Abstract
Based on its ability to produce lactic acid from glucose in mineral salt medium under anaerobic conditions, genetic modifications on Corynebacterium glutamicum Res 167 were carried out with the aim of producing optical pure D-lactic acid, involving the knockout of L-lactate dehydrogenase gene from C. glutamicum and the heterologous expression of D-lactate dehydrogenase gene from Lactobacillus bulgaricus into C. glutamicum. D-lactic acid production of the genetically engineered strain C. glutamicum Res 167Δldh/ldhA was 17.92 g/l (optical purity higher than 99.9%) after 16 h fermentation, which was 32.25% higher than the lactic acid production of the parental strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As an important synthetic precursor of many chiral compounds, lactic acid has been widely used in chemical, agriculture, food, medicine and environmental protection industries (John et al. 2007; Vallander and Erikson 1985; Wee et al. 2006). Moreover, lactic acid is a potential alternative of the non-biodegradable plastics from petrochemicals in poly lactic acid (PLA) synthesis (Akerberg and Zacchi 2000). Depending on its optical property, lactic acid can be divided into D-lactic, L-lactic and racemized DL-lactic acid. Recent research showed that the thermal stability of PLA was enhanced up to 200°C, which was 20°C above the melting temperature of the homo-polymer, when the poly D-lactic acid and poly L-lactic acid were mixed under a molar ratio of 1:1 (Sawai et al. 2007). Studies on D-lactic acid production become more and more popular since the above phenomenon was observed.
Currently, D-lactic acid production strains under research mainly include Lactobacillus species, Escherichia coli and Saccharomyces cerevisiae. Being the first industrialized strains such as L. delbrueckii (Hofvendahl and Hahn-Hägerdal 2000), L. coryniformis (Yáñez et al. 2003) and L. lactis (Joshi et al. 2010) etc. for D-lactic acid production, lactobacilli have drawn most researchers’ focus (Lu et al. 2009). So far as we known, the highest yield (up to 1.0 g D-lactic acid/g glucose) and productivity (up to 18 g/l/h) were obtained by L. delbrueckii (Tashiro et al. 2010). However, the fermentation processes by these strains required complicated nutrients-contained media thus increased the cost of the substrate consumption and downstream separation. To solve this problem, researchers tried to produce D-lactic acid by engineered E. coli (Zhou et al. 2003), which could use minimal salt medium for cell growth. But the alternative approaches lacked promise not only for engineered E. coli which had a low productivity and a low tolerance to lactic acid (Portnoy et al. 2008); but also for engineered S. cerevisiae whose separation cost was low but yield and productivity was low, either (Tokuhiro et al. 2009). Thus the major problem now is to realize the high efficient D-lactic acid production based on cheap resources.
Corynebacterium glutamicum has been widely used for production of amino acid derivatives in industries (Kinoshita et al. 1957; Leuchtenberger et al. 2005). Under anaerobic conditions, growth of C. glutamicum was repressed but organic acids (mainly lactic acid, succinic acid and acetic acid) were produced with high conversion yield and productivity in mineral salt medium containing glucose as carbon source (Inui et al. 2004; Okino et al. 2005). If we can take advantage of these abilities of C. glutamicum and direct the carbon flux to D-lactic acid production as much as possible, by using molecular biology methods to block the byproducts production (such as L-lactic acid, succinic acid and acetic acid) during the organic acid metabolism, the maximum production of D-lactic acid may be achieved in a low-cost and high-efficient way. However, there are few studies and reports in this area to date (Okino et al. 2008).
In this paper, genetic modifications on C. glutamicum were carried out in the aim of obtaining an engineered strain to produce high-purity D-lactic acid, involving two steps: first, the knockout of L-lactate dehydrogenase gene from C. glutamicum and second, the heterologous expression of D-lactate dehydrogenase gene from L. bulgaricus into C. glutamicum. The present work was a primary but important step to construct a high-efficiently and low-costly D-lactic acid producing strain in a long term study.
