as a carbon source to a final concentration of 0.5 g l−1.
Optimization of cell immobilization protocol
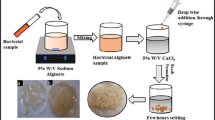
Cell immobilization in gellan gum was carried out essentially following the method described by Moslemy et al. (2003). Gellan gum (0.75%; w/v) was initially added to 100 ml deionized water and heated to 75°C to completely dissolve the gum. Then, 0.06% (w/v) CaCl2 was added to the gum mixture and slowly cooled to 45°C. The pH of the solution was adjusted to pH 7.0 using 0.1 M NaOH. Acinetobacter sp. strain AQ5NOL 1 cultures were centrifuged for 10 min at 7,000 rpm at room temperature to obtain the pellet. 3.5 g wet weight of the resulting bacterial pellet was dispersed in the gum mixture and continuously stirred. Beads were formed by using a peristaltic pump and dropping the gum mixture through a modified pipette tip into canola oil containing 0.1% Span 80. The uniformly-sized beads were then separated from the oil by transferring them into 500 ml of 0.1% (w/v) CaCl2. After 2 h, the beads were repeatedly rinsed with 0.1% (v/v) Tween 80 solution.
Three parameters were optimized for the degradation of phenol by the immobilized bacteria. Firstly, to determine the optimum gellan gum concentration, six different concentrations ranging from 0.6 to 0.85% (w/v) of gellan gum were used. Secondly, for optimization of bead size, 0.75% (w/v) gellan gum was used to prepare beads ranging in size of 1, 2, 3, 4, 5 and 6 mm diameter (equivalent to surface areas of 3.14, 12.56, 28.26, 50.24, 78.50 and 113.04 mm2, respectively). Thirdly, in the determination of the optimum initial cell loading, different quantities of beads ranging from 100 to 400 beads were used. The optimization experiments were conducted in 100 ml of MSM containing 500 mg l−1 phenol. The working solution was incubated for 24 h on an orbital shaker (150 rpm) and phenol concentration in each bottle was measured.
Phenol degradation by free and immobilized cells
Different phenol concentrations ranging from 100 to 1,900 mg l−1 were tested for both the immobilized and free cell systems. To 100 ml of liquid MSM containing the different concentrations of phenol, free cells and immobilized cells were added in separate containers and incubated in a rotary shaker at room temperature at 150 rpm. Phenol concentration was measured hourly and daily until complete degradation of phenol was achieved. Phenol media without the presence of bacteria were used as controls. The experiments were carried out in triplicate.
Reusability of immobilized cells
300 gellan gum beads were added to 100 ml of liquid MSM containing 500 mg l−1 phenol. The immobilized cells were incubated for 18 h on an orbital shaker at 150 rpm. Residual phenol was measured during this period. After 18 h of incubation, the medium was discarded and the beads were thoroughly rinsed with distilled water before they were put into new fresh phenol medium. The steps were repeated at 18 h cycles until phenol degrading ability of the immobilized cells showed a significant decrease in activity.
Statistical analysis
All the experiments were carried out in triplicates. The data shown in the corresponding figures are the mean values of the experiment and expressed as mean ± standard error of mean (SEM). The optimization data were statistically analyzed using One-way ANOVA.
Results and discussion
Optimized conditions of immobilized cells
Figure 1 shows the effect of gellan gum concentration on phenol degradation by immobilized Acinetobacter sp. strain AQ5NOL 1. Phenol degradation was observed to be low at gellan gum concentration below 0.7% (w/v), high between gellan gum concentration of 0.7 and 0.75% and very low above 0.8% (w/v). The optimum gellan gum concentration for phenol degradation by the immobilized bacteria is 0.75% (w/v). Thus, subsequent experiments were carried out using gellan gum concentration of 0.75% (w/v).
Cell immobilization is a promising approach in the biodegradation of toxic compounds because it offers several advantages over free cells namely; enhancement of biodegradation activity as compared to free cells, protection of the cells by the matrix (Weir et al. 1995; Smit et al. 1996; Cassidy et al. 1997), increased density of the cells in the matrix (Lee et al. 1994) and enhancement of physiological activities such as enzyme induction (Chung et al. 2003).
In comparison with other natural polymers, gellan gum is more robust and stable than calcium alginate (Moslemy et al. 2002; Wang et al. 2007). Moreover, gellan gum is recommended for use in fermentation as it is non-toxic and mechanically and thermally stable (Norton and Lacroix 1990; Camelin et al. 1993). The optimum gellan gum concentration is essential as it affects the mechanical strength and pore size of the beads. As inherent with most types of gels such as polyacrylamide, agarose and alginate gels, the gel concentration is reflective of the pore size. Also, pore size affects the diffusion of substrates and leakage of cells from the beads.
