Abstract
Three endangered plant species, Plantago atrata and Pulsatilla slavica, which are on the IUCN red list of plants, and Senecio umbrosus, which is extinct in the wild in Poland, were inoculated with soil microorganisms to evaluate their responsiveness to inoculation and to select the most effective microbial consortium for application in conservation projects. Individuals of these taxa were cultivated with (1) native arbuscular mycorrhizal fungi (AMF) isolated from natural habitats of the investigated species, (2) a mixture of AMF strains available in the laboratory, and (3) a combination of AMF lab strains with rhizobacteria. The plants were found to be dependent on AMF for their growth; the mycorrhizal dependency for P. atrata was 91%, S. umbrosus-95%, and P. slavica-65%. The applied inocula did not significantly differ in the stimulation of the growth of P. atrata and S. umbrosus, while in P. slavica, native AMF proved to be the less efficient. We therefore conclude that AMF application can improve the ex situ propagation of these three threatened taxa and may contribute to the success of S. umbrosus reintroduction. A multilevel analysis of chlorophyll a fluorescence transients by the JIP test permitted an in vivo evaluation of plant vitality in terms of biophysical parameters quantifying photosynthetic energy conservation, which was found to be in good agreement with the results concerning physiological parameters. Therefore, the JIP test can be used to evaluate the influence of AMF on endangered plants, with the additional advantage of being applicable in monitoring in a noninvasive way the acclimatization of reintroduced species in nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The preservation of rare and endangered plant species is the main goal of numerous conservation projects, and is an obligation for a number of countries bound by international agreements (Kaźmierczakowa and Zarzycki 2001). Several active plant protection techniques are applied to multiply plant material ex situ and to maintain natural populations of these species. Soil microorganisms, especially arbuscular mycorrhizal fungi (AMF), are considered to be crucial for proper plant performance (Smith and Read 1997; Turnau and Haselwandter 2002). This is why monitoring of soil and the selection of appropriate microbial strains to inoculate cultivated plants could be of particular value. AMF may be useful in the development of effective methods for the maintenance and propagation of threatened plant species and may significantly improve the success of plant conservation actions (Fisher and Jayachandran 2002; Turnau and Haselwandter 2002; Fuchs and Haselwandter 2004).
Our previous investigations concerned the mycorrhizal status of numerous rare, endemic, or endangered plant species from the Tatra Mts. (Western Carpathians) as well as AMF related to them in the natural habitats (Zubek et al. 2005, 2008). The research enabled us to select plant species strongly colonized by AMF. Among them were Plantago atrata Hoppe subsp. carpatica (Soó) Soó, Pulsatilla slavica G. Reuss, and Senecio umbrosus Waldst. et Kit., which all belong to the group of taxa of special concern. P. atrata subsp. carpatica (Plantaginaceae) is considered as a Pan-Carpathian endemic, and is listed on the Polish, Slovakian, and IUCN Red List of Threatened Plants. The second species, P. slavica (Ranunculaceae) is a Western-Carpathian endemic, which is also threatened in Poland, Slovakia, and on a global scale (Piękoś-Mirkowa et al. 1996; Kaźmierczakowa and Zarzycki 2001). The third one, the central-European species S. umbrosus (Asteraceae), is included in the red lists of Czech Republic and Slovakia as well as Poland. In Poland, it is classified as extinct in the wild (Mirek 1991; Kaźmierczakowa and Zarzycki 2001). Presently, these three species are maintained and multiplied ex situ, and the reintroduction of S. umbrosus is planned in the original area of occurrence in the Tatra Mts. (Kaźmierczakowa and Zarzycki 2001). Our findings from the mycorrhizal survey created the possibility of AMF application in the conservation programs of these valuable taxa in order to improve their cultivation and propagation.
The aim of the present study was to test three inocula on P. atrata, P. slavica, and S. umbrosus in order to evaluate plant responsiveness to inoculation and to select the most effective microbial consortium for application in conservation projects. Comparison of the effectiveness of native AMF strains (isolated from the Tatra Mts.) and AMF available in the laboratory was conducted. The impact of additional inoculation with two strains of rhizobacteria on plants and the development of mycorrhiza were also studied.
The effect of the microbial inoculation on P. atrata, P. slavica, and S. umbrosus was evaluated by physiological methods, namely, by shoot biomass and mycorrhizal colonization assessments, as well as by biophysical methods, termed as JIP test. This test translates the polyphasic chlorophyll (Chl) a fluorescence transient OJIP exhibited by plants upon illumination to biophysical parameters of the photosynthetic machinery, evaluating plants’ vitality. The JIP test, which has been proven to be a very useful, noninvasive, tool for the investigation of stress effects on plants (for reviews, see Strasser et al. 2000, 2004), has been also successfully used for the evaluation of the beneficial role of mycorrhization (Tsimilli-Michael et al. 2000; Pinior et al. 2005; Biró et al. 2006; Strasser et al. 2007; Tsimilli-Michael and Strasser 2008).
