Abstract
Some process has been proposed for azo dye degradation and anaerobic bioreactors are one of them, since for their reduction, the dye has to be the electron acceptor. An anaerobic fixed bed bioreactor packed with activated carbon (AC) is proposed to degradate the Reactive Red 272 azo dye. In the present paper a dye degradation mechanism in an anaerobic environment is explained. It is very important to consider the interaction dye-microorganism-AC, because the groups in the AC surface take part in the reaction besides being an excellent carrier for microorganism and an adsorbent for the dye. The aromatic compounds produced in the dye reduction are partially degraded as a function of inlet dye concentration and reactor residence time. In anaerobic environment the aromatic compounds are decomposed through hydroxylation, carboxylation and redox reactions, due to enzymatic reactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The textile industry uses large volumes of water in its process; therefore, large quantities of wastewater are generated, containing a high load of pollutants, especially dyes. The kinds of dyes that are most extensively used are azo dyes; the main characteristic of these dyes is a double bond between two nitrogen atoms. A dye molecule can contain more than one azo group and that are bonded to aromatic groups.
Azo dye degradation is carried out in two steps: (1) the breaking or reduction of the azo bond and (2) the partial or complete mineralization of the aromatic or aliphatic amines generated (reduction products). It is very important to analyze the compounds in the reactor effluent and the reduction mechanism of the dye because the intermediary products of many azo dyes as benzidine, 2-naphtilamine and other aromatic amines can be toxic, carcinogenic or hazardous in some way.
In biological treatment process, the microorganisms degrade initially the more simple soluble substrates and afterwards the complex organic compounds. In general this process is carried out bye enzymes and redox reactions, and can take place inside or outside the cell. Hydrolysis is the first step to degrade complex molecules; this consists in the addition of water molecules to the complex compounds by bacteria, with the aim of breaking the chemical bonds and to reduce to more simple compounds. This is catalyzed by extracellular enzymes (Gerardi 2006).
Azo dyes are complex molecules and can contain different aromatic groups. The aromatic groups are very stable due to resonance and to the π conjugated bond characteristic.
In anaerobic environment, the ring opening of the aromatic structures is carried out by hydroxylation and carboxylation reactions (Griebler et al. 2004) by the addition of –OH and –COOH or CO2 to the molecule (Fig. 1) from the dextrose anaerobic degradation products as fumarato and succinate (Meckenstock et al. 2004); then a series of redox reactions are carried out to degrade the complex molecule to carboxylic acids or other lower weight compounds as acetate, carbon dioxide and methane.
Anaerobic degradation mechanism of naphthalene and substituted naphthalene (Meckenstock et al. 2004)
This kind of degradation reactions is carried out by radical enzymes; these are enzymes that contain radicals or catalyze reactions with radicals as intermediates (Buckel and Golding 2006). The enzyme contribute to the degradation transporting reducing equivalents (e−) and specific groups (–OH, –COOH, fumarato, succinate), so the degradation redox reactions can occur and the compounds formed in the fading of the water can be obtained.
Some of the main kinds of bacteria that take part in these reactions are: the nitro-reducers that convert the p-cresol to hydroxybenzylic alcohol and hydroxybenzoate, and the sulfate-reducers that attack the methyl groups oxidizing it by the addition of fumarate (Schink 2006), as in Fig. 1.
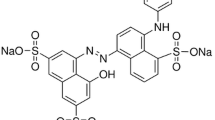
The objective of this paper is to present some proposed pathways for the degradation reaction of the azo dye Reactive Red 272 (Fig. 2) in an upflow anaerobic fixed bed bioreactor, with the aim of understand and identify the reduction mechanism and the degradation of intermediates of these kind of complex compounds.
Materials and methods
Upflow anaerobic fixed bed reactor
The upflow reactor was made from Pyrex glass and it contains a fixed bed of AC (activated carbon) of 42% of working volume, 1.24 l y 541.17 g of AC. The reactor is outlined in Fig. 3 and their characteristics are shown in Table 1. At the beginning of the operation there was an adsorption stage to saturate the AC with the dye and do not allocate the fading of the solution to just possible adsorption on to AC, after that, the rector was inoculated with an adapted anaerobic sludge (microorganism consortium) by recirculation of water with 10% of adapted sludge for 15 days, 11.586 mg SS/g CA were adsorbed at the beginning, forming a biofilm in the AC surface. Dextrose and yeast extract are the carbon and nitrogen source to the microorganism.
