Abstract
Tripartite interactions among Paenibacillus lentimorbus NRRL B-30488 (B-30488), Piriformospora indica DSM 11827 (DSM 11827) and their consortia (B-30488:DSM 11827:: 1:1) with native rhizobial population in the rhizosphere of Cicer arietinum L. (Chick pea) was tested for enhancing nodulations and plant growth promotion. Number of nodules and dry weight per plant significantly enhanced (P = 0.05), which is further evident by N, P, and K uptake by plants and were found to be maximum in B-30488 treated followed by B-30488: DSM 11827 and DSM 11827, as compared with uninoculated control, in 60 days grown chickpea plants. Microbial community structure in the rhizosphere of the four treatments was assessed, using Biolog Eco and MT plates. Principal component analysis (PCA) of carbon source utilization pattern on Biolog Eco plates did not show any clustering among the four samples indicating that in case of individually DSM 11827 and B-30488 treated chickpea rhizosphere there was significant change in microbial community structure, compared with lesser changes in un-inoculated and B-30488 and DSM 11827 consortium treated chickpea rhizosphere microflora. Additional carbon sources tested using Biolog MT plates, higher activity of lignin, chitin, and cellulose utilizing microbial communities in the rhizosphere being stimulated by root exudates treated with B-30488 alone or in consortia with DSM 11827, and, in turn, should encourage beneficial symbiotic or mutualistic microorganisms that can act as plant growth promoting and biocontrol agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colonization of plant root system is the very first step in nearly all interactions between plants and soil borne microbes. The rhizosphere, defined as the volume of soil adjacent to and influenced by plant roots, represents a region of intense microbial activity. The development of rhizosphere microbial communities is influenced by the plant, but in turn, microorganisms exert profound effects on plant growth. The rhizosphere of plants is therefore one of the most fascinating microbial habitats for basic and applied studies in the field of environmental microbiology, as it is shaped by the soil, the plant and the microorganisms (Nautiyal et al. 2002, 2008). Plant roots become quickly colonized by a diverse microflora of soil-borne bacteria and fungi that may have either beneficial or deleterious effects on the plant. This rhizosphere effect is primarily due to the influx of mineral nutrients to the plant roots through mass flow and diffusion, alongside the efflux and accumulation of plant root exudates. Thus, most rhizosphere bacteria and fungi are highly dependent on associations with plants that are clearly regulated by root exudates (Nautiyal et al. 2002; Bais et al. 2004; Bonfante and Anca 2009; Hamer and Makeschin 2009; Schroeckh et al. 2009; Zubek et al. 2009). Among the soil microbes, bacteria, and fungi are most dominant and their associations with plants contribute to the development and activity of microbial communities in soils. They may also regulate physiological processes in ecosystems by decomposing organic matter, fixing atmospheric nitrogen, secreting growth promoting substances, increasing the availability of mineral nutrients and protecting against plant pathogens (Bais et al. 2006; Bonfante and Anca 2009; Schroeckh et al. 2009). Plant studies have shown that the beneficial effects of plant growth promoting microorganisms on plants can be enhanced by co-inoculation with other microorganisms. Co-inoculation, frequently, increased growth and yield, compared to single inoculation (Vestberg et al. 2004; Hildebrandt et al. 2006; Rajendrana et al. 2008). Mixed inoculation with bacteria and arbuscular-mycorrhizal fungi creates synergistic interactions that may result in a significant increase in growth, in the phosphorus content in plants, enhanced mycorrhizal infection, and an enhancement in the uptake of mineral nutrients (Marschner and Dell 1994; Vestberg et al. 2004; Bonfante and Anca 2009). For the present study we chose for Paenibacillus lentimorbus (earlier designated as Bacillus lentimorbus; Pettersson et al. 1999) and Piriformospora indica. Among rhizobacteria Paenibacillus has been the focus of intensive research and has already found applications in environmental conservation and improving plant and soil health (DasGupta et al. 2006). While Piriformospora indica, an endophytic fungus of the Sebacinaceae family, colonises the roots of a wide variety of plant species and promotes their growth, in a manner similar to mycorrhizal fungi (Shahollari et al. 2005).
The aim of this study was to elucidate the plant growth promotional abilities and tripartite interactions among bacteria (Paenibacillus lentimorbus NRRL B-30488) and fungus (Piriformospora indica DSM 11827) in the chickpea (Cicer arietinum L.) rhizosphere and what implications of these interactions might be for plant growth and microbial community structure.