Materials and methods
Plant material
The seeds of P. atrata, P. slavica, and S. umbrosus were provided by the Mountain Botanic Garden of the Polish Academy of Sciences in Zakopane. They were germinated on Petri dishes according to optimised protocols by Piękoś-Mirkowa and Kaczmarczyk (1990).
Inocula preparation
Three kinds of inocula were applied in the experiment; (1) a mixture of AMF species available in the laboratory, (2) native AMF isolated from the Tatra Mts., and (3) AMF lab strains together with rhizobacteria.
The inoculum composed of AMF species available in the laboratory contained the following isolates: Glomus claroideum N. C. Schenck & S. M. Sm. [BEG96], Glomus constrictum Trappe [262–5(6), the collection of C. Walker], Glomus geosporum (Nicol. & Gerd.) C. Walker [25–4, the collection of C. Walker], Glomus intraradices N. C. Schenck & S. M. Sm. [E-1–99, BIORIZE Sarl France], Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe [BEG12].
The dual inoculum was a combination of the aforementioned AMF lab strains with bacteria. Two isolates, Azospirillum brasilense Sp7 and Paenibacillus validus DSM3037 (kindly provided by Prof. H. Bothe from the Institute of Botany, University of Cologne, Germany), were applied. The bacteria were grown on solid media, then were harvested and dilluted in sterile, distilled water. The bacterial inocula contained 107 CFU ml−1 as determined using the serial dilution method (Johnson and Case 2004).
Native AMF were isolated from the natural habitats of the investigated plant species in the Tatra Mts. The soil samples were collected from selected locations in the Polish Tatra Mts. (Western Carpathians). In the case of P. atrata and P. slavica, the soils were excavated from the immediate vicinity (within 20 cm) of several individuals occurring in natural populations (in order not to cause additional threat to the taxa). As S. umbrosus is considered extinct in the wild in Poland, the sampling procedure was carried out in the site where the species used to grow in the Tatras. The special permission for sampling was obtained from the authorities of the Tatra National Park (TPN). The locations of sampling were as follows: P. atrata-Krzesanica Mt., 2,110 m asl, 49°13′54″ N, 19°54′33″ E; P. slavica-Koryciska Wielkie ravine, 1,130 m asl, 49°16′12″ N, 19°48′27″ E; S. umbrosus-Szeroki Żleb gully in Chochołowska valley, 1,130 m asl, 49°15′06″ N, 19°48′12″ E. The procedure of trap cultures establishment, isolation, and identification of AMF spores was reported by Zubek et al. (2008). The three native inocula contained the following isolates (UNIJAG.PL) (Zubek et al. 2008): P. atrata inoculum [Acaulospora bireticulata F. M. Rothwell & Trappe, Ac. paulinae Blaszk., Entrophospora baltica Blaszk., Madej & Tadych, G. claroideum N. C. Schenck & S. M. Sm., G. constrictum Trappe, Glomus trimurales Koske & Halvorson]; P. slavica inoculum [Archaeospora gerdemannii (S. L. Rose, B. A. Daniels & Trappe) J. B. Morton & D. Redecker, Archaeospora trappei (R. N. Ames & Linderman) J. B. Morton & D. Redecker, G. constrictum Trappe, Glomus deserticola Trappe, Bloss & J. A. Menge, Glomus macrocarpum Tul. & C. Tul., Scutellospora dipurpurescens J. B. Morton & Koske]; S. umbrosus inoculum [Acaulospora gedanensis Blaszk., A. mellea Spain & N. C. Schenck, Glomus caledonium (Nicol. & Gerd.) Trappe & Gerd., G. claroideum N. C. Schenck & S. M. Sm., G. constrictum Trappe].
The bulk substratum from the trap cultures was harvested to produce inocula for the experiments. The fungi were multiplied in pots (3 l) on sterile substratum (sand/expanded clay 2:1, v/v) with Zea mays and Plantago lanceolata as host plants. After 4 months of cultivation, the shoots were harvested, and the inocula were dried. One month later, the quality control of the prepared inocula was performed. For this purpose, P. lanceolata seedlings were inoculated (50 g of dried inoculum) and cultivated in 500 ml pots for 6 weeks, then the roots were stained and examined for AM colonization according to Trouvelot method (Trouvelot et al. 1986). More than 90% of P. lanceolata root length was colonized by AMF in the case of all four inocula; no other root endophytes were found in the material.
Experiment design
One-week-old seedlings of P. atrata, P. slavica, and S. umbrosus were planted in pots (1,300 ml) on sterile substrata that were inoculated with three kinds of inocula (native AMF related to each of the species, AMF laboratory strains, and the combination of AMF lab strains with bacteria). Additionally, the autoclaved fungal inoculum (AMF laboratory strains) was used as a control. In total, 40 individuals of each species were grown; ten replicates for a treatment. The substrata, whose characteristics are reported in Table 1, were collected from the Mountain Botanic Garden of the Polish Academy of Sciences in Zakopane, from the places where the species are cultivated.