Identification of degradation products
The compounds in the reactor effluent were identified under different process conditions at the reactor effluent: increasing the inlet dye concentration in the reactor influent from 250 to 500 mg/l, and under different half residence time (RTm) keeping constant the inlet dye concentration in 250 mg/l. Effluent samples were taken and extracted with ethyl acetate in agitated flask for 24 h, afterward, the extract was rota-vaporated and analyzed in a gas chromatographer coupled with a mass spectrometer (GC-MS, Perkin Elmer Clarus 500). The operation conditions of temperature-time for the analysis in the GC-MS were: 40° for 8 min and afterwards a 5 min slope until reaching 250° and keeping it for 30 min.
Analytical techniques
Absorbance was measured to 506 nm in the samples to follow the change in dye concentration using a spectrophotometer UV/VIS Perkin Elmer, Lambda25, and COD (Chemical Oxygen Demand) was measured by closed reflux method (APHA-AWWA-WPCF 1989).
Results and discussion
The results obtained by the continuous operation of the reactor showed that the proposed reactor works efficiently to remove the reactive azo dye with high removal percentages of almost 99%. COD removal rates were from 16.3 to 55.9%; these values indicate the degradation grade because when the dye molecule is reduced, occur the fading of the water, but the reduction products of the dye are complex organics that can be harder to degrade than the dye, as aromatic amines and substituted naphthalene. Table 2 shows some results of the degradation tests in the reactor and the parameters used in each one.
The activated carbon (AC) used as a carrier provides a suitable surface for microorganism growth and enhance the biodegradation because the dye is adsorbed to their surface and then reduced through a reaction carried out by the interaction of microorganism, extracellular enzymes and the groups in the AC surface. The dye is transported from the bulk to the bioparticle (biomass and activated carbon) surface and the dye reduction take place in the biofilm and in the AC surface. This effect and the data of adsorption of the dye to the AC were explained in a previous paper (Gonzalez-Gutierrez and Escamilla-Silva 2007).
In the first step of the degradation mechanism of the dye, it is carried out a reduction of the molecule by the breaking of the azo bond due to the transfer of electrons (reducing equivalents), by means of a redox mediator that can be the quinolic groups in the activated carbon surface and extracellular enzymes or coenzymes (Fig. 4); when the dye is reduced in this first step, it looses color. Dextrose added to the water is a source of carbon for microorganism and electron donor for dye reduction in the reactor.
The compounds identified by GC-MS in the reactor effluent, change with the reactor operation conditions. Under the best operation conditions with a good COD removal rate (around 50%) it was achieved good degradation efficiency, the identified compounds corresponded mainly to carboxylic acids, esters and alcohols, and in smallest amount to aromatic acids. The more frequently identified compounds in the effluent in this case are shown in Table 3.
As the organic load increases because a raise in dye concentration or a reduction in the half residence time of the reactor, the COD removal efficiency was reduced, and as a result the amount of aromatic compounds identified in the effluent was raised. In this case the identified compounds correspond to substituted aromatic acids, benzene-substituted rings and naphthalene-hydrated rings, as a result that were not enough conditions to take the degradation to a mayor extent, these are shown in Table 4. While dye concentration at the inlet continues increasing, the same compounds are found in the reactor effluent, although the abundance of some of them goes increasing as naphthalene, benzene acetic acid, xylene, ethyl benzene.
Despite the amount of aromatic compounds found in the reactor effluent, no aromatic amines were identified. Some authors state that aromatic amines are the most difficult compounds to degrade in azo dyes reduction (Field 2002; dos Santos et al. 2003; Supaka et al. 2004; HeFang et al. 2004; Plum and Rehorek 2005; Sponza and Isik 2005; van der Zee and Villaverde 2005; Khehra et al. 2006; Kumar et al. 2006); this indicate that the proposed process is efficient even at high dye concentration (400–500 mg/l). Anaerobic degradation of nitro aromatic compounds or aromatic amines has been demonstrated by other authors (Donlon et al. 1996; Kudlich et al. 1999; Chen 2002; Chen et al. 2006). Therefore, in the best operation conditions of the bioreactor with a COD removal of 50%, it is possible to degradation most of the aromatic compounds produced in the reduction of the reactive azo dye.
As was stated before, in anaerobic environment the aromatic compounds degradation is carried out by hydroxylation and carboxylation reactions, adding to the aromatic molecule –OH, CO2, –COO as well as fumarate (C4H2O4 2−) and succinate (C4H4O4 2−) groups, with the aim that the necessary redox reactions can be achieved. According to this and to the achieved results in the identification of the products at the bioreactor effluent, some degradation pathways are proposed for the partial mineralization of the Reactive Red 272 Azo dye. Nevertheless, the produced compounds in the dye reduction reactions can be degraded in different pathways by enzymes, metabolism and co-metabolism of the cells.