Materials and methods
Microorganisms
A plant growth–promoting Paenibacillus lentimorbus NRRL B-30488 (B-30488) was grown and maintained on nutrient broth (NB) or nutrient agar (NA) (HI-MEDIA Laboratories, Bombay, India), as described earlier (DasGupta et al. 2006). Strain B-30488 has been deposited under the Budapest treaty into Agricultural Research Service (ARS) patent culture collection, US Department of Agriculture, Illinois. Piriformospora indica Verma, Varma, Kost, Rexer and Franken, a plant root-interacting fungus, P. indica DSM 11827 (DSM 11827) was grown on plates or in liquid culture containing a complete medium as described previously and has been deposited at the Deutsche Sammlung fu¨r Mikroorganismen und Zellkulturen, Braunschweig, Germany (Varma et al. 1999).
Greenhouse experiment and microbial inoculations
The plant experiments were conducted in greenhouse conditions at the National Botanical Research Institute, Lucknow, India (latitude/longitude 11° 24′ N/79° 044′ E) as described earlier (Nautiyal et al. 2008). In vitro pot experiment using chickpea (Cicer arietinum L.) consisted of following four treatments: un-inoculated seeds as control; seeds inoculated with B-30488; DSM 11827, and with B-30488 and DSM 11827 consortium. Six replicates of each treatment, with four plants in each pot, were maintained for throughout the experiment in about 23 cm diameter earthen pots. B-30488 cell suspension (9.0 log10 CFU/mL) of B-30488 was used to inoculate as described earlier (Nautiyal et al. 2008). For DSM 11827 treatment, fungal mycelium was obtained from liquid culture after removal of the medium, and was washed with an excess of distilled water and then mycelium was fragmented in a blender for 10 s and then 2 g of crushed mycelium was mixed with the soil. Consortium of B-30488 and DSM 11827 both the cultures were mixed to the soil together as mentioned for individual treatment, considered as 1:1 ratio. Moisture level of the soil was maintained at 20% for throughout the growing season by irrigating the pots as and when required. After 60 days of plant growth plants were harvested and plant biomass data (plant height, no of nodules and dry weight) were recorded for six replicates with two plants from each pot.
The harvested 12 plants chosen at random were rinsed with Milli-Q water and oven-dried at 75 °C for 72 h. The dried shoot tissues were ground and then digested using concentrated HNO3 (Page et al. 1982) for the determination of K using an atomic absorption spectrometer. Total N and P were extracted by digesting shoot tissue with 3 mL concentrated H2SO4 and 1 mL H2O2 at 360 °C, and determined by the Berthelot reaction and molybdenum blue method, respectively (Page et al. 1982). The amount of NaHCO3-extractable P (available inorganic P) from the soil was determined by extracting samples with 0.5 M NaHCO3 (pH 8.5) at a solution/solid ratio of 20:1 for 30 min (Olsen and Sommers 1982).
Microbial diversity using carbon source utilization pattern
Biolog Eco and MT plates (Biolog, Inc., Hayward, CA, USA) were used to determine the carbon source utilization pattern of the four rhizosphere soil samples as described earlier (Campbell et al. 1997). The MT plates were prepared using the manufacturer’s instructions (Biolog Inc., Hayward, CA 94545, U.S.A.), in that 0.3 mg of each carbon source was dispensed into each well. All stock solutions were, however, prepared in ethanol to enhance substrate solubility, to sterilize the substrate and to assist in the drying down procedure. After dispensing, the solutions in the wells were dried down, at room temperature in a laminar sterile air flow cabinet, prior to use. For all additional 11 carbon sources i.e., trehalose, proline, pectin, lysine, lignin, glycolic acid, glutamine, glutamic acid, chitin, cellulose and betaine individual carbon source stock solutions were prepared aseptically and for each carbon source 3 wells were filled by dispensing 15 μL of a 2% solution. For individual treatments, rhizosphere soil samples (10 g) were shaken in 90 mL of sterile 0.85% saline MQW for 60 min and then makeup a final dilution 10−3. After incubation 150 μL of sample were inoculated in each well of Biolog Eco plates and incubated at 30 °C. The rate of utilization is indicated by the reduction of tetrazolium, a redox indicator dye, which changes from colorless to purple. Data were recorded for day 1–7 at 590 nm. Microbial activity in each microplate, expressed as average well color development (AWCD) was determined as described by Garland (1996). Diversity and evenness indexes were calculated as described by Staddon et al. (1997) and Nautiyal (2009). Principal component analysis (PCA) was performed on data divided by AWCD as described by Garland and Mills (1991). Formulas used for diversity calculations have been described earlier (Staddon et al. 1997; Nautiyal 2009; Mishra and Nautiyal 2009). Principal component analysis (PCA) was performed on 4th day data divided by the AWCD, as described by Garland and Mills (1991). Statistical analyses were performed using SPSS 16.0 and Statistica 7.0.