The dried inoculum (100 g/pot) was mixed with the substratum. In the case of plants co-inoculated with the bacteria, the liquid bacterial inocula (3 ml) of each of two strains-A. brasilense Sp7 and P. validus DSM3037 (107 CFU ml−1), were used per plant root system. The plants were grown for 4 months in the Mountain Botanic Garden in Zakopane-Antałówka (930 m asl, 49°17′34″ N, 19°58′39″ E). The chlorophyll a fluorescence measurements were conducted, and the plants were harvested. The roots were stained, and the shoots were dried.
Mycorrhizal colonization assessment
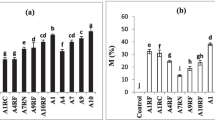
Mycorrhizal colonization analysis was conducted according to the Trouvelot method (Trouvelot et al. 1986). The parameters analyzed were mycorrhizal frequency (F), relative mycorrhizal root length (M), and relative arbuscular richness (A).
To assess the influence of bacterial inoculation on the activity of fungal mycelium inside roots, the alkaline phosphatase activity (ALP) staining was performed in the case of P. atrata and S. umbrosus using a fluorescence microscope. The pigmented rhizoderm of P. slavica roots made it impossible to conduct this test. In the case of this species, aniline blue staining was applied.
Alkaline phosphatase activity
The material was stained according to Van Aarle et al. (2001) method. The roots were washed in tap water, rinsed in distilled water, cut into 1 cm fragments, placed on microscopic slides, and treated for 15 min with the reagents of ELF97 Endogenous Phosphatase Detection Kit (Molecular Probes). After staining, the roots were incubated in the rinse buffer (30 mM Tris, 1.5 M NaCl, and 0.05% Triton X-100, pH 8.0) for 15 min. Finally, the mounting medium from the kit was used, and the slides were closed with coverslide. The material was analyzed with Nikon Eclipse 800 fluorescence microscope.
Aniline blue staining
The roots were prepared according to the modified Phillips and Hayman (1970) method. After careful washing in running tap water, the roots were cleared in 10% KOH for 24 h and subsequently rinsed in water. The material was acidified in 5% lactic acid in water for 24 h, then stained with 0.01% aniline blue in 80% lactic acid for 24 h, and finally stored in 80% lactic acid. The roots were cut into 1 cm pieces and were mounted on slides in glycerol/lactic acid (5:1). Mycorrhizal colonization analysis was conducted using Nikon Eclipse 800 microscope with Nomarski interference contrast optics.
Plant growth assessment
The shoots were washed in tap water in order to remove soil particles. Then, the material was dried and weighed using electronic analytical balance (Radwag, WPA 60/c/1) with the precision of 0.0001 g. The mycorrhizal dependency (Md), indicating how much a plant species depends on AMF for its growth, was determined according to Van der Heijden (2002), by the following formula:
where
- a :
-
mean dry mass of plants inoculated with AMF,
- b :
-
mean dry mass of non-inoculated plants,
- n :
-
number of treatments in which plants were inoculated with AMF.
Evaluation of plants’ vitality
Measurement of Chl a fluorescence transient OJIP
Chl a fluorescence transients OJIP were measured with a HandyPEA-fluorimeter (Hansatech Instruments, King’s Lynn Norfolk, PE 30 4NE, UK). The transients were induced by red light (peak at 650 nm) of 3,000 μmol photons m−2 s−1 provided by an array of three light-emitting diodes and recorded for 1 s with 12 bit resolution. The data acquisition was every 10 μs (in the interval from 10 μs to 0.3 ms), every 0.1 ms (0.3–3 ms), every 1 ms (3–30 ms), every 10 ms (30–300 ms), and every 100 ms (300 ms to 1 s). The measurements were conducted on fully expanded leaves, still attached on the plants (16–20 replicates for each treatment), which were dark-adapted for 30 min prior to measuring.
The JIP test
For each species and treatment, the average OJIP fluorescence transient, as representative of the case, was analyzed according to the JIP test (for a review, see Strasser et al. 2004), with the ‘Biolyzer’ software (Laboratory of Bioenergetics, University of Geneva, Switzerland). We applied two types of data processing:
-
1.
Utilization of selected original data for the calculation of biophysical parameters by the JIP test equations: the selected data were: the maximal measured fluorescence intensity, F P, equal here to F M since the excitation intensity is high enough to ensure the closure of all active reaction centers (RCs) of photosystem (PS) II; the fluorescence intensity at 20 μs, considered as the intensity F 0 when all RCs are open; the fluorescence intensities at 2 ms (J-step; F J), and at 30 ms (I-step; F I); the fluorescence intensities at 50 and 300 μs (\(F_{50{\text{ $ \mu $ s}}} \) and \(F_{300{\text{ $ \mu $ s}}} \)); the complementary area (area) above the fluorescence curve, i.e., the area between the curve, the horizontal line F = F M, and the vertical lines at t = 20 μs and at \(t = t_{{{\text{F}}_{{\text{M}}} }} \) (the time at which FM is reached).