The effect of surface area of the beads on phenol degradation by the immobilized cells was evaluated by measuring phenol degradation over a range of gellan gum bead sizes of 1 to 6 mm diameter at 0.75% (w/v) gellan gum concentration (Fig. 2). The highest phenol degradation rate was achieved at 28.26 mm2 surface area (3 mm beads) compared with the other surface areas. Phenol degrading activity was markedly reduced over the larger surface areas tested. Since the quantity of bacteria was kept constant during the preparation of the gellan gum beads, the surface area is indicative of cell density of the immobilized bacteria. The result showed that phenol degradation was reduced at higher cell densities.
Using gellan gum concentration of 0.75% (w/v) and bead size of surface area 28.26 mm2, the effect of bead number on phenol degradation by the immobilized bacteria was evaluated (Fig. 3). At lower bead numbers, phenol degradation was observed to be low but it peaks at bead number of 250–300 after which phenol degradation was again observed to be decreased. Optimum phenol degradation was achieved by 300 beads each of 28.26 mm2 surface area. Cell density is essential for optimum degradation of phenol by the immobilized bacteria. High cell density leads to a greater demand for oxygen and nutrient and results in a reduced degradation of phenol (Beshay et al. 2002).
Comparison of phenol degrading activities of freely-suspended cells and immobilized cells
Immobilized and free bacteria at optimized conditions were tested for their phenol-degrading activity over time at different initial phenol concentrations ranging from 100 to 1,900 mg l−1 (Fig. 4). At 100 mg l−1 (Fig. 4a), both free and immobilized bacteria exhibited similar phenol degrading characteristics and phenol was completely degraded in 3 h. As the phenol concentration is increased, immobilized bacteria showed a faster phenol degrading activity than the free cells (Fig. 4b, c, d). Immobilized bacteria completely degrade phenol within 108, 216 and 240 h at 1,100, 1,500 and 1,900 mg l−1 phenol concentration whereas the free bacteria took 240 h to completely degrade phenol at 1,100 mg l−1. At higher concentrations, phenol degrading activity of the free bacteria is inhibited.
Effects of different phenol concentrations on the phenol degradation over time of incubation of (empty circle) free and (filled circle) immobilized Acinetobacter sp. Strain AQ5NOL 1. Phenol concentrations selected were; a 100 mg l−1, b 1,100 mg l−1, c 1,500 mg l−1, and d 1,900 mg l−1. Data represents mean ± SEM, n = 3
Encapsulating the bacteria in gellan gum afforded protection to the bacteria since it can survive in high phenol concentration over a longer period compared with the free bacteria. With the optimum gellan gum concentration of 0.75% (w/v), the pore size of the beads probably affects diffusion of phenol into the beads and simultaneously prevents leakage of the bacteria into the solution. Immobilization has been reported to increase degradation activity by altering the metabolic features of the living cells such as enzyme induction, cell growth and yield (Chung et al. 2003).
Figure 5 shows the rates of phenol degradation by free and immobilized bacteria incubated in phenol concentration ranging from 100 to 2,100 mg l−1. Degradation rates for both were initially similar at phenol concentration of 100 mg l−1 but at higher phenol concentrations, the immobilized bacteria exhibited a higher rate of phenol degradation than the free bacteria. The free cells stopped degrading phenol at 1,300 mg l−1 while the immobilized cells continued to degrade phenol up to 1,900 mg l−1 but at a much lower rate. Overall, the rates of phenol degradation by both immobilized and free bacteria decrease gradually as the phenol concentration was increased. Immobilized cells showed better phenol degrading activity than free cells. It was estimated that for immobilized cells, the optimal rate of phenol degradation is at 500 mg l−1 phenol concentration.
Effect of initial phenol concentration on specific degradation rate of free and immobilized Acinetobacter sp. Strain AQ5NOL 1. Free cell, (empty circle); immobilized cell, (filled circle). Cells were grown in MSM medium (pH 7.5) at 30°C with different initial phenol concentrations. Data represents mean ± SEM, n = 3
Figure 6 shows that the phenol-degrading ability of the immobilized cells decreased by 10 and 38% after 46th and 47th cycles, respectively. This indicated that immobilized cells could be reused for at least 45 cycles or approximately 33 days with each cycle comprising 18 h. This finding is better than that shown by immobilized Acinetobacter baumannii SERDANG 1 with 5 times reusability (Adinarayana et al. 2005; Yadzir 2007) and the immobilized consortium of Acinetobacter sp. XA05 and Sphingomonas sp. FG03 with 20 times reusability (Liu et al. 2009).
