Abstract
Halogenated compounds have been incorporated into the environment, principally through industrial activities. Nonetheless, microorganisms able to degrade halophenols have been isolated from neither industrial nor urban environments. In this work, the ability of bacterial communities from oligotrophic psychrophilic lakes to degrade 2,4,6-tribromophenol and 2,4,6-trichlorophenol, and the presence of the genes tcpA and tcpC described for 2,4,6-trichlorophenol degradation were investigated. After 10 days at 4°C, the microcosms showed the ability to degrade both halophenols. Nonetheless, bacterial strains isolated from the microcosms did not degrade any of the halophenols, suggesting that the degradation was done by a bacterial consortium. Genes tcpA and tcpC were not detected. Results demonstrated that the bacterial communities present in oligotrophic psycrophilic lakes have the ability to degrade halophenolic compounds at 4°C and the enzymes involved in their degradation could be codified in genes different to those described for bacteria isolated from environments contaminated by industrial activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Halophenolic compounds are part of a group of pollutants that are incorporated into the environment, largely due to industrial activities. 2,4,6-trichlorophenol (246TCP) is widely used as a wood preservative, herbicide, insecticide, and fungicide (Aranda et al. 2003; Xun and Webster 2004; Sánchez et al. 2004), whereas 2,4,6-tribromophenol (246TBP) is used in the wood industry to control fungi (Gutiérrez et al. 2002) and as a flame retardant (Hassenklöver et al. 2006). Due to their constant use, these compounds are common pollutants of soils and freshwater. Fulthorpe and Schofield (1999) reported that microorganisms recently developed the ability to degrade halogenated aromatic compounds as a response to their use as pesticides, even as sole carbon and energy source (Kharoune et al. 2002; Sánchez et al. 2004; Yamada et al. 2008). Nevertheless, several studies have shown that bacteria isolated from environments not exposed to this type of pollutant are able to degrade organochlorinates (Kamagata et al. 1997; Sánchez et al. 2004). Furthermore, in nature, several organisms have been described as producers of chlorinated and brominated organic compounds (Gribble 1992, 1998; Whitfield et al. 1999; Ahn et al. 2003; Vetter and Janussen 2005). Thus, bacterial communities associated with these organisms might be exposed to the presence of halophenolic compounds of biological origin.
In the aerobic degradation of chlorophenol compounds, the first steps are carried out by mono- and dioxygenase enzymes. tcpA and tcpC genes have been described for the degradation of 246TCP by Cupriavidus necator JMP134. These genes codify the enzymes 2,4,6-trichlorophenol monooxygenase (TCP-MO) and 6-chlorohydroxyquinol-1,2-dioxygenase (HQDO), respectively. The enzyme TCP-MO is involved in the dehalogenation process of 246TCP, whereas the enzyme HQDO participates in cleavaging of the aromatic ring (Louie et al. 2002; Matus et al. 2003; Sánchez and González 2007). These enzymes were described for the 246TCP degradative pathway. However, Aranda et al. (2003) suggested that the enzymes involved in the degradation of halophenolic compounds do not discriminate between chlorinated or brominated substrates.
The degradation properties of most of the bacteria that have shown the ability to degrade halophenols have been characterized at temperatures between 20 and 30°C (Fulthorpe et al. 1996; Kamagata et al. 1997; Kharoune et al. 2002; Aranda et al. 2003; Godoy et al. 2003; Matus et al. 2003; Sánchez et al. 2004). However, little information is available regarding the activity of the aerobic psychrophilic bacteria participating in the metabolism of halophenols such as 246TBP.
The aim of this work was to study the ability to degrade 246TBP by aerobic heterotrophic bacterial communities from psychrophilic and oligotrophic lakes, (Soto 2002; Woelfl et al. 2003) of the Chilean Patagonia; and also to investigate if this ability is due to the presence of the tcpA and tcpC genes described for 246TCP degradation.