Materials and methods
Strains, plasmids, media and cultivation
All strains and plasmids used in this study were listed in Table 1. E. coli DH5α was used for plasmids propagation, which was cultured in Luria–Bertani (LB) medium containing (per liter): 10 g peptone, 5 g yeast extract and 10 g NaCl (pH 7.2–7.5) at 37°C. L. bulgaricus was used as the ldhA source, which was cultured in de Man-Rogosa-Sharp (MRS) medium containing (per liter): 20 g glucose, 10 g peptone, 10 g meat extract, 5 g yeast extract, 2 g K2HPO4, 5 g NaAc, 2 g triammonium citrate, 0.58 g MgSO4·7H2O, 0.25 g MnSO4·H2O and 1 ml Tween 80 (pH 6.2–6.4) at 40°C. All corynetacteria strains used were derived from C. glutamicum Res 167 and cultured in nutrient-rich medium (A-medium) containing (per liter): 40 g glucose, 2 g urea, 2 g yeast extract, 7 g casamino acid, 7 g (NH4)2SO4, 0.5 g KH2PO4, 0.5 g K2HPO4, 0.5 g MgSO4·7H2O, 6 mg FeSO4·7H2O, 4.2 mg MnSO4·H2O, 0.2 mg biotin and 0.2 mg thiamine (pH 7.0) at 30°C. Steam sterilization of the above media was performed under 0.05 MPa, 115°C for 30 min. Appropriate concentrations of antibiotics were supplemented in corresponding media when genetic manipulations were carried out: for E. coli, 50 μg/ml of kanamycin (final concentration, similarly hereinafter) and 20 μg/ml of chloramphenicol; for C. glutamicum, 25 μg/ml of kanamycin and 10 μg/ml of chloramphenicol. For transformation, C. glutamicum Res 167 was cultured in Brain Heart Infusion-Sorbitol (BHIS) medium containing: 7.4 g brain heart infusion, 18.2 g sorbitol in 200 ml distilled water (pH 7.2–7.5) at 30°C, sterilized by filtration (filer pore size of 0.22 μm).
General DNA manipulation
To clone corresponding DNA, C. glutamicum and L. bulgaricus were treated with 4 mg/ml lysozyme at 37°C for 30 min. PCR reaction system contained 50 ng DNA, 0.2 mM deoxynucleoside triphosphates, 2% dimethylsulfoxide in LA Taq polymerase buffer with MgCl2 and 4 U of LA Taq polymerase (Takara Biotechnology Co., Dalian, China) for 30 cycles at temperatures of 94°C for denaturation (1 min), 52°C for annealing (30 s), and 72°C for extension (1.5 min). Genetic manipulations such as restriction and ligation were carried out as described by Sambrook and Russell (2001). C. glutamicum were transformed by electroporation as described by van der Rest et al. (1999). Transformation of E. coli was performed by heat shock as described by Sambrook and Russell (2001).
Construction of recombinant plasmids
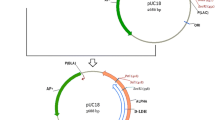
Plasmids for gene disruption and expression in C. glutamicum were derived from pK18mobsacB and pXMJ19, respectively. Primers used for DNA amplification were listed in Table 2. To clone ldh1 and ldh2 (cloning conditions described in above section), C. glutamicum Res 167 genomic DNA was used as PCR template with two pairs of primers uldh1, dldh1 and uldh2, dldh2 (After obtaining ldh (945 bp) sequence of C. glutamicum from NCBI, two homology arms ldh1 (723 bp) and ldh2 (784 bp) were designed by Primer Premier 5.0. The pair of primers uldh1 and dldh1 was used to generate ldh1 and the pair of primers uldh2 and dldh2 was used to generate ldh2). The overlapped PCR products were amplified with a pair of primers uldh1 and dldh2 using ldh1 and ldh2 mixture as PCR template. The PCR-amplified fragment of ldh1-ldh2 whose 3′-A overhang was added before ligation was sub-cloned into pEASY-T1 (procedures performed according to the manufacture’s instructions), obtaining the recombinant plasmid pEASY-T1-ldh. The HindIII-EcoRI target fragment was cloned into the suicide vector pK18mobsacB cut with the same enzymes, creating ldh gene knockout vector pK18mobsacB-ldh (Fig. 1). ldhA was amplified using L. bulgaricus ATCC 11842 genomic DNA as PCR template with a pair of primers uldhA and dldhA. The PCR-amplified fragment of ldhA whose 3′-A overhang was added before ligation was sub-cloned into pEASY-T1 (procedures performed according to the manufacture’s instructions), obtaining the recombinant plasmid pEASY-T1-ldhA. The XbaI-SacI target fragment was cloned into the shuttle vector pXMJ19 cut with the same enzymes, creating ldhA gene expression vector pXMJ19-ldhA (Fig. 2). All cloned DNA fragments were confirmed to be correct by sequencing.