In conclusion, the phenol degrading ability of a locally isolated strain, Acinetobacter sp. strain AQ5NOL 1, immobilized in gellan gum has been optimized. It is able to degrade up to 1,900 mg l−1 phenol and could be reused for 45 cycles. These positive attributes will make it a suitable candidate for bioremediation of phenol and its derivatives from waste waters. Work is in progress to purify the phenol degrading enzyme and study the potential of immobilized Acinetobacter sp. strain AQ5NOL 1 in the treatment of industrial effluents containing phenols.
References
Adav SS, Chen MY, Lee DJ, Ren NQ (2007) Degradation of phenol by Acinetobactor strain isolated from aerobic granules. Chemosphere 67:1566–1572
Adinarayana K, Jyothi B, Ellaiah P (2005) Production of alkaline protease with immobilized cells of B. subtilis PE-11 in various matrices by entrapment technique. AAPS Pharm Sci Tech 6:391–397
Aguayo J, Barra R, Becerra J, Martínez M (2009) Degradation of 2, 4, 6-tribromophenol and 2, 4, 6-trichlorophenol by aerobic heterotrophic bacteria present in psychrophilic lakes. World J Microbiol Biotechnol 25:553–560
Aksu Z, Bulbul G (1999) Determination of effective diffusion coefficient of phenol in Ca-alginate immobilized P. putida beads. Enzyme Microbiol Technol 25:344–348
American Public Health Association (1998) Standard methods for the examination of water and wastewater. 20th Edition. Method 5530. pp 540–544
Ashtaputre AA, Shah AK (1995) Studies on viscous, gel forming exopolysaccharide from Sphingomonas paucimobilis GS1. Appl Environ Microbiol 61:1159–1162
Beshay U, Abd-El-Haleem D, Moawad H, Zaki S (2002) Phenol biodegradation by free and immobilized Acinetobacter. Biotechnol Lett 24:1295–1297
Buckley JD, Meadows AT, Kadin ME, LeBeau AM, Siegel S, Robinson LL (2000) Pesticide exposures in children with non-Hodgkin lymphoma. Cancer 2:2315–2321
Camelin I, Lacroix C, Paquin C, Prevost H, Cachon R, Divies C (1993) Effects of chelants on gellan gum rheological properties and setting temperature for immobilization of living Bifidobacteria. Biotechnol Prog 9:291–297
Cassidy MB, Mullineers H, Lee H, Trevors JT (1997) Mineralization of pentachlorophenol in a contaminated soil by Pseudomonas sp. UG30 cells encapsulated in k-carrageenan. J Ind Microbiol Biotechnol 19:43–48
Čejková A, Masák J, Jirk V, Veselý M, Pátek M, Nevera J (2005) Potential of Rhodococcus erythropolis as a bioremediation organism. World J Microbiol Biotechnol 21:317–321
Chatterjee S, Das SK, Chakravarty R, Chakrabarti A, Ghosh S (2010) Interaction of malathion, an organophosphorus pesticide with Rhizopus oryzae biomass. J Hazard Mater 174:47–53
Chung TP, Tseng HY, Juang RS (2003) Mass transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Process Biochem 38:1497–1507
Dursun AY, Tepe O (2005) Internal mass transfer effect on biodegradation of phenol by Ca-alginate immobilized Ralstonia eutrophe. J Hazard Mater 126:105–111
González-Gutiérrez LV, González-Alatorre G, Escamilla-Silva EM (2009) Proposed pathways for the reduction of a reactive azo dye in an anaerobic fixed bed reactor. World J Microbiol Biotechnol 25:415–426
Ha J, Engler CR, Wild JR (2009) Biodegradation of coumaphos, chlorferon, and diethylthiophosphate using bacteria immobilized in Ca-alginate gel beads. Biores Technol 100:1138–1142
Hooiveld M, Heederick DJJ, Kogevinas M, Boffetta P, Needham LL, Patterson DG, Bas BJ, Bas BH (1998) Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenol, and contaminants. Am J Epidemiol 147:891–901
Hori TSF, Avilez IM, Inoue LK, Moraes G (2006) Metabolical changes induced by chronic phenol exposure in matrinxã Brycon cephalus (teleostei: characidae) juveniles. Comp Biochem Physiol 143:67–72
Kumaran P, Paruchuri YL (1997) Kinetics of phenol biotransformation. Water Res 31:11–22
Lee CM, Lu CJ, Chuang MS (1994) Effects of immobilized cells on the biodegradation of chlorinated phenols. Water Sci Technol 30:87–90