Materials and methods
Study sites and sampling
Samples (1l) were taken from four lakes (Alto Reino, Las Dos Torres, Venus, Orilla Camino) in the Chilean Patagonia (44–46° S, 72–73° W). The samples were stored at 4°C until being processed in the laboratory. The presence of organohalogenated compounds in the water samples was determined by gas chromatography with an electron capture detector (GC-ECD) and gas-mass chromatography (GC-MS). For this, 10-ml samples were extracted with n-hexane (three times) and concentrated to 500 μl. The chromatographic analysis (GC-ECD) was carried out with a Shimadzu 9A with a Shimadzu C-R7A Chromatopac integrator. An HP-5 capillary column (30 m × 0.53 mm) (J&W Scientific) was used. Carrier N2 (30 psi), split less 1:100, injection temperature 275°C, oven temperature 210°C. The GC-MS was carried out in an Agilent 5890 Series II gas chromatograph with a Hewlett Packard 5,972 mass detector equipped with a capillary column (HP-5MS; 30 m × 0.25 mm) (J&W Scientific). Lindane, DDT, myrex, aldrin, dieldrin and heptachlorine, 2,4,6-trichlorophenol and 2,4,6-tribromophenol were used as standards for GC-MS.
Bacterial counts
The total bacterial community in the water samples was determined by fixing 50 ml of water with 3% formalin. Fixed samples were stained with acridine orange (125 μg/ml) and the bacterial counts were done by triplicate using epifluorescent microscopy, (Hobbie et al. 1977). The viable bacterial counts were done by inoculating, by triplicate, 20 μl aliquots of serial dilutions on the surface of petri dishes with R2A agar (HeMedia), and incubated at 4 or 30°C for 48–120 h (Herbert 1990).
Halophenol degradation test in water microcosms
The ability of the bacterial populations present in the water samples to degrade 246TBP and 246TCP was studied by preparing microcosms in 250 ml Erlenmeyer flasks. For this purpose, to 75 ml of the water sample, 25 ml of threefold concentrated mineral saline medium (MSM) was added (Aranda et al. 2003). In addition, each Erlenmeyer flask was added with 20 μg/ml of 246TCP or 246TBP. To determine if the degradation of halophenols is carried out by biological process, a negative control with autoclaved water sample was performed as described above. The Erlenmeyer flasks were then incubated at 4 or 20°C and stirred constantly (100 rpm). Every 48 h, 1 ml aliquots were taken for UV spectroscopy to analyze the aromatic ring cleavage (Aranda et al. 2003) and viable bacterial counts (Herbert 1990). After the complete degradation of halophenols, the microcosms were again added with 246TBP or 246TCP (20 μg/ml) and incubated at 4°C, and bacterial counts and degradative kinetics were evaluated as described above. Also, 1 ml of the samples was stored to confirm the complete degradation of halophenols. The presence of halogenated compounds was evaluated through high resolution liquid chromatography (HPLC-DAD) (Agilent Series 1100, California, USA) and a diode arrangement detector using a Lichospher 100 RP-18.5 μm column (250 × 4 mm) (Merck). About 20 μl of the samples obtained at the beginning and end of the experiment were injected. The chromatographic conditions were mobile phase acetonytrile 50% (vol/vol) in 30 mM ammonium acetate, flow 1 ml/min, column temperature 38°C.
Metabolic profile study of the bacterial microcosms
The diversity of carbon sources that can be used by the bacterial communities present microcosms were determined by using the Biolog Ecoplate™ system (Biolog Inc., CA, USA). Once the complete degradation of halophenols was detected, each well of the microplate was inoculated with 100 μl of 246TBP of degrading microcosm, and incubated at 4°C for 10 days. Richness was considered as the number of oxidized carbon substrates and the microbial activity, expressed as average well-color development were determined as described by Gomez et al. (2006).
Bacterial isolation and degradation study
Aliquots (100 μl) were taken from the microcosms in which a decrease in the concentration of 246TBP or 246TCP was detected. These samples were spread onto R2A agar, and incubated for 72 h at 4 or 30°C. Later, all the different macroscopic colonies were selected and characterized according to their staining affinity as Gram positive or Gram negative.