From the reduction of the dye showed in Fig. 4, two products are formed: RRP1 and RRP2. The degradation pathway proposed for the product RRP1 is showed in Fig. 5. The product RRP1 is divided in two main fractions: Chlorotriazine-Ethyl Phenyl Amine (EPhA), and the naphthalene-substituted group. The Chlorotriazine separates from the EPhA and is enzymatically carboxylated, and afterwards the ring opens and ammonium and acetic acid are produced.
In the Naphthalene-substituted molecule, the amino groups are substituted by –OH, through a nucleophilic substitution mechanism, and later loose the protons to give place to ketone groups. Subsequently, sulphone groups are reduced to sulphide through reductases and one of the rings opens, at this moment the sulphide groups are released as a consequence of the resonance and the compound marked as RRP1-2-A is produced (Fig. 5).
The degradation pathway for the RRP1-2-A is described in Fig. 6. The proposed pathway for this compound occurs through Claisen conversions and are produced CO2 and Acetic, Formic and Heptanedioic acid, which are low molecular weight compounds and can be metabolized by the microorganisms; 2-benzylmalonic acid is produced also in this stage in a parallel pathway and the route for its decomposition is shown in Fig. 7.
Claisen conversion is a mechanism that happens to the aryl-phenyl-ethers chemically by heating; this consist in the movement of six electrons and a transient high energy compound is formed which is quickly tautomerized to produce the final compound. The Claisen mechanism is used usually to refer to changes in aryl-phenyl-ethers arrays, including the tautomerism stages in the production of phenolic compounds (Miller 1998).
The proposed degradation pathway of 2-benzylmalonic acid results in the production of benzenedicarboxylic acid-mono (2-ethyl-hexyl) ester (BAME) through the addition of fumarate and transpositions; this compound was found almost in every sample at the reactor effluent. In addition, in this stage are produced: Benzyl acetic acid, Benzoic acid, Phenol and Pthalic acid, and after the ring opening, the Octanedioic acid which can be easily metabolized and split in other low molecular weight organic acids. At this stage of the degradation reactions of the dye, are produced the compounds identified more frequently in the reactor effluent as the BAME, Benzylacetic acid and Phenol, and through the degradation of the Octanedioic acid can be formed the Hexanoic and acetic acids or the Pentanoic and Propionic acids which were the organic acids found regularly in the water samples.
In Fig. 8 are shown the degradation pathways for the RRP2 product; at this point, are outlined four possible fractions in which the molecule could be broken. Two probable fractions are aromatic sulphone-amines; in their molecule, the amino groups are displaced by –OH groups through nucleophilic substitution mechanism, then, the sulphone groups are convert to sulphide and afterwards are released. Phenol and Resorcinol are produced afterwards the last reductions and substitutions, which generate the corresponding quinone to give place to the ring opening. Phenol and derived products can be produced starting from different pathways in the dye degradation mechanism; the proposed route for its decomposition is analyzed in Fig. 9.
The RRP2 product can be decomposed in other two fractions that correspond to the aliphatic part of the molecule: an acid bromide and an acid amino-bromide. Generally, acid halides are derived from carboxylic acids used in the synthesis of some compounds as esters, amides and aromatic acids. The halogen atom of an acid halide subtracts inductively the electronic density of the carbon in the carbonyl group, increasing its electrophilic nature and turning the molecule predominantly reactive for the nucleophilic substitution of the acid group, therefore, the halide acts as good group to be removed (Wade 2004).
Based in the last statement there were proposed some degradation routes for the acid bromides; the bromide is nucleophilically substituted for the hydroxyl group (–OH) and produce the corresponding acid, or by the amino group (−NH2) producing amides. The bromide in the position 2 of the molecule can be replaced also by the hydroxyl or amino group, producing amino-amides.
Phenols are highly reactive substrates in aromatic electrophilic substitution since the non bonded electrons of the hydroxyl group stabilize the sigma complex that is formed due to an electrophilic attack in the ortho or para position. Therefore, the hydroxyl group is highly activator and ortho–para director (Wade 2004). The proposed pathway to degrade phenol consist in forming a carboxylic group through the addition of fumarate or CO2 (electrophilic addition), which are bonded to the molecule in the para position. This mechanism occurs in the contrary way of the Phthalic acid production shown in Fig. 7, were the carboxylic group enters in the Meta position because –COOH is Meta director.