Liu YJ, Zhang AN, Wang XC (2009) Biodegradation of phenol by using free and immobilized cells of Acinetobacter sp. XA05 and Sphingomonas sp. FG03. Biochem Eng J 44:187–192
Machado KMG, Matheus DR, Monteiro RTR, Bononi VLR (2005) Biodegradation of pentachorophenol by tropical basidiomycetes in soils contaminated with industrial residues. World J Microbiol Biotechnol 21:297–301
Moslemy P, Neufeld RJ, Guiot SR (2002) Biodegradation of gasoline by gellan gum- Encapsulated bacterial cells. Biotechnol Bioeng 80:175–184
Moslemy P, Neufeld RJ, Millette D, Guiot SR (2003) Transport of gellan gum microbeads through sand: an experimental evaluation for encapsulated cell bioaugmentation. J Environ Manag 69:249–259
Norton S, Lacroix C (1990) Gellan gum gel as entrapped matrix for high temperature fermentation process-rheological study. Biotechnol Tech A 4:351–356
Ozdemir G, Ceyhan N, Manav E (2005) Utilization in alginate beads for Cu(II) and Ni(II) adsorption of an exopolysaccharide produced by Chryseomonas luteola TEM05. World J Microbiol Biotechnol 21:163–167
Prieto MB, Hidalgo A, Rodriguez FC, Serra JL, Llama MJ (2002) Biodegradation of phenol in synthetic and industrial wastewater by Rhodococcus erythropitics. UPV-1 immobilized in air-stirred reactor with clarifier. Appl Microbiol Biotechnol 58:853–859
Revankar MS, Lele SS (2006) Increased production of extracellular laccase by the white rot fungus Coriolus versicolor MTCC 138. World J Microbiol Biotechnol 22:921–926
Sadouk Z, Tazerouti A, Hacene H (2009) Biodegradation of diesel oil and production of fatty acid esters by a newly isolated Pseudomonas citronellolis KHA. World J Microbiol Biotechnol 25:65–70
Sejákova Z, Dercová K, Tóthová L (2009) Biodegradation and ecotoxicity of soil contaminated by pentachlorophenol applying bioaugmentation and addition of sorbents. World J Microbiol Biotechnol 25:243–252
Shukor MY, Ahmad SA, Nadzir MMM, Abdullah MP, Shamaan NA, Syed MA (2010) Molybdate reduction by Pseudomonas sp. strain DRY2. J Appl Microbiol 108:2050–2058
Smit E, Lee H, Trevors JT, Elsas JDV (1996) Interaction between a genetically engineered Pseudomonas fluorescens and bacteriophage øR2f in soil: effect of nutrients, alginate encapsulation, and the wheat rhizosphere. Microb Ecol 31:125–140
Song L, Hua R, Zhao Y (2005) Biodegradation of fenoxaprop-p-ethyl by bacteria isolated from sludge. J Hazard Mater 118:247–251
Syed MA, Sim HK, Khalid A, Shukor MY (2009) A simple method to screen for azo-dye-degrading bacteria. J Environ Biol 30:89–92
Wang Y, Tian Y, Han B, Zhao HB, Bi JN, Cai BL (2007) Biodegradation of phenol by free and immobilized Acinetobacter sp. strain PD12. J Environ Sci 19:222–225
Weir SC, Dupuis SP, Providenti MA, Lee H, Trevors JT (1995) Nutrient enhanced survival of and phenanthrene mineralization by alginate encapsulated and free Pseudomonas sp. UG14Lr cells in creosote-contaminated soil slurries. Appl Microbiol Biotechnol 43:946–951
Whitely AS, Bailey MY (2000) Bacterial community structure and physiological state within an industrial phenol bioremediation system. Appl Environ Microbiol 66:2400–2407
Yadzir ZHM (2007) Characterization, identification and application of Acinetobacter baumannii SERDANG 1 for phenol biodegradation. M.Sc. Dissertation, Universiti Teknologi Petronas. Malaysia
Zhou M, Liu Y, Zeng G, Li X, Xu W, Fan T (2007) Kinetic and equilibrium studies of Cr(VI) Biosorption by Dead Bacillus licheniformis Biomass. World J Microbiol Biotechnol 23:43–48
Acknowledgments
This work was supported by Research Grant Scheme (RUGS) 2009, Universiti Putra Malaysia (91851).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, S.A., Shamaan, N.A., Arif, N.M. et al. Enhanced phenol degradation by immobilized Acinetobacter sp. strain AQ5NOL 1. World J Microbiol Biotechnol 28, 347–352 (2012). https://doi.org/10.1007/s11274-011-0826-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0826-z