The parameters chosen to be calculated in the present study, all referring to the condition of the sample at time zero (onset of fluorescence induction; all RCs open), were: (a) the quantum yields of primary photochemistry (reduction of primary electron quinone acceptor Q A), \({{{\text{TR}}_0 } \mathord{\left/ {\vphantom {{{\text{TR}}_0 } {{\text{ABS}}}}} \right. \kern-\nulldelimiterspace} {{\text{ABS}}}} = 1 - {{F_0 } \mathord{\left/ {\vphantom {{F_0 } {F_{\text{M}} }}} \right. \kern-\nulldelimiterspace} {F_{\text{M}} }}\) (= ϕ Po; maximum yield since all RCs are open), of electron transport from \(Q_{\text{A}}^ - \) to plastoquinone pool (PQ), \({{{\text{ET}}_0 } \mathord{\left/ {\vphantom {{{\text{ET}}_0 } {{\text{ABS}}}}} \right. \kern-\nulldelimiterspace} {{\text{ABS}}}} = 1 - {{F_{\text{J}} } \mathord{\left/ {\vphantom {{F_{\text{J}} } {F_{\text{M}} }}} \right. \kern-\nulldelimiterspace} {F_{\text{M}} }}\), and of the reduction of PSI end electron acceptors, \({{{\text{RE}}_0 } \mathord{\left/ {\vphantom {{{\text{RE}}_0 } {{\text{ABS}}}}} \right. \kern-\nulldelimiterspace} {{\text{ABS}}}} = 1 - {{F_{\text{I}} } \mathord{\left/ {\vphantom {{F_{\text{I}} } {F_{\text{M}} }}} \right. \kern-\nulldelimiterspace} {F_{\text{M}} }}\); (b) the efficiencies/probabilities that an electron moves from \(Q_{\text{A}}^ - \) to PQ, \({{{\text{ET}}_0 } \mathord{\left/ {\vphantom {{{\text{ET}}_0 } {{\text{TR}}_0 }}} \right. \kern-\nulldelimiterspace} {{\text{TR}}_0 }} = {{\left( {F_{\text{M}} - F_{\text{J}} } \right)} \mathord{\left/ {\vphantom {{\left( {F_{\text{M}} - F_{\text{J}} } \right)} {\left( {F_{\text{M}} - F_0 } \right)}}} \right. \kern-\nulldelimiterspace} {\left( {F_{\text{M}} - F_0 } \right)}}\), and from the reduced PQ to the PSI end electron acceptors, \({{{\text{RE}}_0 } \mathord{\left/ {\vphantom {{{\text{RE}}_0 } {{\text{ET}}_0 }}} \right. \kern-\nulldelimiterspace} {{\text{ET}}_0 }} = {{\left( {F_{\text{M}} - F_{\text{I}} } \right)} \mathord{\left/ {\vphantom {{\left( {F_{\text{M}} - F_{\text{I}} } \right)} {\left( {F_{\text{M}} - F_{\text{J}} } \right)}}} \right. \kern-\nulldelimiterspace} {\left( {F_{\text{M}} - F_{\text{J}} } \right)}}\); (c) the amount of active reaction centers per absorption (arbitrary units; proportional to the probability that a PSII antenna Chl a molecule is functioning as RC), \({{{\text{RC}}} \mathord{\left/ {\vphantom {{{\text{RC}}} {{\text{ABS}}}}} \right. \kern-\nulldelimiterspace} {{\text{ABS}}}} = {{\left[ {\left( {1 - {{F_0 } \mathord{\left/ {\vphantom {{F_0 } {F_{\text{M}} }}} \right. \kern-\nulldelimiterspace} {F_{\text{M}} }}} \right)\left( {F_{\text{J}} - F_0 } \right)} \right]} \mathord{\left/ {\vphantom {{\left[ {\left( {1 - {{F_0 } \mathord{\left/ {\vphantom {{F_0 } {F_{\text{M}} }}} \right. \kern-\nulldelimiterspace} {F_{\text{M}} }}} \right)\left( {F_{\text{J}} - F_0 } \right)} \right]} {\left[ {4\left( {F_{300{\text{ $ \mu $ s}}} - F_{50{\text{ $ \mu $ s}}} } \right)} \right]}}} \right. \kern-\nulldelimiterspace} {\left[ {4\left( {F_{300{\text{ $ \mu $ s}}} - F_{50{\text{ $ \mu $ s}}} } \right)} \right]}}\); (d) the total electron carriers per reaction center, \({{{\text{EC}}_0 } \mathord{\left/ {\vphantom {{{\text{EC}}_0 } {{\text{RC}}}}} \right. \kern-\nulldelimiterspace} {{\text{RC}}}} = {{{\text{Area}}} \mathord{\left/ {\vphantom {{{\text{Area}}} {\left( {F_{\text{M}} - F_0 } \right)}}} \right. \kern-\nulldelimiterspace} {\left( {F_{\text{M}} - F_0 } \right)}}\); (e) the performance indexes PIABS and PItotal, which are products of terms expressing “potentials” for photosynthetic performance (partial potentials) at the sequential energy bifurcations from exciton to PQ reduction and to the reduction of PSI end acceptors respectively:
-
2.