A substitute electron donor as –OH in the phenol molecule activates mainly the ortho and para positions, and a substitute electron subtractor as the group –COOH disables these positions.
After the electrophilic addition the corresponding redox reactions take place to take the molecule through different routes to the formation of Benzoquinone, p-cresol or hydroxybenzoic acid. Phenols oxidize producing aromatic ketones or diketones named quinones (Wade 2004); once the quinone is formed, the ring opening takes place to produce carboxylic acids.
The p-cresol or the hydroxybenzoic acid formed previously are dehydrogenated to form the carbonyl in the –OH position and afterwards is carried out an enzymatic hydrogenation and the ring opening producing a dicarboxylic acid that can be metabolized by bacteria to produce CO2 and lower weight carboxylic acids.
Ye et al. (2004) presented a review about the biodegradation of nitroaromatic compounds by aerobic and anaerobic media and the degradation pathways that they present for similar compounds, especially for triazines that are difficult to degrade, agree with the routes presented in this work. Tauber et al. (2005) presented a research in which they degraded azo dyes using fungus and ultrasound and they identify as the reduction products: Phenol, CO2, and carboxylic acids as Oxalic, Malonic, Formic, Propionic and Acetic acid; they also state that the degradation of Phenol produces Catechol, Hydroquinone and Benzoquinone, which are also proposed in this work as Phenol degradation intermediaries to carboxylic acids.
From the products showed in Fig. 9, the p-cresol and the propanoic acid were found usually in the water samples at the reactor effluent.
In the proposed degradation pathways are not described all the identified products in the reactor effluent because, as was stated before, it can be carried out several routes due to the cell metabolism or due to extracellular enzymatic reactions, besides, the identification studies were applied only to the water at the effluent instead of analyze the water at different reactor height, in order to identify other higher weight intermediaries to outline the degradation mechanism of the dye. However, there were carried out studies at different conditions, in such a way that, changing the residence time in the reactor and the dye inlet concentration the reaction is restricted, and then we achieve a lower degradation grade at higher dye concentration and at lower residence time; therefore, the aromatic compound identified under these conditions (Table 4) are the main reaction intermediaries.
One of the major compounds identified is the butanamide (3-methyl and 3, 3-dimethyl) that would be produced from the degradation of the previously formed Ethyl-Phenyl-Amine (Fig. 5), amides (Fig. 8), carboxylic acids or from the amino acids of the media. Amides are derived from the acids that are formed through the combination of an acid and ammonia or an amine (Wade 2004), therefore, given the conditions inside the reactor (carboxylic acids and ammonia are produced), these compounds can be produced.
Concluding remarks
A degradation pathway for the degradation of the azo dye Reactive red 272 was explained and proposed based in hydroxylation, carboxylation and redox reactions carried out by enzymes and by means of the interactions with the activated carbon as a carrier and with the substrate, in this case, dextrose.
The achieved degradation grade is a function of the dye concentration at the influent as well as the reactor residence time. Therefore, the degradation grade and the presence of aromatic compounds and amines can be manipulated handling these factors in the treatment of textile wastewater effluents, and so, avoid problems in further treatment process or dispose.
References
APHA-AWWA-WPCF (1989) Standard methods for the examination of water and wastewater, 17va ed. In: Clesceri LS, Greenberg AE, Trussell RR (eds) American Public Health Association, USA
Buckel W, Golding BT (2006) Radical enzymes in anaerobes. Annu Rev Microbiol 60:27–49. doi:10.1146/annurev.micro.60.080805.142216
Chen B-Y (2002) Understanding decolourization characteristics of reactive azo dyes by Pseudomonas luteola: toxicity and kinetics. Process Biochem 38:437–446. doi:10.1016/S0032-9592(02)00151-6
Chen B-Y, Chen S-Y, Lin M-Y, Chang J-S (2006) Exploring bioaugmentation strategies for azo-dye decolourization using a mixed consortium of Pseudomonas luteola and Escherichia coli. Process Biochem 41(7):1574–1581. doi:10.1016/j.procbio.2006.03.004
