Abstract
Lactic acid (LA) is an important and versatile chemical that can be produced from renewable resources such as biomass. LA is used in the food, pharmaceutical, and polymers industries and is produced by microorganism fermentation; however, most microorganisms cannot directly utilize biomass such as starchy materials and cellulose. Here, we summarize LA production using several kinds of genetically modified microorganisms, such as LA bacteria, Escherichia coli, Corynebacterium glutamicum, and yeast. Using gene manipulation and metabolic engineering, the yield and optical purity of LA produced from biomass has been significantly improved. In this review, the drawbacks as well as improvements of LA production by fermentation is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, fossil resources are widely used to produce electricity, heat, and transportation fuels, as well as the vast majority of chemicals. During the twentieth century, continuous scientific and technological developments have led to ongoing refinements in these areas, resulting in highly optimized and efficient technologies (Christensen et al. 2008). Unfortunately, such refinements have contributed to the exhaustion of fossil resources and serious environmental problems, as represented by global warming. From this standpoint, the replacement of petroleum-derived transportation fuels and chemicals with those from biomass is vital for sustaining the growth of the chemical industry and society (Dodds and Gross 2007).
Lactic acid (LA) is an important and versatile chemical that can be produced from biomass and used as an acidulant, flavor enhancer, and preservative in the food, pharmaceutical, leather, and textile industries, as well as for the production of base chemicals (Hofvendahl and Hahn-Hägerdal 2000). LA has attracted considerable attention for polymerization to poly LA (PLA). As LA has two optical isomers (D- and L-LA) and its optical purity is crucial to the physical properties of PLA, the production of enantiomeric pure D- or L-LA is an important goal. In addition, stereocomplex PLA, which is composed of both poly D- and L-LA, is known to have high thermostability. Stereocomplex-type polymers show a high melting point (ca. 230 °C) that is approximately 50 °C higher compared with the respective single polymers (Ikada et al. 1987). Therefore, there is demand for both D- and L-LA, but LA produced by microbial fermentation employing LA bacteria (LAB), instead of by chemical synthesis, generally results in a mixture of both D- and L-LA (Wee et al. 2006).
Although demand for PLA has expanded, its current production capacity of 450 million kilograms per year (Christensen et al. 2008) is dwarfed by the 200 billion kilograms per year of total plastics produced. This low production volume is due for the most part to high manufacturing costs. On an industrial scale, the manufacturing cost of the LA monomer is targeted to be less than 0.8 US$/kg, as the selling price of PLA must decrease by roughly half of its present price of 2.2 US$/kg to compete with fossil-fuel-based plastics (Wee et al. 2006). Thus, the majority of the cost of manufacturing PLA is occupied by LA monomer production costs.
One bottleneck for cost-efficient LA production is the cost of pretreatment of raw materials. Currently, starchy materials are promising raw materials as they are relatively abundant and low in price. However, they must be saccharificated by physicochemical and enzymatic treatment using amylolytic enzymes such as α-amylase and glucoamylase because most LAB cannot utilize starchy materials directly (Narita et al. 2004). As pretreatments make the whole process cost-inefficient, direct fermentation of starchy materials would reduce the cost of LA production. Utilization of lignocellulose is also expected in the future. Although lignocellulose is inedible and represents one of the most abundant and inexpensive biomass sources in the world, its utilization is more difficult than starchy materials. This is because lignocellulose contains as its main component cellulose, which is a persistent polymer whose degradation requires physicochemical pretreatments and multi-enzymatic reactions employing endoglucanase (EG), cellobiohydrolase (CBH), and β-glucosidase (BGL; Okano et al. 2009c). Its secondary abundant component, hemicellulose, contains considerable amounts of pentose sugars such as xylose and arabinose, which are unavailable to most microorganisms (Tanaka et al. 2002).
The other bottlenecks for cost-efficient LA production are the separation and purification processes of LA after fermentation. A low pH has inhibitory effects on cell growth and LA production during LA fermentation, and most Lactobacillus sp. cannot grow and produce LA below pH 4, although the pKa of LA is 3.78 (Adachi et al. 1998). Therefore, neutralizing agents such as CaCO3, NaOH, or NH4OH must be added to keep the pH at a constant value. This requires processing for the regeneration of precipitated lactates (Porro et al. 1995). Moreover, the use of complex media such as yeast extract and corn steep liquor hampers not only separation but also purification of LA.
Thus, there are many challenges for industrial mass production of LA as a raw material for PLA production. However, satisfying these requirements is very difficult through the traditional use of LAB. Therefore, improving LAB via gene modification has become an essential and interesting research area. In addition, movements to use other microorganisms such as Escherichia coli, Corynebacterium glutamicum, and yeast for LA production via gene modification have also arisen (Zhou et al. 2003a; Okino et al. 2008; Ishida et al. 2005). Although LAB has attracted the most attention as an LA producer, other organisms can also produce LA with high yield and productivity.
In this review, we mainly focused on LA production using genetically modified (GM)-microorganisms and discussed recent achievements, perspectives, and limits that concern the production of enantiomeric pure LA from renewable resources.