Construction of the recombinant strains
The gene knockout plasmid pK18mobsacB-ldh was transformed by electroporation into C. glutamicum Res 167 and the positive strains were selected by kanamycin resistance. Because pK18mobsacB could not duplicate in C. glutamicum, only the recombinant strain could survive on the petri dish with kanamycin (Schäfer et al. 1994). Positive strains were picked up and cultured in LB liquid nutrient medium for 16 h, then the strain suspensions was spread onto solid medium with 20% sucrose. Only wild-type C. glutamicum and recombinants with double crossover homologous recombination could survive on this solid medium because the C. glutamicum strains with sacB gene were not able to growing. Pairs of primers up1, down1, uldh1, dldh2 and u1, d1 were used to do bacterial colony PCR to select the ldh gene knockout recombinant C. glutamicum Res 167Δldh among the ones with sucrose resistance.
The gene expression plasmid pXMJ19-ldhA was transformed by electroporation into C. glutamicum Res 167Δldh and the positive strains were selected by chloramphenicol resistance. As pXMJ19 was an E. coli-C. glutamicum shuttle vector, recombinants harboring pXMJ19-ldhA could survive on the petri dish with chloramphenicol. Positive strains were picked up and cultured in LB liquid nutrient medium with 10 μg/l chloramphenicol for 16 h. Pairs of primers uldhA, dldhA and upXMJ, dpXMJ were used to do bacterial colony PCR to select the ldhA gene expression recombinant C. glutamicum Res 167Δldh/ldhA.
Lactic acid production
For lactic acid/D-lactic acid production, C. glutamicum Res 167/Res 167Δldh/ldhA were cultured in A-medium at 30°C for 8 h with a constant rotation speed of 200 rpm. 500 ml of the culture were harvested (OD600 = 0.8–1.0) by centrifugation (5,000 × g, 4°C, 10 min). The cell pellet was subsequently washed once with mineral salt medium (BT-medium) containing (per liter): 0.5 g KH2PO4, 0.5 g K2HPO4, 0.5 g MgSO4·7H2O, 6 mg FeSO4·7H2O, 4.2 mg MnSO4·H2O, 0.2 mg biotin and 0.2 mg thiamine (pH 7.5 before sterilization), which was steam sterilized under 0.05 MPa, 115°C for 30 min. All of the washed cells were re-suspended in 50 ml of BT-medium with 4% (w/v) glucose in a lidded 100 ml bottle and incubated in a rotary shaker (THZ-C, Taicang Experiment Equipment, China) at 30°C for 16 h with a constant rotation speed of 150 rpm. The pH was monitored using a pH controller (PH-101, Hotec Instruments Co., Taiwan) and maintained at pH 7.0 by automatic supplementing of NH4OH. The fermentation experiments were all done in three repeats under the same operating conditions.