Utilization of the whole fluorescence transients: the transients were normalized between F 0 and \(F_{300{\text{ $ \mu $ s}}} \) and between F 0 and F J; hence, the kinetics of the relative variable fluorescence \(W_{20 - 300{\text{ $ \mu $ s}}} = {V \mathord{\left/ {\vphantom {V {V_{300{\text{ $ \mu $ s}}} }}} \right. \kern-\nulldelimiterspace} {V_{300{\text{ $ \mu $ s}}} }} = {{\left( {F - F_{\text{0}} } \right)} \mathord{\left/ {\vphantom {{\left( {F - F_{\text{0}} } \right)} {\left( {F_{300{\text{ $ \mu $ s}}} - F_0 } \right)}}} \right. \kern-\nulldelimiterspace} {\left( {F_{300{\text{ $ \mu $ s}}} - F_0 } \right)}}\) and \(W = {V \mathord{\left/ {\vphantom {V {V_{\text{J}} }}} \right. \kern-\nulldelimiterspace} {V_{\text{J}} }} = {{\left( {F - F_0 } \right)} \mathord{\left/ {\vphantom {{\left( {F - F_0 } \right)} {\left( {F_{\text{J}} - F_0 } \right)}}} \right. \kern-\nulldelimiterspace} {\left( {F_{\text{J}} - F_0 } \right)}}\) were derived, respectively (where \(V = {{\left( {F - F_0 } \right)} \mathord{\left/ {\vphantom {{\left( {F - F_0 } \right)} {\left( {F_{\text{M}} - F_0 } \right)}}} \right. \kern-\nulldelimiterspace} {\left( {F_{\text{M}} - F_0 } \right)}}\)). Then, the \(W_{20 - 300{\text{ $ \mu $ s}}} \) and W kinetics of the non-inoculated plants were subtracted from those of inoculated plants; the difference kinetics reveal the L- and K-bands, respectively, hidden in the original transients OJIP, which provide additional information.
Statistical analysis
Data for mycorrhizal parameters and shoot dry weight were analyzed with the Kruskal–Wallis test (p < 0.05). The analyses were conducted using STATISTICA ver. 7.1 (Statsoft).
Results
AMF colonization
Arbuscular mycorrhizae with arbuscules, which are the structural and functional criterion of the symbiosis, were observed in the case of all inoculated plants. Other root endophytes were not detected in the investigated material. No endophytes were observed in the control material treated with autoclaved AMF inoculum.
In the case of P. atrata and S. umbrosus, there were no differences in the effectiveness of root colonization between all used inocula (Table 2). In the case of P. slavica, native AMF proved to be less effective in root colonization than laboratory strains (Table 2).
The additional co-inoculation with bacteria had no significant impact on the colonization of roots of investigated plant species. Only in the case of P. slavica, the mean values of M and A parameters of individuals treated with the dual inoculum were slightly higher than the rates of these parameters obtained from the analysis of roots inoculated with the AMF lab strains. However, these differences were not statistically significant (Table 2).
Staining for alkaline phosphatase activity, providing information on the viability of the fungal partner within roots, showed that additional inoculation with bacteria did not also influence the vitality of fungal mycelium. The values of all mycorrhizal parameters estimated on the basis of alkaline phosphatase activity did not differ significantly from the data obtained from the assessment of fungal total root colonization (autofluorescence of mycelium) (data not presented).
Plant growth
Mycorrhizal plants were characterized by increased biomass in comparison to nonmycorrhizal controls (Fig. 1). This effect was especially pronounced in the case of S. umbrosus and P. atrata where the mean dry shoot weights of inoculated plants were 19- to 22- and 11- to 14-fold higher, respectively, than the biomass of the individuals which were not inoculated (Table 3); nevertheless, in these mycorrhizal plants, the shoot weights did not differ between inoculated treatments. The inoculation of P. slavica individuals with native AMF had no significant effect on the shoot biomass in comparison to the plants which were treated with autoclaved inoculum. Individuals inoculated with AMF lab strains and bacteria had the highest biomass, but the means did not differ statistically from those of plants treated only with lab AMF (Table 3).
The investigated plant species were strongly dependent on AMF for their growth. The mycorrhizal dependency (Md) for P. atrata was 91%, S. umbrosus-95%, and P. slavica-65%.
Analysis of the Chl a fluorescence transients OJIP by the JIP test
Dark-adapted leaves of all species exhibited the typical Chl a fluorescence transient OJIP, upon illumination with red actinic light (650 nm; 3,000 μmol photons m−2 s−1). Figure 2 depicts the transients from P. slavica, exhibited by non-inoculated plants (control; closed circles) and by plants inoculated with native AMF (open triangles), lab AMF (closed squares), and lab AMF with bacteria (open diamonds). Each transient, plotted on logarithmic time scale from 20 μs to 1 s, is the average of raw fluorescence transients from 16–20 samples. As shown in Fig. 2, there are some differences among the transients from the different treatments, which are rather small. In order to reveal hidden differences related with the growth conditions, the difference kinetics \(\Delta \left[ {W_{20 - 300{\text{ $ \mu $ s}}} } \right]\) and Δ[W] (see “Materials and methods” section) were calculated and plotted in the left and right panels, respectively of Fig. 3.