Lactic acid bacteria
LAB has been traditionally used for LA production and is still the predominant candidate for industrial LA production as a raw material for PLA. Varying by species, enantiomeric pure L-LA can be produced by species such as Lactobacillus rhamnosus and Lactococcus lactis, while D-LA can be produced by species such as Lactobacillus delbrueckii (Hofvendahl and Hahn-Hägerdal 2000). In addition, LBA can produce LA with high yield and high productivity (Litchfield 1996). Interestingly, in some environments, many wild amylolytic LAB (ALAB) have been isolated (Giraud et al. 1994; Guyot et al. 2000; Narita et al. 2004). Using such ALAB, many researchers have attempted direct LA production from starchy materials. As shown in Table 1, although direct LA production from starch has been achieved, LA productivity is not practical according to most reports. Lactobacillus manihotivorans LMG 18010 can produce L-LA from starch with a high optical purity of 98.0%, but its yield is significantly low at 0.67 (gram per gram of consumed sugar; Guyot et al. 2000), and Lactobacillus plantarum A6 can produce LA with a high yield of 0.84, but it produces a mixture of L- and D-LA that is not suitable for PLA synthesis (Guyot et al. 2000). In one of the few successful studies, Streptococcus bovis 148 was found to produce L-LA from raw corn starch with a high yield of 0.88 and a relatively high optical purity of 95.6% (Narita et al. 2004). However, screening for useful ALAB is both time-consuming and difficult.
To overcome such problems, recent studies have applied recombinant strategies. Okano et al. (2007) attempted expression of α-amylase from S. bovis 148 (AmyA; Satoh et al. 1993) that efficiently degraded raw starch with the aid of a C-terminal starch-binding domain (Matsui et al. 2007) in L. lactis IL 1403 that produces L-LA with high yield and high optical purity (Hofvendahl and Hahn-Hägerdal 1997). The resulting recombinant L. lactis strain IL 1403/pCUSαA successfully secreted AmyA and directly produced L-LA from soluble starch with a high yield of 0.89 and an optical purity of 99.2% (Table 1). This successful result provided a new methodology to achieve efficient and direct LA production from starch through the combination of an efficient LA producer and known useful amylolytic enzymes.
D-LA as well as L-LA can be produced from starch based on recombinant strategies. Since there has been no report about D-LA-producing ALAB, and the fact that gene manipulation of D-LA-producing LAB is known to be difficult (Serror et al. 2002), the construction of D-LA-producing LAB from gene manipulated D,L-LA-producing strains has been attempted. Okano et al. (2009b) disrupted the L-lactate dehydrogenase (LDH) gene of L. plantarum NCIMB 8826. Since the resulting strain L. plantarum ΔldhL1 possesses only D-LDH, L. plantarum ΔldhL1 produces exclusively D-LA from glucose, and its optical purity is significantly high 99.7% (Table 1). Moreover, after introduction of an AmyA-expressing plasmid into L. plantarum ΔldhL1, the resulting strain L. plantarum ΔldhL1/pCUSαA produced 73.2 g/l of D-LA from raw corn starch with a high yield of 0.85 and an optical purity of 99.6% (Table 1). Thus, direct enantiomeric pure LA production from starch has become feasible.
Direct fermentation from cellulose is an important challenge. Although simultaneous saccharification and fermentation of cellulose has been attempted (Yáñez et al. 2003; Yoon 1997), there has been no report demonstrating direct LA fermentation from not only cellulose but also from cellooligosaccharides consisting of more than four glucose units (Adsul et al. 2007). By introducing an expression plasmid for EG from Clostridium thermocellum (CelA) in L. plantarum ΔldhL1, direct D-LA production from cellopentaose and cellohexaose was achieved (Okano et al. 2009c). The resulting strain L. plantarum ΔldhL1/pCU-CelA produced 1.27 g/l of D-LA from a medium containing 2 g/l of cellopentaose and cellohexaose (Table 1). Moreover, by addition of BGL from Aspergillus aculeatus (BGL1) produced by recombinant Aspergillus oryzae, L. plantarum ΔldhL1/pCU-CelA produced 1.47 g/l of D-LA from β-glucan (Table 1). Such results demonstrate a strong possibility of direct LA production from β-glucan and other cellulosic compounds by co-expression of CelA and other cellulolytic enzymes.
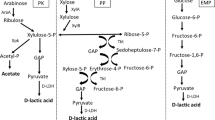
For utilization of hemicellulose, utilization of pentose sugars such as xylose and arabinose is a major problem. Some LAB such as Lactobacillus pentosus (Bustos et al. 2005), Lactobacillus brevis (Chaillou et al. 1998), L. plantarum (Helanto et al. 2007), and Leuconostoc lactis (Ohara et al. 2006) are known to ferment either or both arabinose and xylose. The metabolic pathway of pentose is shown in Fig. 1. In these microorganisms, arabinose and xylose are converted to xylulose-5-phosphate (X5P) through several enzymatic reactions (Helanto et al. 2007; Tanaka et al. 2002). This common intermediate, X5P, is converted to equimolar amounts of LA and acetic acid (AA; 3:2 in gram order) via the phosphoketolase pathway (PK pathway; Tanaka et al. 2002). This low yield of LA is an undesirable feature. Recently, Tanaka et al. (2002) have shown that L. lactis IO-1 has another pathway for X5P assimilation, the pentose phosphate pathway (PP pathway), in addition to the PK pathway. Since 5 mol of LA is produced from 3 mol of X5P through this pathway, L. lactis IO-1 is capable of producing L-LA at high yield of 0.68 over 0.60 that is the theoretical yield via the PK pathway (Table 2). This interesting finding indicated the possibility of homogenous LA fermentation from pentose sugars by metabolic engineering. In L. plantarum ΔldhL1 that can originally assimilate arabinose via the PK pathway and exclusively produces D-LA (Okano et al. 2009b), redirection of the PK pathway to the PP pathway was examined. The endogenous phosphoketolase 1 gene (xpk1) that encodes phosphoketolase was replaced with a heterologous transketolase gene (tkt) from L. lactis IL 1403 (Okano et al. 2009a). In the resulting strain L. plantarum ΔldhL1-xpk1::tkt, AA production was almost abolished, and this strain produced 38.6 g/l of D-LA from 50 g/l of arabinose with a high yield of 0.82, and the optical purity of D-LA was 99.9% while produced AA was only 0.4 g/l (Table 2).