Since to only a Gram negative nor fermentative bacteria was isolated, the biochemical properties of the isolated strains were investigated using the codified system API 20 NE (Bio Merieux). Then, the ability of isolated bacterial strains to degrade 246TBP or 246TCP was evaluated. For this, the isolated strains were cultured in R2A broth for 48 h and washed 3 times with MSM. The bacterial strains were inoculated at a cellular density of 1 × 107 CFU/ml in Erlenmeyer flasks with 100 ml MSM as sole carbon source or supplemented with glucose (0.3 mM) (Aranda et al. 2003), and 20 μg/ml of 246TBP or 246TCP and incubated at 4 and 30°C. Viable bacterial counts were done daily for five days and the degradation of the halogenated compounds was evaluated through UV spectroscopy.
The same strains inoculated as pure strains were also inoculated as a mixture of all of them in 100 ml of MSM in a 250 ml Erlenmeyer flask containing 20 μg/ml of 246TBP or 246TCP, at a density of 1 × 107 CFU/ml of each one of them, in order to evaluate their degrading ability as sole carbon source of the mixture.
DNA extraction
The DNA from the bacteria in the microcosms was purified using the Power Soil DNA Isolation Kit (Mo Bio Laboratories). DNA was extracted from Cupriavidus necator JMP134 and from Cupriavidus sp. PZK strain (Matus et al. 2003) as a positive control for the amplification of tcpA and tcpC genes using the Ultra Clean Microbial DNA Isolation Kit (Mo Bio Laboratories). The amount of DNA was determined spectrophotometrically by using a 260/280 ratio.
Amplification of tcpA and tcpC genes
The DNA obtained from the water samples and the isolated strains were checked for the presence of the genes described for the degradation of 246TCP. This was done by using the degenerated primers (TftDF1/TftDR1 and HQF/HQR) described by Louie et al. (2002) and the specific primers (MON-F/MON-R and HQ-F/HQ-R) reported by Godoy et al. (2003) and Matus et al. (2003) for tcpA and tcpC genes, respectively. The amplification conditions for the primers MON-F/MON-R and HQ-F/HQ-R and TftDF1/TftDR1 and HQF/HQR were the same as those described by Godoy et al. (2003) and Matus et al. (2003) and by Louie et al. (2002), respectively.
Results and discussion
Physico-chemical characterization of the study areas
The lakes of the study area are glacial in origin, deep, and oligotrophic (Woelfl et al. 2003), with highly transparent waters and low levels of suspended solids and dissolved organic carbon (Soto 2002).
The results obtained from the GC-ECD and GC-MS analyses of the water samples suggest the presence of two unidentified halogenated phenolic compounds whose retention times were 10.4 and 24.7 min with 247 and 303 units of mass, respectively (data not showed). No industrial activity exists in the area surrounding the water bodies in which these halogenated compounds were found. Hence, their presence could be related to biological activity since some organisms are known to produce a chemical defense system when faced with competitors (Gribble 1992; Bantleon et al. 1994; Peters et al. 2003). Moreover, the combustion of vegetable biomass during forest fires could form a variety of halogenated organic compounds including phenolics (Gribble 1998). However, anthropogenic contamination could not be ignored, since some persistant organic pollutans have the ability to be transported from distant areas (Castro-Jiménez et al. 2008).
Viable and total bacterial counts
The total bacterial count by eplifluorescence microscopy was 1 × 105 cells/ml, whereas the viable bacteria did not exceeded 1 × 103 CFU/ml (Table 1). According to these results, the proportion of bacteria that can be recovered for cultivation was less than 1%. Amann et al. (1995) reported that, in aquatic environments, that it is possible to cultivate only a small proportion (no more than 1%) of the population of microorganisms since most of the bacteria are found in an inactive or dormant metabolic state.