Results and discussion
The plant growth–promotion potential of B-30488, DSM 11827 and their consortia (B-30488:DSM 11827 :: 1:1) was tested for enhancing nodulations and plant growth promotion as compared with uninoculated control using chickpea as host plant under greenhouse conditions. Treatments of B-30488; DSM 11827, and their consortia significantly enhanced (P = 0.05) plant height 34.44, 19.73 and 35.32%; no of nodules 138.86, 94.01 and 133.95%; dry weight 64.56, 44.30 and 53.16%, respectively, compared with un-inoculated plants (Table 1). The most efficient treatment in terms of plant height, dry weight, was the B-30488 followed by consortium, and DSM 11827 over uninoculated control (Table 1). Interesting observation was the ability of B-30488, DSM 11827 and consortium to enhance the nodulation ability by native chickpea rhizobia (Table 1). Several PGPRs have been reported to enhance ability of the native rhizobia to enhance nodulation (Tilak et al. 2006; Remans et al. 2007; Castro-Sowinski et al. 2007; Mishra et al. 2009). To enhance the performance of the Rhizobium- legume symbiosis, the practice of inoculating legume seeds with carrier-based inoculants of the desired rhizobia is widely practised. But root nodulation by an introduced rhizobial inoculant is dependent on numerous soil and environmental factors, and very often the applied inoculant strain has to overcome intense competition from the native soil rhizobia and other antagonistic microorganisms that colonize the rhizosphere. A variety of approaches, including genetic engineering of the rhizobial symbiont for enhanced nodule occupancy, have been tried out. An alternative to such technology-intensive approaches is the utilization of other bacterial or fungal species that positively influence plant growth, to enhance the nodule occupancy of the introduced rhizobia (Kondorosi et al. 1982; Martinez et al. 1987).
The plant-growth promotion ability of B-30488; DSM 11827, and their consortia were also evident based on the significantly enhanced (at P < 0.05) N content 43.99, 26.32, 35.15%; P content 64.28, 42.85, 85.71%; K content 61.53, 76.92, 38.46% increase in chickpea plant tissue, respectively compared with uninoculated control. Numerous bacteria have already been shown to directly stimulate the growth of plants using varied mechanisms, including improving the mineral and water uptake, changing the hormonal balance of plants, and generally improving the plant growth (Compant et al. 2005; Egamberdiyeva 2007).
Studies of rhizosphere structure and functioning have proved difficult because of the complexity of this soil compartment. However, advances in the development of analytical tools such as to use Biolog plates, which permit microbial communities to be characterized according to their physiological profile (community-level physiological profile, CLPP) calculated from the different utilization patterns of many carbon and nitrogen sources, determined by a redox reaction that changes color after inoculation and incubation of the microbial communities (Insam and Goberna 2004; Garland 2006; Bonfante and Anca 2009; Hamer and Makeschin 2009). Microbial community structure in the rhizosphere of the four treatments was assessed, using Biolog Eco and MT plates. Biolog MicroPlates test the ability of a microorganism to utilize or oxidize compounds from a preselected panel of carbon sources. The test yields a characteristic pattern of purple wells which constitutes a “Metabolic Fingerprint” of the test organism(s). The Eco plates are intended for environmental samples; they contain 31 different carbon sources in a triplicated pattern, whereas the addition of carbon sources using MT plates will increase its efficiency. Patterns of carbon source utilization were used to calculate diversity indexes of Shannon, Simpson, McIntosh, and related evenness indices. Significant differences among diversity and evenness indices measured, were found in all the treatments compared with un-inoculated control using Waller-Duncan test at P = 0.05. Inoculation of B-30488 makes the maximum increase in the chickpea rhizosphere functional diversity followed by consortia and DSM 11827. The Principal components (PC) score plots describe the characteristics of the samples and help to understand their distribution and clustering. The PCs scores plot (PC-I and PC-II) shows the spatial distribution among the samples. Principal component analysis (PCA) of carbon source utilization pattern on Biolog Eco plates did not make any cluster among the four samples, as they were distributed separately among each other at 