Homogenous LA production from xylose was also achieved by disruption of the phosphoketolase 2 gene (xpk2) that is presumed to be expressed in the presence of xylose and with the introduction of a plasmid for the expression of a xylose isomerase gene (xylA) and xylulose kinase gene (xylB) from L. pentosus NRIC 1069 in L. plantarum ΔldhL1-xpk1::tkt (Okano et al. 2009a). Using the resultant strain L. plantarum ΔldhL1-xpk1::tkt-Δxpk2, 41.2 g/l of D-LA was produced from xylose with a high yield of 0.89 and an optical purity of 99.2%, while produced AA was only 1.0 g/l (Table 2). As described above, homogenous LA production can be accomplished by metabolic engineering of the X5P assimilation pathway. There are a few LAB, such as L. lactis SHO-47 and SHO-54, that can assimilate xylooligosaccharides such as xylobiose to xylohexaose (Ohara et al. 2006). By introducing such characteristics into a homogenous LA producer for pentose, homogenous LA production from larger components of hemicellulose could be possible.
Direct production of optically pure LA from a huge variety of mono-, oligo-, or polysaccharides may therefore be possible with a high yield. Thus, cost-efficient LA production is feasible from the view of LA fermentation. However, LAB requires complex nutrients due to their limited ability to synthesize B-vitamins and amino acids (Hofvendahl and Hahn-Hägerdal 2000), and this results in cost-inefficient LA purification. From both a view of metabolic engineering and purification, solutions to this problem should be studied.
E. coli
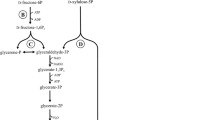
Compared to LAB, E. coli does not require a complex medium and can grow in a simple mineral salt medium. In addition, E. coli can naturally utilize both hexose and pentose sugars (Zhou et al. 2003a). The main problem for LA production in E. coli is improving LA yield, since E. coli produces a mixture of organic acids (D-LA, AA, succinic acid (SA), and formic acid (FA)) and ethanol to accommodate the reducing equivalents generated during glycolysis (Fig. 2; Zhou et al. 2003a).
Successful D-LA production using recombinant E. coli was first reported by Chang et al. (1999). A mutation only in the phosphotransacetylase gene (pta) significantly improved LA yield. Mutant strain JP201 produced approximately sixfold more D-LA than parental strain RR1. Interestingly, not only production of AA but also production of FA and ethanol were almost blocked. This phenomenon is considered to be due to reduced levels of pyruvic acid (PA)-FA lyase (PFL) in the pta mutant (Chang et al. 1999). As a result, production of AA, FA, and ethanol was suppressed, and accumulating PA provoked activation of LDH (Tarmy and Kaplan 1968). In fed-batch fermentation from glucose, this strain produced 60 g/l of D-LA (Table 3) with a yield of 0.8, while SA was simultaneously produced (ca. 9 g/l). To prevent accumulation of SA, a mutation in the gene for phosphoenolpyruvate carboxylase (ppc), the branch point leading to SA synthesis, was introduced into JP201. Using the resultant pta ppc double mutant, JP203, D-LA production was carried out. As expected, SA production was almost inhibited, and JP203 produced 62.2 g/l of D-LA with a high yield of 0.9 (Table 3). Although successful D-LA production was achieved, the ppc mutant has an auxotrophic requirement for tricarboxylic acid pathway intermediates or amino acids. This is one of the drawbacks when using E. coli as a host for LA production.
On the other hand, Zhou et al. (2003a) suggested another strategy for homogenous LA fermentation that has no auxotrophic requirement. They succeeded in inhibiting FA, AA, and ethanol production by a mutation in the PFL gene (pflB) of E. coli W3110. In addition, SA production was inhibited by a mutation in fumarate reductase (frdBC), not ppc, while the resultant strain SZ40 remained prototrophic. Although SZ40 produced 51.8 g/l of D-LA with an extremely high yield of 0.99 and an optical purity of >99% in M9 mineral salts medium, measurable amounts of AA and ethanol were also produced (0.26 and 0.32 g/l, respectively; Table 3). In addition, the growth of SZ40 was significantly hampered by the mutation, and fermentation time reached 192 h. To eliminate the production of AA and ethanol, a further mutation was introduced into SZ40. Mutations in the alcohol dehydrogenase gene (adhE) and acetate kinase (ackA) led to inhibition of ethanol and AA production, and the resultant strain SZ63 produced 48.6 g/l of D-LA with a high yield of 0.98 (Table 3). Surprisingly, SZ63 had partially improved growth inhibition, and fermentation time was reduced to 168 h (Table 3). This improvement was assumed to be due to increasing pools of acetyl phosphate and acetyl coenzyme A. Thus, a prototrophic, homogenous, fermentative E. coli was constructed. Another favorable feature is that SZ63 contained no antibiotic resistance genes or plasmids.