Halophenol degradation in the water samples
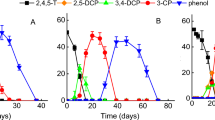
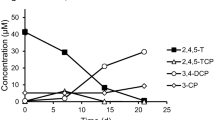
Under both temperature conditions, there was an adaptation period during which no degradation of the assayed halogenated compounds was detected (Fig. 1). Sánchez et al. (2004) reported that bacterial communities in soils not previously exposed to 246TCP, degraded chlorophenol (50 μg/ml) after 30 days of incubation. Furthermore, the bacterial communities isolated from unpolluted soils can degrade 2,4-dichlorophenoxiacetic acid, but this requires more than 10 days (Kamagata et al. 1997). In this work, at 20°C, degradation began after 6 days of incubation whereas, at 4°C, it was detected after 12 days. Once the degradation had begun, the time required for the total removal of the halophenols was approximately 4 days. This difference suggests that the degradation could be carried out by mesophilic bacteria since the adaptation period was shorter at 20°C than at 4°C. Hereby, a slower metabolic response could be explained by the lower incubation temperature. Nevertheless, a degradation process with psicrotolerant bacterial cells involved cannot be rejected since similar degradation rate was observed—but not calculated—at both incubation temperatures 4 or 20°C. The successive re-inoculations done with the halophenols revealed that the bacterial populations studied after the first assay did not present an adaptation period when they were inoculated with the halophenols being studied and, thus, 12 h after the halophenol was added, 10% of it had been removed. Nonetheless, differences in degradative profile was observed between microcosms added with 246TBP; on the other hand, microcosms ammended with 246TCP showed similar degradation rates (Fig. 2). The results obtained by HPLC confirmed that after 48 h incubation both 246TCP and 246TBP were completely degraded in all microcosms. A similar result was informed by Kharoune et al. (2002) in a bacterial consortioum able to degrade 246TCP. The authors indicated that the consortioum required a 15 day adaptation period to begin degradation, and after successive additions of chlorophenol, the degradation rate increased four times without requiring an adaptation period.
Kinetics of degradation at 4°C in the microcosmos previously adapted to the presence of halophenols. a Kinetics of 246TBP degradation; b kinetics of 246TCP degradation. (■) Lake Alto Reino; (♦) Lake Las Dos Torres; (▲) Lake Orilla Camino; (●) Lake Venus. The bacterial counts are shown as empty shapes and the degradation as solid shapes
It should be noted that, in the studied microcosms, the complete removal of the halophenols was achieved under conditions considered to be psychrophilic (4°C) and that these, once adapted, did not differ from the degradative activity of those incubated at 20°C. These results could be considered as an adaptation process since the degradation rates increased due to repeated exposure to halophenols. This observation has been reported in soils, estuaries and continental waters, and could be explained as the result of the selection of microorganisms capable of using the halophenols by combinations of inducible enzymes (Aelion et al. 1987).
Metabolic profile of the microcosms
After the degradation of 246TBP, the metabolic profile of the microcosms prepared with the water samples from the studied lakes was determined according to the Biolog Ecoplate™ system. These results indicated differences between the carbon sources used by each community. Moreover, none of the microcosms used more than 89% of the carbon sources available through the Biolog Ecoplate™ system (Fig. 3). Choi and Dobbs (1999) reported the use of more than 95% of the substrates by bacterial communities present in continental and oceanic water samples. Although differences exist in terms of the type of substrate used, overall, the average metabolic responses shown by each microbial community were similar (OD590 nm) 0.461, 0.459, 0.556, and 0.402 for Orilla Camino, Venus, Alto Reino and Las Dos Torres, respectively). According to Gomez et al. (2006) microbial activity obtained in microcosm of patagonic lakes was nearly two fold lower than that obtained by them in soil communities. This difference could be due to the incubation temperature of the samples since the bacterial metabolism could be lower at 4°C than at the temperature assayed by Gomez et al. (2006). Though there are differences between the viable bacterial counts of the degrading microcosms, Garland and Mills (1991) indicated that color production in the BIOLOG community-level assay is caused by growth of bacteria within wells after inoculation rather than respiration of the inoculated community. Nevertheless, the results demonstrated that the bacterial communities present in microcosms are able to totally degrade both 246TBP and 246TCP. These results together with the different carbon sources oxidized by the degrading microsoms suggest that they could be considered as different communities.