79.56 and 10.49% on the PCA vector 1 and 2 axis (Fig. 1). DSM 11827 and B-30488 were found to be most separated, while un-inoculated and consortia could loosely be grouped together by using PCA. Results indicate that in case of B-30488 treated chickpea rhizosphere there was maximum change in microbial community structure, compared with lesser changes in un-inoculated, DSM 11827 and consortium treated chickpea rhizosphere microflora. Analyzing carbon source utilization patterns by microbial samples using Biolog plate’s shows promise as a means of assessing microbial community structure, which examines the functional capabilities of the microbial population and the resulting data can be analyzed using multivariate techniques to compare metabolic capability of communities. Such information allows examination of the natural variation and diversity of microbial communities (Nautiyal 2009). Most importantly, this technique offers the potential to monitor changes in microbial diversity caused by management practices (Table 2). In general the patterns were not entirely consistent among the different diversity measures indicating complexity of microbial communities in vivo and our limitations in measuring the same under in vitro conditions. The CLPP method is based upon the metabolic capabilities of bacteria during growth in the wells of the microtiter plates at nutrient rich conditions compared to the conditions in soil. Inoculum preparation, incubation conditions, reading intervals and data treatment have all been the subject of many investigations. In spite of the various criticisms, the method remains to be widely used, to a large extent due to the ease and speed of performance (Winding and Hendriksen 2007, Mishra and Nautiyal 2009).
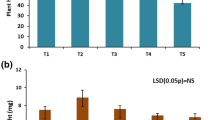
The MT MicroPlate provides a standardized micro-method for performing carbon source utilization tests and the user has complete flexibility in selecting the carbon sources and in configuring the tests (Campbell et al. 1997; Staddon et al. 1997; Hamer and Makeschin 2009). Additional carbon sources were tested using Biolog MT plates in which the wells have no carbon source but have the buffered nutrient medium and the tetrazolium indicator dye. Plant roots in the soil represent a rich source of diverse, abundant, and somewhat reliable substrates through the secretion of root exudates. These exudates consist of a wide array of compounds and simple substrates from low molecular weight compounds such as sugars, phenolics, amino acids, organic acids, and other secondary metabolites, to higher molecular weight compounds such as proteins and mucilage (Bais et al. 2006). Depending on the exact nature of the compound in the root exudates, they may play a role in activation of microbial genes responsible for recognition and initiation of symbiotic association, act as an antimicrobial plant defense, activate or disrupt key microbial genes responsible for biofilm formation, or they may simply act as an easy source of moisture, nutrients, and energy. We opted for 11 carbon sources trehalose, proline, pectin, lysine, lignin, glycolic acid, glutamine, glutamic acid, chitin, cellulose and betaine (Fig. 1). The additional carbon sources were chosen to represent compounds reported in literature as plant root exudates, providing abiotic and pathogenic stress tolerance to make the tests ecologically more relevant for testing soil microbial communities and rhizosphere isolates. Of the 11 carbon sources tested proline, lysine, glutamine, and glutamic acid were maximally utilized in the rhizosphere of B-30488 and DSM 11827 consortium followed by DSM 11827, B-30488; and un-inoculated plants, while reverse was applicable for lignin, chitin, cellulose, and betaine while trehalose and glycolic acid had no correlation (Fig. 2). In general proline, lysine, glutamine, and glutamic acid are associated with imparting abiotic stress tolerance (Ashraf and Foolad 2007; Yancey 2005) while lignin, chitin, and cellulose are associated with providing defense against pathogenic fungi (Caño-Delgado et al. 2003; Maksimov et al. 2003). Understanding how community processes affect ecosystem processes is a central challenge in ecology, and microbial communities offer a potentially powerful forum for advancing this understanding as variation in microbial community structure may have effects on ecosystem processes. Higher activity of lignin, chitin, and cellulose utilizing microbial communities in the rhizosphere being stimulated by root exudates, and, in turn, that should encourage beneficial symbiotic or mutualistic microorganisms