L-LA production was also achieved using derivatives of SZ63 (Zhou et al. 2003b). A part of the D-LDH gene of E. coli (ldhA) was replaced with the L-LDH gene of Pediococcus acidilactici (ldhL). Although the resultant strain SZ79 produced 43.1 g/l of L-LA with a high yield of 0.91, SZ79 showed poor growth and fermentation time reached 408 h (Table 3). This is considered to be due to weak expression of the ldhL gene. Mutants of SZ79 that showed improved growth were readily isolated, and one mutant, SZ85, exhibited a 30-fold increase in L-LDH activity in comparison to SZ79. Several mutations in the upstream, coding, and terminator regions of ldhL were confirmed in SZ85. Using SZ85, more rapid L-LA fermentation than that of SZ79 was achieved. SZ85 produced 45.5 g/l of L-LA with a high yield of 0.95 and an optical purity of 99.5% in 120 h of fermentation in M9 mineral salts medium (Table 3). Moreover, L-LA fermentation from xylose in M9 mineral salts medium was also achieved, and 40.0 g/l of L-LA was produced with yield of 0.93 in 312 h of fermentation (Table 3).
Taken together, E. coli can produce both D-LA and L-LA with high optical purity and extremely high yields even in mineral salts medium. In addition, the assimilation capacity of pentose sugars is an attractive feature. However, the low productivity of LA and low acidic tolerance (normally cultivated around pH 7.0) should be improved.
C. glutamicum
C. glutamicum is an aerobic Gram-positive bacterium that has been widely used for the industrial production of amino acids such as L-glutamate and L-lysine (Hermann 2003; Leuchtenberger et al. 2005). Under oxygen deprivation, cell growth of this bacterium is arrested, while it retains the capability to produce mix-organic acids such as L-LA, SA, and AA from glucose in mineral salts medium. Using this phenomenon, Inui et al. (2005) suggested a novel system for organic acids production containing LA that based on the use of a reactor filled to a high density with cells derived from an aerobic culture, leading to a bioprocess with high volumetric productivity. Using the C. glutamicum R strain (Yukawa et al. 2007), L-LA production was achieved with high volumetric productivity of 42.9 g/l/h at a cell concentration of 60 g/g dry cell, although significant SA was simultaneously produced (11.7 g/l/h; Okino et al. 2005).
D-LA was also produced using the same system. By expression of the D-LDH encoding gene from L. delbrueckii in the C. glutamicum ΔldhA strain, which is an internal L-LDH null mutant, D-LA production from glucose was achieved (Okino et al. 2008). In fed-batch fermentation, the C. glutamicum mutant produced 120 g/l of D-LA with a high optical purity of >99.9% at a cell concentration of 60/g dry cell at 30 h.
Although LA production from hexose sugars such as glucose and sucrose is possible, utilization of pentose sugars such as xylose and arabinose is not possible. To enable C. glutamicum to utilize xylose, Kawaguchi et al. (2006) examined expression of xylA from E. coli that encodes xylose isomerase, and xylB from E. coli that encodes xylulokinase in the C. glutamicum R strain using a multi-copy plasmid under the control of the constitutive promoter trc. Only expression of xylA (CRX1 strain) enables xylose assimilation, as C. glutamicum R has a putative endogenous xylulokinase gene (Kawaguchi et al. 2006). The fact that the xylulokinase gene-null mutant, CRX3, hardly grows in xylose-containing medium supports the existence of xylulokinase in C. glutamicum R (Kawaguchi et al. 2006). Such a xylulokinase gene is also observed in the genome of C. glutamicum ATCC 13032, although its function has not been investigated (Kalinowski et al. 2003). Both the expression of xylA and xylB (CRX2) further improved the growth rate (Kawaguchi et al. 2006). Using CRX2, L-LA production from xylose was achieved (29 mmol/l/h) with a yield of 0.53 accompanied with SA production (productivity of 17 mmol/l/h and yield of 0.25). Moreover, L-LA production from mixed sugars of glucose and xylose was also achieved, while repression of xylose metabolism by glucose was found.
Arabinose utilization has also been examined. The expression of E. coli genes araA, araB, and araD encoding arabinose isomerase, ribulokinase, and ribulose-5-phosphate 4-epimerase, respectively, was carried out in the C. glutamicum R strain (Kawaguchi et al. 2008). In the resultant strain CRA1, arabinose was successfully consumed (3.4 mmol/h/g dry cell) and L-LA was produced, while SA and AA were simultaneously produced. Moreover, L-LA production from mixed sugars of glucose and arabinose was also achieved, although the arabinose consumption rate was significantly lower (0.06 g/h/g dry cell) than that of glucose (0.76 g/h/g dry cell).
In real lignocellulose hydrolysate, oligosaccharides such as cellobiose are also found in addition to monosaccharides of hexose and pentose sugars (Katahira et al. 2006), and they should be simultaneously utilized. Sasaki et al. (2008) constructed a recombinant C. glutamicum strain that simultaneously consumed glucose, xylose, and cellobiose. Chromosomal integration of a xylA-xylB gene cluster from E. coli under the control of the trc promoter was first carried out in the C. glutamicum R strain. The X5 strain that possesses five copies of the xylA-xylB genes in non-essential regions for cell growth in the genome showed rapid xylose consumption (41.2 mmol/l/h) compared to CRX2 (19.3 mmol/l/h). In order to apply cellobiose utilization to strain X5, bglF 317A-bglA genes, which encode the phosphoenolpyruvate phosphotransferase system β-glucoside-specific enzyme IIBCA component and phospho-β-glucosidase, respectively, controlled by the constitutive tac promoter, were integrated into the chromosome of strain X5. The bglF 317A-bglA genes were obtained from the C. glutamicum R-CEL strain which is a cellobiose adaptive mutant (Kotrba et al. 2001), and a single mutation of V317A of bglF enabled cellobiose assimilation (Kotrba et al. 2003). The resultant strain X5C1 strain could simultaneously consume glucose, xylose, and cellobiose and produced LA, SA, and AA (Sasaki et al. 2008). Surprisingly, xylose was consumed at a constant rate different from that of fermentation using the CRX2 strain (Kawaguchi et al. 2006).