Analysis of glucose, lactic acid and cell concentrations
Samples were centrifuged (10,000 × g, 4°C, 10 min) and the supernatants were analyzed for sugars and organic acids. Glucose concentration was measured by a biosensor analyzer (SBA-40B, Institute of Biology, Shandong Province Academy of Sciences, China). Lactic acid concentration (D-lactic acid concentration + L-lactic acid concentration) was determined by high performance liquid chromatography (HPLC, Series 1200, Agilent Technologies, USA), equipped with a ZORBSX SB-C18 column (250 mm × 4.6 mm, Agilent Technologies, USA) and a UV detector (210 nm). The mobile phase was 5 mM H2SO4 at a flow rate of 1 ml/min. The oven temperature was 65°C. D-lactic acid concentration was measured by a lactate assay kit (Sigma Diagnostics Lactate Procedure No. 735, Sigma Co., St. Louis, MO, USA). Then L-lactic acid concentration was the difference between the measured lactic acid concentration and D-lactic acid concentration. The optical purity of the produced lactic acid was calculated by: (D-lactic acid concentration − L-lactic acid concentration)/lactic acid concentration ×100% (Okano et al. 2010). Cell concentration was measured through optical density at 600 nm (OD600) by an ultraviolet spectrophotometer (ZF1-II, Shanghai Jiapeng Tech. Co., China).
Results and discussion
Confirmation of the recombinant strains
Confirmation of ldh deletion in the mutant C. glutamicum Res 167Δldh by PCR and electrophoresis was shown in Fig. 3. It was expected that with pair of primers uldh1 and dldh2, there should be an about 1,500 bp product (ldh1-ldh2) which was amplified from C. glutamicum Res 167Δldh. If reverse mutation occurred in the strain, there would be an about 2,500 bp product (ldh1-ldh-ldh2). With pair of primers up1 and down1, there should be an about 1,000 bp product (internal fragment of ldh1-ldh2) which was amplified from C. glutamicum Res 167Δldh. If reverse mutation occurred, there would be an about 2,000 bp product (internal fragment of ldh1-ldh-ldh2). With pair of primers u1 and d1, there should be no product amplified from C. glutamicum Res 167Δldh. If reverse mutation occurred, there would be an about 1,000 bp product (ldh). From Fig. 3, it can be seen that there were the 1,500 bp bands with pair of primers uldh1 and dldh2 in lanes 1–4 and the 1,000 bp bands with pair of primers up1 and down1 in lanes 9–12; while no bands with pair of primers u1 and d1 were obtained in lanes 5–8. These PCR and electrophoresis results were in good consistent with the expected results, which meant that the L-lactate dehydrogenase gene of C. glutamicum Res 167 had been successfully knocked out in C. glutamicum Res 167Δldh.
Confirmation of ldh gene knockout in the mutant C. glutamicum Res 167Δldh by PCR and electrophoresis (lanes 1–4: amplifying ldh1-ldh2 with pair of primers uldh1 and dldh2; lanes 5–8: amplifying ldh with pair of primers u1 and d1; lanes 9–12: amplifying internal fragment of ldh1-ldh2 with pair of primers up1 and down1; lane M: 500 bp plus ladder)
Confirmation of ldhA expression in the recombinant C. glutamicum Res 167Δldh/ldhA by PCR and electrophoresis was shown in Fig. 4a (amplifying ldhA with pair of primers uldhA and dldhA) and Fig. 4b (amplifying ldhA and part of the plasmid with pair of primers upXMJ and dpXMJ, selecting bacterial colonies 1–4, 6–7, 9 and 11 of Fig. 4a with obvious PCR products). It was expected that if the shuttle vector pXMJ19-ldhA was successfully transformed into C. glutamicum Res 167Δldh, there should be an about 1,000 bp product (ldhA) with pair of primers uldhA and dldhA and an about 1,500 bp product (ldhA and part of the plasmid) with pair of primers upXMJ and dpXMJ; otherwise, there would be no products for both cases. It can be seen from Fig. 4a that there were the 1,000 bp bands with pair of primers uldhA and dldhA in eight lanes (1–4, 6–7, 9 and 11; the 1,000 bp band in lane X was the PCR product of ldhA amplified from E. coli harboring pXMJ19-ldhA as positive control); and it was also confirmed in Fig. 4b that there were the 1,500 bp bands with pair of primers upXMJ and dpXMJ in all eight lanes. These PCR and electrophoresis results were in good consistent with the expected results, which meant that the plasmid pXMJ19-ldhA had been successfully transformed into C. glutamicum Res 167Δldh, obtaining the final engineered strain C. glutamicum Res 167Δldh/ldhA in this work. Strain of colony 3 of Fig. 4b was picked up for further D-lactic acid fermentation experiments.