Chl a fluorescence transients (OJIP) of dark-adapted leaves of P. slavica plants inoculated with native AMF (open triangles), lab AMF (closed squares), and lab AMF with bacteria (open diamonds), or non-inoculated (control; closed circles). Each transient, plotted on logarithmic time scale from 20 μs to 1 s, presents the average of raw fluorescence transients from 16–20 samples, induced by red actinic light (650 nm; 3000 μmol photons m−2 s−1; HandyPEA-fluorimeter)
Difference fluorescence kinetics derived from the OJIP kinetics of P. atrata, P. slavica, and S. umbrosus plants, inoculated with native AMF (triangles), lab AMF (squares), and lab AMF with bacteria (diamonds), or non-inoculated (control; circles). For each species, the kinetics of the relative variable fluorescence of the untreated plant (control) was subtracted from those of the treated plants. The left panels depict the differences of the kinetics of the relative variable fluorescence between F 0 and F 300μs, i.e., \(\Delta \left[ {W_{20 - 300{\kern 1pt} {\text{ $ \mu $ s}}} } \right] = \Delta \left[ {{V \mathord{\left/ {\vphantom {V {V_{300{\text{ $ \mu $ s}}} }}} \right. \kern-\nulldelimiterspace} {V_{300{\text{ $ \mu $ s}}} }}} \right] = \Delta \left[ {{{\left( {F - F_0 } \right)} \mathord{\left/ {\vphantom {{\left( {F - F_0 } \right)} {\left( {F_{300{\text{ $ \mu $ s}}} - F_0 } \right)}}} \right. \kern-\nulldelimiterspace} {\left( {F_{300{\text{ $ \mu $ s}}} - F_0 } \right)}}} \right]\), revealing the L-band; the right panels depict the differences of the kinetics of the relative variable fluorescence between F 0 and F J, i.e., \(\Delta \left[ W \right] = \left[ {{V \mathord{\left/ {\vphantom {V {V_{\text{J}} }}} \right. \kern-\nulldelimiterspace} {V_{\text{J}} }}} \right] = \left[ {{{\left( {F - F_0 } \right)} \mathord{\left/ {\vphantom {{\left( {F - F_0 } \right)} {\left( {F_{\text{J}} - F_0 } \right)}}} \right. \kern-\nulldelimiterspace} {\left( {F_{\text{J}} - F_0 } \right)}}} \right]\), revealing the K-band
As demonstrated in Fig. 3, the difference kinetics for all species reveal the L-band (0.1–0.15 ms) and the K-band (at about 0.3 ms). The L-band is negative for all species and treatments. According to the analysis by Strasser and co-workers (Strasser et al. 2004, 2007), this means that the extent of the energetic connectivity among PSII units is bigger in the inoculated than in the non-inoculated plants. This increase reveals a beneficial impact of symbiosis, since energetic connectivity increases the utilization of excitation energy (Strasser 1978, 1981); moreover, increased connectivity is related with improved stability of a photosynthetic system (Strasser et al. 2004, 2007). The K-band is also negative for all species and treatments, indicating a higher activity of the oxygen evolving system (Strasser et al. 2004, 2007) in inoculated plants. Accordingly, the different amplitudes of the L- and K-bands reflect different extents of the positive impact of all types of inoculation.
The average OJIP transients were also translated to biophysical parameters with the JIP test equations. Figure 4 depicts the impact of inoculation by lab AMF on the three species (P. atrata-open triangles, P. slavica-open circles, and S. umbrosus-closed diamonds) evaluated by the behavior pattern of the following parameters of PSII: the quantum yields TR0/ABS, ET0/ABS, and RE0/ABS; the probabilities/efficiencies ET0/TR0 and RE0/ET0; the total electron transport carriers per reaction center EC0/RC; the reaction centers per absorption (or per antenna Chl a) RC/ABS; the partial potentials TR0/(ABS − TR0), ET0/(TR0 − ET0), and RE0/(ET0 − RE0); the performance indexes PIABS and PItotal. For each species, the values of the calculated parameters were normalized on those of the control plant; hence, the deviation of the behavior pattern of the inoculated plants from that of the non-inoculated (presented by the regular dodecagon; thick black line) demonstrates the fractional impact of the inoculation by lab AMF on each species.