Thus, production of optically pure LA from a variety of sugars has become possible using mineral salts-based media with high volumetric productivity and extremely high optical purity. Utilization of polysaccharides may be feasible. Direct lysine production from starch using GM-amylase secretion or display on the cell surface of C. glutamicum has already been reported (Tateno et al. 2007a, b). By applying such technology to this GM-C. glutamicum system, direct LA production from polysaccharides may be possible. Similar to E. coli, the acid tolerance of C. glutamicum is extremely low as its LA fermentation operates around a pH of 7.0. The low yield of LA accompanied with SA and AA production also should be improved.
Yeast
Compared to bacterial species, yeasts such as Saccharomyces cerevisiae, which produce ethanol in anaerobic cultivation, are robust and more tolerant to low pH (Skory 2003). This may enable construction of a non-neutralizing fermentation process and eliminate the regeneration of precipitated lactates. In ethanol fermentation, PA is converted into ethanol by two enzymatic reactions by pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH). Recent research has shown that a transgenic yeast expressing heterologous L-LDH can produce LA from PA. Figure 3 shows the LA production strategy using this yeast. Such metabolically engineered yeasts were first reported by Dequin and Barre (1994) and Porro et al. (1995). They examined the expression of the Lactobacillus casei or bovine LDH genes using a multicopy plasmid and recombinant S. cerevisiae strains and succeeded in producing 10 to 20 g/l of LA (Table 4), although a significant amount of ethanol was simultaneously produced. A similar result was obtained using wine yeast as a host (Dequin et al. 1999; Table 4). In such transgenic yeasts, improvement of LA yield by inhibiting ethanol production is an important goal, and several metabolic engineering approaches have been examined.
There are three structural PDC genes, PDC1, PDC5, and PDC6, in the S. cerevisiae genome (Hohmann 1991), and several mutants lacking single- or multi-PDCs have been reported. Adachi et al. (1998) constructed a pdc1 mutant and expressed the bovine LDH gene under the ADH1 promoter. However, no major effect was observed as the ethanol yield was only slightly decreased (from 0.35 to 0.26) and the LA yield was slightly increased (from 0.16 to 0.20; Table 4). This is due to incomplete inactivation of PDC activity as the PDC1 deletion leads to a great increase in PDC5 promoter-driven mRNA expression, while PDC5 seems to be not, or only poorly, expressed in wild-type cells (Hohmann and Cederberg 1990). In PDC1 mutants, approaches that improve LDH activity have been examined. Ishida et al. (2005) used the S. cerevisiae OC-2T strain (Saitoh et al. 1996) as a host, which is a diploid and homothallic yeast, and two copies of the bovine LDH gene were integrated into the PDC1 locus. As a result, LDH was expressed under the control of the native PDC1 promoter, and PDC1 was completely inactive. The resulting strain YIBO-7A showed approximately fivefold higher LDH activity than that of the OC-2T strain expressing LDH using a multicopy plasmid and produced 50.6 g/l of LA with a high yield of 0.65 (Table 4). LA production was further improved by increasing the copy number of LDH. Saitoh et al. (2005) integrated four more copies of LDH into the genome of YIBO-7A, and the resulting strain possessing six copies of LDH produced 68.0 g/l of LA from 100 g/l of glucose (Table 4), which is 1.28 times higher than that produced by YIBO-7A. Moreover, when LA fermentation was carried out in a sugar juice-based medium containing 200 g/l of glucose, 122 g/l of L-LA was produced with an extremely high optical purity of >99.9%. D-LA can also be produced using the same strategy. D-LDH of Leuconostoc mesenteroides was integrated instead of bovine LDH into the OC-2T strain (Ishida et al. 2006b). The resultant strain produced 61.5 g/l of D-LA with a yield of 0.61 and an extremely high optical purity of 99.9%.
Double inactivation of pdc1 and pdc5 (Hohmann and Cederberg 1990; Ishida et al. 2006a) or triple inactivation of pdc1, pdc5, and pdc6 (Hohmann 1991) has also been reported. These resultant mutants had strongly impaired growth on glucose medium. In fact, the pdc1 pdc5 double mutant of S. cerevisiae with two copies of the bovine LDH gene in the PDC1 locus produced 82.3 g/l of LA with a high yield of 0.82, and ethanol production was repressed to only 2.8 g/l (Ishida et al. 2006a; Table 4). However, it took a long incubation time of 192 h. Thus, double activation of pdc1 and pdc5 led to serious growth inability. To achieve both a high yield of LA and productivity, the use of a pdc1 and adh1 mutant has been suggested. Among the five ADHs, the ADH1 gene product is the major enzyme responsible for conversion of acetaldehyde to ethanol (Leskovac et al. 2002). While a single adh1 mutant expressing the Rhizopus oryzae LDH gene has been reported (Skory 2003), the adh1 mutation led to poor growth and a decrease in LA yield (from 0.44 to approximately 0.20). This was attributed to the accumulation of acetaldehyde to toxic level as a result of the ADH1 disruption. Tokuhiro et al. (2009) hypothesized that by decreasing ADH activity in the pdc1 mutant, acetaldehyde accumulation would be lowered because the metabolic flux from pyruvate to acetaldehyde is reduced by the PDC1 disruption; therefore, a pdc1 adh1 double mutant that possesses four bovine LDH genes was constructed. The resultant strain produced 71.8 g/l of LA with a high yield of 0.74 in 63 h (Table 4), which is quite rapid compared to fermentation using the pdc1 pdc5 double mutant (192 h), although the yield of LA was slightly lower than that of the pdc1 pdc5 double mutant (Table 4).