Microbial activity of bacterial communities present in microcosms added with 246TBP a Orilla Camino; b Venus; c Alto Reino; d Las Dos Torres. (1) Water; (2) β-mehtyl-d-glucoside; (3) d-galactonic acid γ-lactone; (4) l-arginine; (5) pyruvic acid methyl ester; (6) d-xylose; (7) d-galacturonic acid; (8) l-asparagine; (9) Tween 40; (10) i-erythritol; (11) 2-hydroxy benzoic acid, (12) l-phenylalanine; (13) Tween 80; (14) d-mannitol; (15) 4-hydroxy benzoic acid; (16) l-serine; (17) α-cyclodextrin; (18) N-acetyl-d-glucosamine; (19) γ-hydroxybutyric acid; (20) l-threonine; (21) Glycogen; (22) d-glucosaminic acid; (23) Itaconic acid; (24) Glycyl-l-glutamic acid; (25) d-cellobiose; (26) Glucose-1-phosphate; (27) α-ketobutyric acid; (28) Phenylethyl-amine; (29) α-d-lactose; (30) d,l-α-glycerol phosphate; (31) d-malic acid; (32) Putrescine
Isolation of strains and degradation studies
A total of 14 Gram negative bacterial strains were isolated from the degrading microcosms. Only one isolated strain was identified at genus level and corresponded to Sphingomonas (84.4%). On the other hand, four of all isolated strains were identified at species level; Stenotrophomonas maltophilia (99.9%), Crhyseobacterium indologenes (97.9%), Brevundimonas vesicularis (98%) and Burkholderia cepacia (99.9%). None Gram positive bacterial strain was isolated from any degrading microcosms.
Although in microcosms 246TBP and 246TCP were degraded, none of the 14 bacterial strains isolated were able to degrade these compounds, when in pure or mixed culture, as the sole carbon source or supplemented with glucose. Thus, the degradation of 246TBP or 246TCP could be associated with the action of bacterial consortia in which two or more strains provide essential factors that allow them to degrade the halophenols, as proposed by Ronen et al. (2005). On the other hand, the results indicated that only 0.1% of bacterial cells were recovered in culture. According to this results, the possibility that bacteria with degradative abilities were not cultivable in the culture media used (R2A agar) cannot be rejected.
Detection of tcpA and tcpC genes in microcosms
The tcpA and tcpC genes were not detected in the DNA obtained from the microcosms (Fig. 4). Similar results were found for Sphingopyxis chilensis S37, a bacterium that degrades 246TBP and 246TCP (Aranda et al. 2003) and also lacks tcpA or tcpC genes (Matus et al. 2003). This suggests that the degradation is carried out by enzymes codified in genes other than those described for the degradation of 246TCP in C. necator JMP134.
Detection of the genes tcpA and tcpC with specific and degenerated primers in water samples. a and c amplification of tcpA with specific and degenerated primers, respectively. c and d amplification of tcpC with specific and degenerated primers, respectively. Lane (1) Lake Orilla Camino; (2) Lake Venus; (3) Cupriavidus necator JMP134; (4) negative control; (5) Cupriavidus necator PZK; (6) Lake Alto Reino; (7) Lake Las Dos Torres; (S) 1 kb (c) or 100 bp (a, b, d) DNA standards
The ability to degrade 246TBP and 246TCP in the absence of tcpA and tcpC genes in the microcosms suggests a genetic diversity related to the degradation of halophenolic compounds. Aerobic heterotrophic bacteria have the ability to degrade both 246TBP and 246TCP, using enzymes codified by genes other than those described for 246TCP degradation by bacteria from environments contaminated by industrial activities. These genes should be studied in order to obtain their complete characterization.
Conclusions
The results demonstrate that in the Patagonic psychrophilic lakes, the proportion of bacteria recovery in culture is low. Nonetheless, the aerobic bacteria of these lakes without industrial or urban activity have the ability to degrade efficiently both 246TBP and 246TCP added as a sole carbon and energy source. However, we can not exclude that bacterial cells present in the microcosms lakes may have the ability to use either an endogenous carbon source as a polyhydroxyalanoate as describe Godoy et al. (2003) or organic material release by cellular lysis (Pinchuk et al. 2008). Thus, these bacterial communities could be participating in self-remediation processes of toxic compounds like halophenols incorporated into the environment by biological or industrial activities.