that can act as plant growth promoting and biocontrol agents. However, betaine an important metabolite involved in imparting abiotic tolerance was not grouped with proline, lysine, glutamine, and glutamic acid but with lignin, chitin, and cellulose, instead. Therefore, our results comparing the discriminant ability of carbon sources shows variable results. The reasons why certain carbon sources increase the discrimination of this technique may be as discriminatory power of multivariate techniques lies not in the use of many different carbon sources, but in the use of combinations of carbon sources (Campbell et al. 1997; Staddon et al. 1997). As such plant root exudates are a complex mixture of chemicals and organic compounds secreted into the soil by the roots that drive underground interactions and the exact composition of the exudates is determined by many factors, including species and nutritional status of the plant, soil structure and micronutrient status (Bais et al. 2006), which makes it further more difficult to opt for carbon sources which might, therefore, be expected to differentiate to a greater extent between microbial populations. Nevertheless, we have demonstrated significant discrimination ability of proline, lysine, lignin, glutamine, and cellulose in the rhizosphere of B-30488 and DSM 11827 consortium and un-inoculated plants, illustrating that these carbon sources have significant advantages over the existing sources in the GN plate of using fewer tests, greater efficiency, economy, and increases the separation power providing an indirect view of microbial communities represent a viable means for increasing current knowledge of temporal and spatial dynamics, as the microbial communities associated to the root system are key factors in the context of sustainable systems since they regulate the availability of plant nutrient. Our understanding of the dynamic interactions and synergisms between plants, bacteria and fungi in the rhizosphere, and the possibility to manipulate them, will provide new tools to promote sustainable agriculture.
Mean absorbance at 590 nm by chickpea rhizosphere microbial population of Un-inoculated control; B-30488; DSM 11827 and B-30488 + DSM 11827 (1:1) after incubation for 3 days on Biolog MT plates containing 11 different carbon sources, ± standard error (SE). (Plants were harvested after 60 days). Different letters showing significant difference at P = 0.05 using Waller–Duncan test
References
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bais HP, Park SW, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information super highway. Trends Plant Sci 9:26–32
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol 57:233–266
Bonfante P, Anca I (2009) Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu Rev Microbiol 63:363–383
Campbell CD, Grayston SJ, Hirst DJ (1997) Use of rhizosphere carbon sources in sole carbon utilisation tests to discriminate soil microbial communities. J Microbiol Meth 30:33–41
Caño-Delgado A, Penfield S, Smith C, Catley M, Bevan M (2003) Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. The Plant J 34:351–362
Castro-Sowinski S, Herschkovitz Y, Okon Y, Jurkevitch E (2007) Effects of inoculation with plant growth-promoting rhizobacteria on resident rhizosphere microorganisms. FEMS Microbiol Lett 276:1–11
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
DasGupta SM, Khan N, Nautiyal CS (2006) Biological control ability of plant growth-promoting Paenibacillus lentimorbus NRRL B-30488 isolated from milk. Curr Microbiol 53:502–505
Egamberdiyeva D (2007) The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol 36:184–189
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28:213–221
Garland JL (2006) Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol Ecol 24:289–300
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol 57:2351–2359
Hamer U, Makeschin F (2009) Rhizosphere soil microbial community structure and microbial activity in set-aside and intensively managed arable land. Plant Soil 316:57–69
Hildebrandt U, Ouziad F, Marner FJ, Bothe H (2006) The bacterium Paenibacillus validus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiol Lett 254:258–267
Insam H, Goberna M (2004) Use of Biolog for the community level physiological profiling (CLPP) of environmental samples. Mol Microbial Ecol Manual IInd Ed 4.01:853–860
Kondorosi A, Kondorosi E, Pankhurst CE, Broughton WJ, Banfalvi Z (1982) Mobilization of a Rhizobium meliloti megaplasmid carrying nodulation and nitrogen fixation genes into other rhizobia and Agrobacterium. Mol Gen Genet 188:433–439