The other approach for LA production involves the use of a Crabtree-negative yeast such as Kluyveromyces lactis. Bianchi et al. (2001) used K. lactis strains lacking the KlPDC1 gene, which is a single PDC gene expressing PDC activity, and transformed them with the bovine LDH gene under the control of the promoter of KlPDC1. Moreover, the pyruvate dehydrogenase E1α subunit gene (KlPDA1) was deleted. Using the resultant strain, 60.0 g/l of L-LA was produced with a high yield of 0.85 in fed-batch fermentation (Table 4). However, similar to the S. cerevisiae pdc1 pdc5 double mutant, it required a long fermentation time (500 h).
In addition to LA fermentation from glucose using GM-yeasts, the use of a variety of sugars is an important goal. Cellobiose is one of the main components of oligosaccharides obtained from cellulose degradation and a potent inhibitor of CBHs (Tokuhiro et al. 2008), which are key enzymes for degradation of crystalline cellulose. However, the yeast S. cerevisiae cannot assimilate cellobiose. Thus, rapid assimilation of cellobiose is of primary importance for developing cellulose-utilizing yeast. Tokuhiro et al. (2008) constructed S. cerevisiae that possesses eight bovine LDH genes in its genome. Also, this yeast has two copies of the BGL1 gene from A. aculeatus (Kawaguchi et al. 1996) fused with the 3′-half of the SAG1 anchor domain in the genome, and BGL1 is stably expressed and displayed on the cell surface. The recombinant successfully produced LA from 95 g/l of cellobiose with a yield of 0.70. Moreover, productivity of LA from cellobiose (2.8 g/l/h) is comparable to that from glucose (3.0 g/l/h). Judging from this result and the direct ethanol fermentation from amorphous cellulose using EG, CBH, and BGL co-displaying yeast, direct LA fermentation from cellulosic materials is possible in immediate future. Also, studies showing the utilization of xylose (Katahira et al. 2006) and arabinose (Wisselink et al. 2007) have already been reported for ethanol fermentation, and these are important findings for LA fermentation from hemicellulose or lignocellulose hydrolysate.
In yeast LA fermentation, the improvement of LA yield that is not accompanied by growth inability and a decreased LA production rate is an essential goal. Further improvement is expected.
Concluding remarks
In this review, the development of GM-microorganisms for enantiomeric pure LA production from renewable resources was highlighted. Recombinant techniques have become necessary in this field. In addition to the construction of efficient fermentation processes, recovery processes of LA such as reverse osmosis, ultrafiltration, electrodialysis, and solvent extraction (Gao et al. 2009) should be concurrently developed. PLA is undoubtedly at the forefront of bio-based polymer innovation, and full-scale production of PLA is expected to contribute to the construction of a bio-refinery society.
References
Adachi E, Torigoe M, Sugiyama M, Nikawa J, Shimizu K (1998) Modification of metabolic pathways of Saccharomyces cerevisiae by the expression of lactate dehydrogenase and deletion of pyruvate decarboxylase genes for the lactic acid fermentation at low pH value. J Ferment Bioeng 86:284–289
Adsul M, Khire J, Bastawde K, Gokhale D (2007) Production of lactic acid from cellobiose and cellotriose by Lactobacillus delbrueckii mutant Uc-3. Appl Environ Microbiol 73:5055–5057
Bianchi MM, Brambilla L, Protani F, Liu CL, Lievense J, Porro D (2001) Efficient homolactic fermentation by Kluyveromyces lactis strains defective in pyruvate utilization and transformed with the heterologous LDH gene. Appl Envion Microbiol 67:5621–5625
Bustos G, Moldes AB, Cruz JM, Domínguez JM (2005) Influence of the metabolism pathway on lactic acid production from hemicellulosic trimming vine shoots hydrolyzates using Lactobacillus pentosus. Biotechnol Prog 21:793–798
Chaillou S, Bor YC, Batt CA, Postma PW, Pouwels PH (1998) Molecular cloning and functional expression in Lactobacillus plantarum 80 of xylT, encoding the D-xylose-H+ symporter of Lactobacillus brevis. Appl Environ Microbiol 64:4720–4728
Chang DE, Jung HC, Rhee JS, Pan JG (1999) Homofermentative production of D- or L-lactate in metabolically engineered Escherichia coli RR1. Appl Environ Microbiol 65:1384–1389
Christensen CH, Rass-Hansen J, Marsden CC, Taarning E, Egeblad K (2008) The renewable chemicals industry. ChemSusChem 1:283–289
Dequin S, Barre P (1994) Mixed lactic acid-alcoholic fermentation by Saccharomyces cerevisiae expressing the Lactobacillus casei L(+)-LDH. Biotechnology 12:173–177
Dequin S, Baptista E, Barre P (1999) Acidification of grape musts by Saccharomyces cerevisiae wine yeast strains genetically engineered to produce lactic acid. Am J Enol Vitic 50:45–50
Dodds DR, Gross RA (2007) Chemicals from biomass. Science 318:1250–1251
Gao MT, Shimamura T, Ishida N, Takahashi H (2009) Application of metabolically engineered Saccharomyces cerevisiae to extractive lactic acid fermentation. Biochem Eng J 44:251–255