References
Aelion C, Swindoll CM, Pfaender F (1987) Adaptation to and biodegradation of xenobiotic compounds by microbial communities from a pristine aquifer. Appl Environ Microbiol 53:2212–2217
Ahn Y, Rhee S, Fennell D, Kerkhof LJ, Hentschel U, Häggblom M (2003) Reductive dehalogenation of brominated phenolic compounds by microorganisms associated with the marine sponge Aplysina aerophoba. Appl Environ Microbiol 69:4159–4166. doi:10.1128/AEM.69.7.4159-4166.2003
Amann R, Wolfgang L, Karl-heinz S (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Aranda C, Godoy F, Becerra J, Barra R, Martínez P (2003) Aerobic secondary utilization of a non-growth and inhibitory substrate 2,4,6-trichlorophenol by Sphingopyxis chilensis S37 and Sphingopyxis-like strain S32. Biodegradation 14:265–274. doi:10.1023/A:1024752605059
Bantleon R, Altenbuchner J, van Pée K (1994) Chloroperoxidase from Streptomces lividans: isolation and characterization of the enzyme and the corresponding gene. J Bacteriol 176:2339–2347
Castro-Jiménez J, Mariani G, Eisenreich SJ, Christoph EH, Hanke G, Canuti E, Skejo H, Umlauf G (2008) Atmospheric input of POPs into lake Maggiore (Northern Italy): PCDD/F and dioxin-like PCB profiles and fluxes in the atmosphere and aquatic system. Chemosphere 73:S122–S130. doi:10.1016/j.chemosphere.2007.06.097
Choi K, Dobbs F (1999) Comparison of two kinds of Biology microplates (GN and ECO) in their ability to distinguish among aquatic microbial communities. J Microbiol Methods 36:203–213. doi:10.1016/S0167-7012(99)00034-2
Fulthorpe R, Schofield L (1999) A comparison of the ability of forest and agricultural soils to mineralize chlorinated aromatic compounds. Biodegradation 10:235–244. doi:10.1023/A:1008365606324
Fulthorpe R, Rhodes A, Tiedje J (1996) Pristine soils mineralize 3-chlorobenzoate and 2,4-dichlorophenoxyacetate via different microbial populations. Appl Environ Microbiol 64:1159–1166
Garland J, Mills A (1991) Classification and characterization of heterotrophic microbial communities on the basis of pattern of community-level sole-carbon-source utilization. Appl Environ Microbiol 57:2351–2359
Godoy F, Bunster M, Matus V, Aranda C, González B, Martínez M (2003) Poly-b-hydroxyalkanoates consumption during degradation of 2,4,6-trichlorophenol by Sphingopyxis chilensis S37. Lett Appl Microbiol 36:315–320. doi:10.1046/j.1472-765X.2003.01315.x
Gomez E, Ferreras L, Toresani S (2006) Soil bacteria functional diversity as influenced by organic amendment application. Bioresour Technol 97:1484–1489. doi:10.1016/j.biortech.2005.06.021
Gribble GW (1992) The natural production of chlorinated compounds—a survey. J Nat Prod 55:1353–1395. doi:10.1021/np50088a001
Gribble GW (1998) Naturally occurring organohalogen compounds. Acc Chem Res 31:141–152. doi:10.1021/ar9701777
Gutiérrez M, Becerra J, Barra R (2002) Tribromophenol empleado en aserraderos: métodos de análisis, características físico-químicas y presencia en componentes ambientales. Bol Soc Chil Quim 47:485–493
Hassenklöver T, Predehl S, Pilli J, Ledwolorz J, Assmann M, Bickmeyer U (2006) Bromophenols, both present in marine organisms and industrial flame retardants, disturb cellular Ca2+ signaling in neuroendocrine cells (PC12). Aquat Toxicol 76:37–45. doi:10.1016/j.aquatox.2005.09.004
Herbert RA (1990) Methods for enumerating microorganisms and determining biomass in natural environments. In: Grigorova R, Norris JR (eds) Methods in microbiology. Techniques in microbial ecology, vol 22. Academic Press, London, p 619
Hobbie JE, Daley RJ, Jasper S (1977) Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33:1225–1228