Confirmation of plasmid pXMJ19-ldhA transformation in the recombinant C. glutamicum Res 167Δldh/ldhA by PCR and electrophoresis (a amplifying ldhA with pair of primers uldhA and dldhA; lane X: E. coli harboring pXMJ19-ldhA as positive control; lane M: 1,000 bp plus ladder. b amplifying ldhA and part of the plasmid with pair of primers upXMJ and dpXMJ; lane M: 1,000 bp plus ladder)
Lactic acid production
Fermentation experiments of the parental strain C. glutamicum Res 167 and genetically engineered strain C. glutamicum Res 167Δldh/ldhA were carried out under the ways as described above, and the results were shown in Fig. 5, including the glucose consumption of both strains, and the lactic acid production of the parental strain as well as D-lactic acid production of the engineered strain. Data shown in this figure were the averaged results of the repeated experiments and the relative standard deviations were all with ±3%, which ensured the reliability of the strains. It can be seen from the figure that the lactic acid production of the parental strain C. glutamicum Res 167 was 13.55 g/l after 16 h fermentation, while the D-lactic acid production of the engineered strain C. glutamicum Res 167Δldh/ldhA was 17.92 g/l after 16 h fermentation, which was about 32.25% higher than the lactic acid production of the parental strain. It should be pointed out that the engineered strain did not produce L-lactic acid and the optical purity of the final product was higher than 99.9%.
In this study, a genetically engineered strain C. glutamicum Res 167Δldh/ldhA was successfully constructed which had the ability to produce high optical pure D-lactic acid with a higher production compared with the lactic acid production of the parental strain, by knockout of the L-lactate dehydrogenase gene and heterologous expression of D-lactate dehydrogenase gene from L. bulgaricus, as validated by the bacterial colony PCR and electrophoresis results. However, this was only the very first step to obtain the high-efficiently and low-costly D-lactic acid producing strain. Current engineered strain was still not comparable in D-lactic acid producing ability with the already industrialized strains whose production was up to 120 g/l (Calabia and Tokiwa 2007), not to mention the production cost since the substrate was glucose and the fermentation process had not been optimized yet in this work. Moreover, compared with other genetically engineered strains reported in literature whose D-lactic acid productions were around 1.27–138 g/l and yields were around 0.53–0.99 (Zhou et al. 2010), the recombinant obtained in this work (D-lactic acid production: 17.92 g/l, yield: 0.82 g/g) also needed further improvement for future utilization.
It was noticed that there were many other byproducts such as succinic acid and acetic acid apart from D-lactic acid in the products. Genetic engineering approach could be adopted to disrupt corresponding genes to reduce or even eliminate the production of these byproducts, increasing the carbon availability toward D-lactic acid. It is also promising to further enhance the production ability of the strain through metabolic engineering application and fermentation technique optimization.