Giraud E, Champailler A, Raimbault M (1994) Degradation of raw starch by a wild amylolytic strain of Lactobacillus plantarum. Appl Environ Microbiol 60:4319–4323
Guyot JP, Calderon M, Morlon-Guyot J (2000) Effect of pH control on lactic acid fermentation of starch by Lactobacillus manihotivorans LMG 18010T. J Appl Microbiol 88:176–182
Helanto M, Kiviharju K, Leisola M, Nyyssölä A (2007) Metabolic engineering of Lactobacillus plantarum for production of L-ribulose. Appl Environ Microbiol 73:7083–7091
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104:155–172
Hofvendahl K, Hahn-Hägerdal B (1997) L-lactic acid production from whole wheat flour hydrolysate using strains of Lactobacilli and Lactococci. Enzyme Microb Technol 20:301–307
Hofvendahl K, Hahn-Hägerdal B (2000) Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb Technol 26:87–107
Hohmann S (1991) Characterization of PDC6, a third structural gene for pyruvate decarboxylase in Saccharomyces cerevisiae. J Bacteriol 173:7963–7969
Hohmann S, Cederberg H (1990) Autoregulation may control the expression of yeast pyruvate decarboxylase structural genes PDC1 and PDC5. Eur J Biochem 188:615–621
Ikada Y, Jamshidi K, Tsuji H, Hyon SH (1987) Stereocomplex formation between enantiomeric poly (lactides). Macromolecules 20:904–906
Ishida N, Saitoh S, Tokuhiro K, Nagamori E, Matsuyama T, Kitamoto K, Takahashi H (2005) Efficient production of L-lactic acid by metabolically engineered Sacchromyces cerevisiae with a genome-integrated L-lactate dehydrogenase gene. Appl Environ Microbiol 71:1964–1970
Ishida N, Saitoh S, Onishi T, Tokuhiro K, Nagamori E, Kitamoto K, Takahashi H (2006a) The effect of pyruvate decarboxylase gene knockout in Sacchromyces cerevisiae on L-lactic acid production. Biosci Biotechnol Biochem 70:1148–1153
Ishida N, Suzuki T, Tokuhiro K, Nagamori E, Onishi T, Saitoh S, Kitamoto K, Takahashi H (2006b) D-lactic acid production by metabolically engineered Sacchromyces cerevisiae. J Biosci Bioeng 101:172–177
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Krämer R, Linke B, McHardy AC, Meyer F, Mockel B, Pfefferle W, Pühler A, Rey DA, Ruckert C, Rupp O, Sahm H, Wendisch VF, Wiegrabe I, Tauch A (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol 104:5–25
Katahira S, Mizuike A, Fukuda H, Kondo A (2006) Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide-assimilating yeast strain. Appl Microbiol Biotechnol 72:1136–1143
Kawaguchi T, Enoki T, Tsurumaki S, Sumitani J, Ueda M, Ooi T, Arai M (1996) Cloning and sequencing of the cDNA encoding β-glucosidase 1 from Aspergillus aculeatus. Gene 173:287–288
Kawaguchi H, Vertès AA, Okino S, Inui M, Yukawa H (2006) Engineering of a xylose metabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72:3418–3428
Kawaguchi H, Sasaki M, Vertès AA, Inui M, Yukawa H (2008) Engineering of an L-arabinose metabolic pathway in Corynebacterium glutamicum. Appl Microbiol Biotechnol 77:1053–1062
Kotrba P, Inui M, Yukawa H (2001) The ptsI gene encoding enzyme I of the phosphotransferase system of Corynebacterium glutamicum. Biochem Biophys Res Commun 289:1307–1313
Kotrba P, Inui M, Yukawa H (2003) A single V317A or V317M substitution in Enzyme II of a newly identified β-glucoside phosphotransferase and utilization system of Corynebacterium glutamicum R extends its specificity towards cellobiose. Microbiology 149:1569–1580
Leskovac V, Trivić S, Pericin D (2002) The three zinc-containing alcohol dehydrogenases from baker’s yeast, Saccharomyces cerevisiae. FEMS Yeast Res 2:481–494
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8
Litchfield JH (1996) Microbiological production of lactic acid. Adv Appl Microbiol 42:45–95
Matsui Y, Okada S, Uchimura T, Kondo A, Satoh E (2007) Determination and analysis of the starch binding domain of Streptococcus bovis 148 raw-starch-hydrolyzing α-amylase. J Appl Glycosci 54:217–222
Narita J, Nakahara S, Fukuda H, Kondo A (2004) Efficient production of L-(+)-lactic acid from raw starch by Streptococcus bovis 148. J Biosci Bioeng 97:423–425
Ohara H, Owaki M, Sonomoto K (2006) Xylooligosaccharide fermentation with Leuconostoc lactis. J Biosci Bioeng 101:415–420
Okano K, Kimura S, Narita J, Fukuda H, Kondo A (2007) Improvement in lactic acid production from starch using α-amylase-secreting Lactococcus lactis cells adapted to maltose or starch. Appl Microbiol Biotechnol 75:1007–1013
Okano K, Yoshida S, Tanaka T, Fukuda H, Kondo A (2009a) Homo D-lactic acid fermentation from arabinose by redirection of phosphoketolase pathway to pentose phosphate pathway in L-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microbiol 75(15):5175–5178
Okano K, Zhang Q, Shinkawa S, Yoshida S, Tanaka T, Fukuda H, Kondo A (2009b) Efficient production of optically pure D-lactic acid from raw corn starch by using genetically modified L-lactate dehydrogenase gene-deficient and α-amylase-secreting Lactobacillus plantarum strain. Appl Environ Microbiol 75:462–467