Kamagata Y, Fulthorpe R, Tamura K, Takami H, Forney L, Tiedje J (1997) Pristine environments harbor a new group of oligotrophic 2,4-dichlorophenoxyacetic acid-degrading bacteria. Appl Environ Microbiol 63:2266–2272
Kharoune L, Kharoune M, Lebeault J (2002) Aerobic degradation of 2,4,6-trichlorophenol by a microbial consortium—selection and characterization of microbial consortium. Appl Microbiol Biotechnol 59:112–117. doi:10.1007/s00253-002-0951-6
Louie T, Webster C, Xun L (2002) Genetic and biochemical characterization of a 2,4,6-trichlorophenol degradation pathway in Ralstonia eutropha JMP134. J Bacteriol 184:3492–3500. doi:10.1128/JB.184.13.3492-3500.2002
Matus V, Sánchez M, Martínez M, González B (2003) Efficient degradation of 2,4,6-Trichlorophenol requires a set of catabolic genes related to tcp genes from Ralstonia eutropha JMP134(pJP4). Appl Environ Microbiol 69:7108–7115. doi:10.1128/AEM.69.12.7108-7115.2003
Peters L, König G, Wright A, Pukall R, Stackebrandt E, Eberl L, Riedel K (2003) Secondary metabolites of Flustra foliacea and their influence on bacteria. Appl Environ Microbiol 69:3469–3475. doi:10.1128/AEM.69.6.3469-3475.2003
Pinchuk G, Ammons C, Culley D, Li S, McLean J, Romine M, Nealson K, Fredrickson J, Beliaev A (2008) Utilization of DNA as a sole source of phosphorus, carbon, and energy by Shewanella spp.: ecological and physiological implications for dissimilatory metal reduction. Appl Environ Microbiol 74:1198–1208. doi:10.1128/AEM.02026-07
Ronen Z, Visnovsky S, Nejidat A (2005) Soil extracts and co-culture assist biodegradation of 2,4,6-tribromophenol in culture and soil by an auxotrophic Achromobacter piechaudii strain TBPZ. Soil Biol Biochem 37:1640–1647. doi:10.1016/j.soilbio.2005.02.001
Sánchez M, González B (2007) Genetic characterization of 2,4,6-trichlorophenol degradation in Cupriavidus necator JMP134. Appl Microbiol Environ 73:2769–2776. doi:10.1128/AEM.02584-06
Sánchez M, Vásquez M, González B (2004) A previously unexposed forest soil microbial community degrades high levels of the pollutant 2,4,6-trichlorophenol. Appl Environ Microbiol 70:7567–7570. doi:10.1128/AEM.70.12.7567-7570.2004
Soto D (2002) Oligotrophic patterns in southern Chilean lakes: the relevance of nutrients and mixing depth. Rev Chil Hist Nat 75:377–393
Vetter W, Janussen D (2005) Halogenated natural products in five species of antarctic sponges: compounds with POP-like properties? Environ Sci Technol 39:3889–3895. doi:10.1021/es0484597
Whitfield F, Drew M, Helidoniotis F, Svoronos D (1999) Distribution of bromophenols in species of marine polychaetes and bryozoans from eastern Australia and the role of such animals in the flavor of edible ocean fish and prawns (Shrimp). J Agric Food Chem 47:4756–4762
Woelfl S, Villalobos L, Parra O (2003) Trophic parameters and method validation in Lake Riñihue (North Patagonia: Chile) from 1978 through 1997. Rev Chil Hist Nat 76:459–474
Xun L, Webster C (2004) A monooxygenase catalyzes sequential dechlorinations of 2,4,6-trichlorophenol by oxidative and hydrolytic reactions. J Biol Chem 279:6696–6700
Yamada T, Takahama Y, Yamada Y (2008) Biodegradation of 2,4,6-tribromophenol by Ochrobactrum sp. strain TB01. Biosci Biotechnol Biochem 72:1264–1271
Acknowledgments
The authors thank Dr. Carlos Smith for his critical review of this manuscript. This study was financed by the projects FONDECYT 1070497 and DIUC Patagonia No. 205.036.029-ISP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguayo, J., Barra, R., Becerra, J. et al. Degradation of 2,4,6-tribromophenol and 2,4,6-trichlorophenol by aerobic heterotrophic bacteria present in psychrophilic lakes. World J Microbiol Biotechnol 25, 553–560 (2009). https://doi.org/10.1007/s11274-008-9923-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9923-z