The impact of inoculation by lab AMF on P. atrata (open triangles), P. slavica (open circles), and S. umbrosus (closed diamonds) plants, evaluated by the behaviour pattern of 12 parameters of PSII. The parameters were derived by the JIP test from the average OJIP fluorescence transients (16–20 samples) exhibited by dark-adapted leaves. For each species, the values were normalized on those of the control plant; hence, the deviation of the behaviour pattern of the inoculated plants from that of the non-inoculated (presented by the regular dodecagon; thick black line) demonstrates the fractional impact of the inoculation by lab AMF on each species. The parameters are: the quantum yields TR0/ABS, ET0/ABS and RE0/ABS; the probabilities/efficiencies ET0/TR0 and RE0/ET0; the total electron transport carriers per reaction centre EC0/RC; the reaction centers per absorption (or per antenna Chl a) RC/ABS; the partial potentials TR0/(ABS − TR0), ET0/(TR0 − ET0), and RE0/(ET0 − RE0); the performance indexes PIABS and PItotal. For the links of the parameters with fluorescence signals, see the “Materials and methods” section
Figure 4 shows similar trends in all three species concerning the fractional deviation of the parameters from the control values, though of different extents. It also shows that, not only \({{{\text{TR}}_0 } \mathord{\left/ {\vphantom {{{\text{TR}}_0 } {{\text{ABS}}}}} \right. \kern-\nulldelimiterspace} {{\text{ABS}}}} = 1 - {{F_0 } \mathord{\left/ {\vphantom {{F_0 } {F_{\text{M}} }}} \right. \kern-\nulldelimiterspace} {F_{\text{M}} }}\), which is commonly used as criterion, but other parameters related with the electron flow toward the Calvin cycle are affected by mycorrhization. A clear beneficial impact is also recognized concerning the density of RCs. As a result, the performance indexes PIABS and PItotal, which incorporate all the processes in the energy cascade from the first absorption events to the reduction of the intersystem electron transport chain and the PSI end electron acceptors, respectively, undergo the widest changes. It is therefore suggested, in accordance with other reports (Strasser et al. 2007; Tsimilli-Michael and Strasser 2008), that they can be used as overall measures of the potential of the plants for energy conservation, in screening, and comparing inoculation effectiveness.
Discussion
The application of rhizosphere microbiota, especially arbuscular mycorrhizal fungi (AMF), in the conservation projects of rare and endangered taxa has been proposed several times (Eriksen et al. 2002; Gemma et al. 2002; Turnau and Haselwandter 2002; Fisher and Jayachandran 2005). Some research clearly showed that AMF enhance performance of threatened plants, e.g., nutrient uptake, growth, or survival (Barroetavena et al. 1998; Fisher and Jayachandran 2002; Panwar and Vyas 2002). In our research, we experimentally studied the influence of microbial consortia on three plant species of a high priority. The present studies provide information on the responsiveness of these valuable species to inoculation during their propagation ex situ. All investigated species reacted positively to inoculation with AMF. However, significant differences between inocula have been found in the case of P. slavica, where native AMF isolates related to the species in the natural habitats proved to be less effective than AMF laboratory strains. The investigations enabled the selection of microbial consortium that might be implemented in restoration and conservation attempts of these species. The mixture of laboratory AMF strains seems to be preferable as a common inoculum for all three studied plants at the stage of ex situ propagation. Nevertheless, as it was highlighted by Fisher and Jayachandran (2002), each endangered species must be studied individually in the particular environmental conditions in order to determine the most effective microbial consortium for the given practical application. There is a possibility that after reintroduction, the laboratory AMF strains would be eliminated and the plants could form symbiotic association mainly with AMF which are present in the natural habitats. Further investigations, including studies performed on reintroduced plants in nature, are needed to sustain that laboratory AMF are the most appropriate for these species. Nevertheless, our findings may contribute to planned reintroduction strategies of S. umbrosus and improve methods of propagating these three threatened taxa. It has been demonstrated that outplanted individuals may have improved acclimatization in the natural sites if they are first colonized by AMF (Sylvia and Williams 1992; Sylvia et al. 1993).
We tested AMF laboratory strains versus native isolates as we had expected some differences in the plant–fungus specificity in root colonization as well as in the efficiency of fungal partners, and also differences in the effectiveness of these isolates in the edaphoclimatic conditions (the experiments were carried out in the Mountain Botanic Garden; 930 m asl). Indigenous AMF isolates originating from soil with conditions similar to these simulated in the experiments have been reported by several authors to be the most effective ones as the strains better adapted to local soil and environmental conditions (Vosátka and Dodd 2002; Orłowska et al. 2005). However, others have shown that AMF strains not related to the certain soil conditions may be equally or even more efficient as symbiotic partners (Vosátka 1995; Enkhtuya et al. 2000). Our findings are in agreement with the secondly mentioned tendencies. In the case of P. atrata and S. umbrosus, the native AMF isolates were equally effective as strains available in the laboratory. Moreover, AMF related to the rhizosphere of P. slavica in the natural habitat proved to be less effective than lab strains.
The less efficient colonization of roots and plant performance stimulation of P. slavica by native AMF may be due to some specificity of AMF. Although AMF, in general, colonize the roots of taxonomically diverse range of plants, physical and functional specificity exists in the symbiosis (McGonile and Fitter 1990; Helgason et al. 2002). If a certain strain of AMF is required for a particular plant species, the lack of a compatible fungus may be the reason of lower root colonization and worse plant performance. In our research, native AMF inoculum contained fungal strains isolated from the rhizosphere of P. slavica in the Tatra Mts. However, due to the extreme rarity of the taxon, the collection of roots during the sampling procedure, which might have caused an additional threat to the population, was not possible. The sampling method could have eliminated the most compatible fungus (or fungi), which was present in roots, but was not necessarily occurring abundantly in the rhizosphere. The second possible explanation is that AMF colonizing P. slavica in the natural site may be incompatible with the species in these particular experimental conditions. Helgason et al. (2002) found that individual AM fungi isolated from the natural habitat of five plant species and occurring within their roots at this site colonized these plants with different intensity in the laboratory cultures. Some of them did not colonize at all, indicating incompatibility under the conditions used in the experiment (Helgason et al. 2002). Finally, the third possible explanation is simply that the mycorrhizal potential of the two tested inocula is different and the symbiosis, and its consequences, developed slower in the plants inoculated with the native AMF.