Okano K, Zhang Q, Yoshida S, Tanaka T, Ogino C, Fukuda H, Kondo A (2009c) D-Lactic acid production from cellooligosaccharides and β-glucan using L-LDH gene-deficient and endoglucanase-secreting Lactobacillus plantarum. Appl Microbiol Biotechnol (in press)
Okino S, Inui M, Yukawa H (2005) Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 68:475–480
Okino S, Suda M, Fujikura K, Inui M, Yukawa H (2008) Production of D-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 78:449–454
Porro D, Brambilla L, Ranzi BM, Martegani E, Alberghina L (1995) Development of metabolically engineered Saccharomyces cerevisiae cells for the production of lactic acid. Biotechnol Prog 11:294–298
Saitoh S, Mieno Y, Nagashima T, Kumagai C, Kitamoto K (1996) Breeding of a new type of baker’s yeast by δ-integration for overproduction of glucoamylase using a homothallic yeast. J Ferment Bioeng 81:98–103
Saitoh S, Ishida N, Onishi T, Tokuhiro K, Nagamori E, Kitamoto K, Takahashi H (2005) Genetically engineered wine yeast produces a high concentration of L-lactic acid of extremely high optical purity. Appl Environ Microbiol 71:2789–2792
Sasaki M, Jojima T, Inui M, Yukawa H (2008) Simultaneous utilization of D-cellobiose, D-glucose, and D-xylose by recombinant Corynebacterium glutamicum under oxygen-deprived conditions. Appl Microbiol Biotechnol 81:691–699
Satoh E, Niimura Y, Uchimura T, Kozaki M, Komagata K (1993) Molecular cloning and expression of two α-amylase genes from Streptococcus bovis 148 in Escherichia coli. Appl Environ Microbiol 59:3669–3673
Serror P, Sasaki T, Ehrlich SD, Maguin E (2002) Electrotransformation of Lactobacillus delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis with various plasmids. Appl Environ Microbiol 68:46–52
Skory CD (2003) Lactic acid production by Saccharomyces cerevisiae expressing a Rhizopus oryzae lactate dehydrogenase gene. J Ind Microbiol Biotechnol 30:22–27
Tanaka K, Komiyama A, Sonomoto K, Ishizaki A, Hall SJ, Stanbury PF (2002) Two different pathways for D-xylose metabolism and the effect of xylose concentration on the yield coefficient of L-lactate in mixed-acid fermentation by the lactic acid bacterium Lactococcus lactis IO-1. Appl Microbiol Biotechnol 60:160–167
Tarmy EM, Kaplan NO (1968) Kinetics of Escherichia coli B D-lactate dehydrogenase and evidence for pyruvate controlled change in conformation. J Biol Chem 243:2587–2596
Tateno T, Fukuda H, Kondo A (2007a) Production of L-lysine from starch by Corynebacterium glutamicum displaying α-amylase on its cell surface. Appl Microbiol Biotechnol 74:1213–1220
Tateno T, Fukuda H, Kondo A (2007b) Direct production of L-lysine from raw corn starch by Corynebacterium glutamicum secreting Streptococcus bovis α-amylase using cspB promoter and signal sequence. Appl Microbiol Biotechnol 77:533–541
Tokuhiro K, Ishida N, Kondo A, Takahashi H (2008) Lactic fermentation of cellobiose by a yeast strain displaying β-glucosidase on the cell surface. Appl Microbiol Biotechnol 79:481–488
Tokuhiro K, Ishida N, Nagamori E, Saitoh S, Onishi T, Kondo A, Takahashi H (2009) Double mutation of the PDC1 and ADH1 genes improves lactate production in the yeast Saccharomyces cerevisiae expressing the bovine lactate dehydrogenase gene. Appl Microbiol Biotechnol 82:883–890
Wee YJ, Kim JN, Ryu HW (2006) Biotechnological production of lactic acid and its recent applications. Food Technol Biotechnol 44:163–172
Wisselink HW, Toirkens MJ, del RF BM, Winkler AA, van Dijken JP, Pronk JT, van Maris AJA (2007) Engineering of Saccharomyces cerevisiae for efficient anaerobic alcoholic fermentation of L-arabinose. Appl Environ Microbiol 73:4881–4891
Yáñez R, Moldes AB, Alonso JL, Parajó JC (2003) Production of D(-)-lactic acid from cellulose by simultaneous saccharification and fermentation using Lactobacillus coryniformis subsp. torquens. Biotechnol Lett 25:1161–1164
Yoon HH (1997) Simultaneous saccharification and fermentation of cellulose for lactic acid production. Biotechnol Bioprocess Eng 2:101–104
Yukawa H, Omumasaba CA, Nonaka H, Kos P, Okai N, Suzuki N, Suda M, Tsuge Y, Watanabe J, Ikeda Y, Vertès AA, Inui M (2007) Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042–1058
Zhou S, Causey TB, Hasona A, Shanmugam KT, Ingram LO (2003a) Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69:399–407
Zhou S, Shanmugam KT, Ingram LO (2003b) Functional replacement of the Escherichia coli D-(-)-lactate dehydrogenase gene (ldhA) with the L-(+)-lactate dehydrogenase gene (ldhL) from Pediococcus acidilactici. Appl Environ Microbiol 69:2237–2244
Acknowledgements
This work was mainly supported by Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe), MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okano, K., Tanaka, T., Ogino, C. et al. Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85, 413–423 (2010). https://doi.org/10.1007/s00253-009-2280-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2280-5