References
Akerberg C, Zacchi G (2000) An economic evaluation of the fermentative production of lactic acid from wheat flour. Bioresour Technol 75:119–126
Bethesda Research Laboratories (1986) BRL pUC host: E. coli DH5α competent cells. Focus 8:9
Calabia BP, Tokiwa Y (2007) Production of D-lactic acid from sugarcane molasses, sugarcane juice and sugar beet juice by Lactobacillus delbrueckii. Biotechnol Lett 29:1329–1332
Hofvendahl K, Hahn-Hägerdal B (2000) Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb Technol 26:87–107
Inui M, Kawaguchi H, Murakami S, Vertès AA, Yukawa H (2004) Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J Mol Microbiol Biotechnol 8:243–254
Jakoby M, Ngougto-Nkili CE, Burkovski A (1999) Construction and application of new Corynebacterium glutamicum vectors. Biotechnol Tech 13:437–441
John RP, Nampoothiri KM, Pandey A (2007) Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl Microbiol Biotechnol 74:524–534
Joshi DS, Singhvi MS, Khire JM, Gokhale DV (2010) Strain improvement of Lactobacillus lactis for D-lactic acid production. Biotechnol Lett 32:517–520
Kinoshita S, Udaka S, Shimono M (1957) Studies of the amino acid fermentation. Part I. Production of l-glutamic acid by various microorganisms. Appl Microbiol 3:193–205
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8
Lu ZD, Lu MB, He F, Yu LJ (2009) An economical approach for D-lactic acid production utilizing unpolished rice from aging paddy as major nutrient source. Bioresour Technol 100:2026–2031
Okano K, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85:413–423
Okino S, Inui M, Yukawa H (2005) Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 68:475–480
Okino S, Suda M, Fujikura K, Inui M, Yukawa H (2008) Production of D-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 78:449–454
Portnoy VA, Herrgard MJ, Palsson BO (2008) Fermentation of d-Glucose by an evolved cytochrome oxidase-deficient Escherichia coli strain. Appl Environ Microbiol 74:7561–7569
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sawai D, Tamada M, Kanamoto T (2007) Development of oriented morphology and mechanical properties upon drawing of stereo-complex of poly (L-lactic acid) and poly (D-lactic acid) by solid-state coextrusion. Polym J 39:953–960
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19 selection of defined deletions in the chromosome of Corynebacterium glutumicum. Gene 145:69–73
Tashiro Y, Kaneko W, Sun YQ, Shibata K, Inokuma K, Zendo T, Sonomoto K (2010) Continuous D-lactic acid production by a novel thermotolerant Lactobaillus delbrueckii subsp. lactic QU 41. Appl Microbiol Biotechnol. doi:10.1007/s00253-010-3011-7
Tokuhiro K, Ishida N, Nagamori E, Saitoh S, Onishi T, Kondo A, Takahashi H (2009) Double mutation of the PDC1 and ADH1 genes improves lactate production in the yeast Saccharomyces cerevisiae expressing the bovine lactate dehydrogenase gene. Appl Microbiol Biotechnol 82:883–890
Vallander L, Erikson KE (1985) Enzymic saccharification of pretreated wheat straw. Biotechnol Bioeng 27:650–659
van der Rest ME, Lange C, Molenaar D (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl Microbiol Biotechnol 52:541–545
Wee YJ, Kim JN, Ryu HW (2006) Biotechnological production of lactic acid and its recent applications. Food Technol Biotechnol 44:163–172
Yáñez R, Moldes AB, Alonso JL, Parajó JC (2003) Production of D(−)-lactic acid from cellulose by simultaneous saccharification and fermentation using Lactobacillus coryniformis subsp. torquens. Biotechnol Lett 25:1161–1164
Zhou S, Causey TB, Hasona A, Shanmugam KT, Ingram LO (2003) Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69:399–407
Zhou L, Tian KM, Chen XZ, Zuo ZR, Shi GY, Wang ZX (2010) Advance in the production of optically pure D-lactic acid by microbial fermentation. China Biotechnol 30:114–124
Acknowledgments
The authors would like to acknowledge the generous donation of the strain C. glutamicum Res 167 and plasmids pK18mobsacB and pXMJ19 by Prof. Shuangjiang Liu of the Institute of Microbiology, Chinese Academy of Sciences. This work was financially supported by the National High Technology Research Development Program of China (No. 2006AA020102), the National Natural Science Foundation of China (No. 20906070 and No. 20976124), the Innovation Foundation of Tianjin University, and Program of Introducing Talents of Discipline to Universities (No. B06006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, X., Liu, P., Li, S. et al. D-lactic acid production by a genetically engineered strain Corynebacterium glutamicum . World J Microbiol Biotechnol 27, 2117–2124 (2011). https://doi.org/10.1007/s11274-011-0675-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0675-9