Donlon BA, Razo-Flores E, Lettinga G, Field JA (1996) Continuous detoxification, transformation, and degradation of nitro phenols in up flow anaerobic sludge blanket (UASB) reactors. Biotech Bioeng 51:439–449. doi:10.1002/(SICI)1097-0290(19960820)51:4<439::AID-BIT7>3.0.CO;2-J
dos Santos AB, Cervantes FJ, Yaya-Beas RE, van Lier JB (2003) Effect of redox mediator AQDS on the decolourisation of a reactive azo dye containing triazine group in a thermophilic anaerobic EGSB reactor. Enzyme Microb Technol 33:942–951. doi:10.1016/j.enzmictec.2003.07.007
Field JA (2002) Limits of anaerobic biodegradation. Water Sci Technol 45(10):9–18
Gerardi MH (2006) Wastewater bacteria. Wastewater microbiology series, Ed. Wiley-Interscience. USA
Griebler C, Safinowski M, Vieth A, Richnow H, Meckenstock R (2004) Combined application of stable carbon isotope analysis and specific metabolites determination for assessing in situ degradation of aromatic hydrocarbons in a tar oil-contaminated aquifer. Environ Sci Technol 38:617–631. doi:10.1021/es0344516
Gonzalez-Gutierrez LV, Escamilla-Silva EM (2007) Anaerobic biodegradation of a reactive red azo dye using an UASB bioreactor packed with activated carbon, World J Microbiol Biotechnol (in review)
HeFang , HuWenrong , LiYuezhong (2004) Biodegradation mechanisms and kinetics of azo dye 4BS by a microbial consortium. Chemosphere 57:293–301. doi:10.1016/j.chemosphere.2004.06.036
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2006) Biodegradation of azo dye C.I. Acid Red 88 by an anoxic-aerobic sequential bioreactor. Dyes Pigment 70:1–7. doi:10.1016/j.dyepig.2004.12.021
Kudlich M, Hetheridge MJ, Knackmuss H-J, Stolz A (1999) Autoxidation reactions of different aromatic o-aminohydroxynaphthalenes that are formed during the anaerobic reduction of sulfonated azo dyes. Environ Sci Technol 33:896–901. doi:10.1021/es9808346
Kumar K, Devi SS, Krishnamurthy K, Gampawar S, Mishra N, Pandya GH, Chakrabarti T (2006) Decolorisation, biodegradation and detoxification of benzidine based azo dye. Bioresour Technol 97:407–413. doi:10.1016/j.biortech.2005.03.031
Meckenstock RU, Safinowski M, Griebler C (2004) Anaerobic degradation of polycyclic aromatic hydrocarbons. FEMS Microbiol Ecol 49:27–36. doi:10.1016/j.femsec.2004.02.019
Miller B (1998) Advanced organic chemistry: reactions and mechanisms. Prentice Hall, USA
Plum A, Rehorek A (2005) Strategies for continuous on-line high performance liquid chromatography coupled with diode array detection and electro spray tandem mass spectrometry for process monitoring of sulphonated azo dyes and their intermediates in anaerobic–aerobic bioreactors. J Chromatogr A 1084:119–133. doi:10.1016/j.chroma.2005.03.001
Schink B (2006) Microbially driven redox reactions in anoxic environments: pathways energetics and biochemical consequences. Eng Life Sci 6(3):228–233. doi:10.1002/elsc.200620130
Sponza DT, Işik M (2005) Toxicity and intermediates of C.I. Direct Red 28 dye through sequential anaerobic/aerobic treatment. Process Biochem 40:2735–2744. doi:10.1016/j.procbio.2004.12.016
Supaka N, Juntongjin K, Damronglerd S, Delia M-L, Strehaino P (2004) Microbial decolorization of reactive azo dyes in a sequential anaerobic–aerobic system. Chem Eng J 99:169–176. doi:10.1016/j.cej.2003.09.010
Tauber MM, Guebitz GM, Rehorek A (2005) Degradation of azo dyes by laccase and ultrasound treatment. Appl Environ Microbiol 71:2600–2607. doi:10.1128/AEM.71.5.2600-2607.2005
van der Zee FP, Villaverde S (2005) Combined anaerobic–aerobic treatment of azo dyes—A short review of bioreactor studies. Water Res 39:1425–1440. doi:10.1016/j.watres.2005.03.007
Wade LG (2004) Química Orgánica, 5 ta edn. Pearson Prentice Hall, España
Ye J, Singh A, Ward OP (2004) Biodegradation of nitroaromatics and other nitrogen-containing xenobiotics. World J Microbiol Biotechnol 20:117–135. doi:10.1023/B:WIBI.0000021720.03712.12
Acknowledgement
The research was funded by Fondos Mixtos—Consejo Nacional de Ciencia y Tecnología del Estado de Guanajuato (Project: 31756).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Gutiérrez, L.V., González-Alatorre, G. & Escamilla-Silva, E.M. Proposed pathways for the reduction of a reactive azo dye in an anaerobic fixed bed reactor. World J Microbiol Biotechnol 25, 415–426 (2009). https://doi.org/10.1007/s11274-008-9906-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9906-0