Maksimov IV, Cherepanova EA, Khairullin RM (2003) Chitin-specific peroxidases in plants. Biochem 68:111–115
Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. J Plant Soil 159:89–102
Martinez E, Palacios R, Sanchez F (1987) Nitrogen-fixing nodules induced by Agrobacterium tumefaciens harboring Rhizobium phaseoli plasmids. J Bacteriol 169:2828–2834
Mishra A, Nautiyal CS (2009) Functional diversity of the microbial community in the rhizosphere of chickpea grown in diesel fuel-spiked soil amended with Trichoderma ressei using sole-carbon-source utilization profiles. World J Microbiol Biotechnol 25:1175–1180
Mishra PK, Mishra S, Selvakumar G, Bisht JK, Kundu S, Gupta HS (2009) Coinoculation of Bacillus thuringeinsis-KR1 with Rhizobium leguminosarum enhances plant growth and nodulation of pea (Pisum sativum L.) and lentil (Lens culinaris L.). World J Microbiol Biotechnol 25:753–761
Nautiyal CS (2009) Self-purificatory Ganga water facilitates death of pathogenic Escherichia coli 157:H7. Curr Microbiol 58:25–29
Nautiyal CS, Johri JK, Singh HB (2002) Survival of rhizosphere- competent biocontrol strain Pseudomonas fluorescens NBRI2650 in the soil and phytosphere. Can J Microbiol 48:588–601
Nautiyal CS, Govindarajan R, Lavania M, Pushpangadan P (2008) Novel mechanism of modulating natural antioxidants in functional foods: Involvement of plant growth promoting rhizobacteria NRRL B-30488. J Agri Food Chem 56:4474–4481
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of Soil Analysis: Part 2. SSSA, Madison, WI, USA, p pp. 403
Page AL, Miller RH, Keeney DR (1982) Methods of Soil Analysis: Part 2. Chemical and Microbiological Properties (2nd edition) Agronomy vol 9. ASA, SSSA Publishing, Madison, WI, p 1159
Pettersson B, Rippere KE, Yousten AA, Priest FG (1999) Transfer of Bacillus lentimorbus and Bacillus popilliae to the genus Paenibacillus with emended descriptions of Paenibacillus lentimorbus comb. nov. and Paenibacillus popilliae comb. nov. Int J Syst Bacteriol 49:531–540
Rajendrana G, Singa F, Desaia AJ, Archana G (2008) Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Bioresource Technol 99:4544–4550
Remans R, Croonenborghs A, Gutierrez RT, Michiels J, Vanderleyden J (2007) Effects of plant growth-promoting rhizobacteria on nodulation of Phaseolus vulgaris L. are dependent on plant P nutrition. Eur J Plant Pathol 119:341–351
Schroeckh V, Scherlach K, Nu¨ tzmann H, Shelest E, Schmidt-Heck W, Schuemann J, Martin K, Hertweck C, Brakhage AA (2009) Intimate bacterial–fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc Natl Acad Sci 106:14558–14563
Shahollari B, Varma A, Oelmüller R (2005) Expression of a receptor kinase in Arabidopsis roots is stimulated by the basidiomycete Piriformospora indica and the protein accumulates in Triton X-100 insoluble plasma membrane microdomains. J Plant Physiol 162:945–958
Staddon WJ, Duchesne LC, Trevors JT (1997) Microbial diversity and community structure of post disturbance forest soils as determined by sole-carbon-source utilization patterns. Microbial Ecol 34:125–130
Tilak KVBR, Ranganayaki N, Manoharachari C (2006) Synergistic effects of plant-growth promoting rhizobacteria and Rhizobium on nodulation and nitrogen fixation by pigeonpea (Cajanus cajan). Eur J Soil Sci 57:67–71
Varma A, Verma S, Sahay NS, Bu¨tehorn B, Franken P (1999) Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol 65:2741–2744
Vestberg M, Kukkonena S, Saaria K, Parikkab P, Huttunenc J, Tainioc L, Devosd N, Weekersd F, Keversd C, Thonartd P, Lemoinee MC, Cordierf C, Alabouvettef C, Gianinazzif S (2004) Microbial inoculation for improving the growth and health of micropropagated strawberry. App Soil Ecol 27:243–258
Winding A, Hendriksen NB (2007) Comparison of CLPP and enzyme activity assay for functional characterization of bacterial soil communities. J Soil Sediment 7:411–417
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Zubek S, Turnau K, Tsimilli-Michael M, Strasser RJ (2009) Response of endangered plant species to inoculation with arbuscular mycorrhizal fungi and soil bacteria. Mycorrhiza 19:113–123
Acknowledgments
Thanks are due to the Director, NBRI for providing necessary facilities. The study was supported by Task Force grant NWP-006 from Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nautiyal, C.S., Chauhan, P.S., DasGupta, S.M. et al. Tripartite interactions among Paenibacillus lentimorbus NRRL B-30488, Piriformospora indica DSM 11827, and Cicer arietinum L.. World J Microbiol Biotechnol 26, 1393–1399 (2010). https://doi.org/10.1007/s11274-010-0312-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0312-z