The bacterial strains from the genera Azospirillum and Paenibacillus have been found to enhance plant performance (growth, photosynthetic activity, nutrient content), act antagonistically toward soil-borne fungal pathogens, increase growth of AMF mycelium, and stimulate mycorrhiza formation (Zaady et al. 1994; Barea et al. 2002, 2004; Bianciotto et al. 2002; Biró et al. 2000; Hildebrandt et al. 2002, 2006). In our studies, the co-inoculation with bacteria had no significant impact neither on the AMF colonization of roots nor the plant performance. In the case of P. slavica, there was a tendency of growth enhancement when dual inoculation was applied; however, the differences were not statistically significant. Nevertheless, our findings are in accordance with other literature data, where also no significant impact or even negative interactions were found when plants were dually inoculated (Sastry et al. 2000; Vosátka and Gryndler 2000).
The additional bacterial inoculation did not also influence the vitality of intraradical mycelium in roots of investigated threatened species. Such stimulation was described by Vivas et al. (2003), where a Bacillus strain improved alkaline phosphatase activity and enhanced mycorrhizal parameters compared with those of roots colonized by AMF alone.
More detailed studies taking into account mono (one bacterial strain), dual (one bacterial strain/AMF), and multilevel (bacterial strains/AMF) co-inoculation of these taxa are needed. The limited number of seeds of the investigated endangered species made it impossible to carry out broader studies on the influence of bacteria on these plants and the potential application of the bacteria in conservation projects.
The analysis of chlorophyll a fluorescence transients using the JIP test has been applied in several studies of the impact of soil microbial activity on plants (see the “Introduction” section). In the present study, we demonstrated that this experimental approach is also a valuable tool to evaluate the condition of endangered plant species influenced by AMF. Our results from the analysis of the fluorescence transients with the JIP test showed that the plants inoculated with any of the three microbial consortia exhibited increased efficiency for energy conservation and increased stability. Differences of the impact of the three consortia were also clearly revealed, in agreement with the tendencies found in the case of plant growth evaluation. Moreover, we could recognize differential effects at different sites of the photosynthetic machinery of the three species.
Our findings demonstrate that the JIP test is a powerful tool for studies of the multilevel beneficial role of symbiosis, with the added advantages of being applicable in a noninvasive and quick way-less than a few seconds are needed for each measurement. The method can be especially recommended in the research on valuable plant material like threatened taxa, which should not be destroyed, like e.g., by biomass measurements. The evaluation of plant vitality with the JIP test enables the control of reintroduced species by monitoring the effects of AMF inoculation on plant acclimatization in the natural sites, even more because it can be applied for early diagnosis of vitality differences (Strasser et al. 2004), before any morphological evidences appear.
References
Barea JM, Gryndler M, Lemanceau P, Schűepp H, Azcón R (2002) The rhizosphere of mycorrhizal plants. In: Gianinazzi S, Schűepp H, Barea JM, Haselwandter K (eds) Mycorrhizal technology in agriculture. From genes to mycorrhiza application. Birkhauser Verlag, Switzerland, pp 1–18
Barea JM, Azcón R, Azcón-Aguilar C (2004) Mycorrhizal fungi and plant growth promoting rhizobacteria. In: Varma A, Abbott D, Werner D, Hampp R (eds) Plant surface microbiology. Springer-Verlag, Berlin, Heidelberg, pp 351–371
Barroetavena C, Gisler SD, Luoma DL, Meinke RJ (1998) Mycorrhizal status of the endangered species Astragalus applegatei Peck as determined from a soil bioassay. Mycorrhiza 8:117–119 doi:10.1007/s005720050222
Bianciotto V, Perotto S, Ruiz-Lozano JM, Bonfante P (2002) Arbuscular mycorrhizal fungi and soil bacteria: from cellular investigations to biotechnological perspectives. In: Gianinazzi S, Schűepp H, Barea JM, Haselwandter K (eds) Mycorrhizal technology in agriculture. From genes to mycorrhiza application. Birkhauser Verlag, Switzerland, pp 19–31
Biró B, Köves-Péchy K, Vörös I, Takács T, Eggenberger P, Strasser RJ (2000) Interactions between Azospirillum and Rhizobium nitrogen-fixers and arbuscular mycorrhizal fungi in the rhizosphere of alfalfa in sterile, AMF-free or normal soil conditions. Appl Soil Ecol 15:159–168 doi:10.1016/S0929-1393(00)00092-5
Biró B, Köves-Péchy K, Tsimilli-Michael M, Strasser RJ (2006) Role of beneficial microsymbionts on the plant performance and plant fitness. In: Mukerji KG, Manoharachary C, Singh J (eds) Microbial activity in the rhizosphere. Soil biology vol. 7 (Varma A-series editor). Springer-Verlag, Berlin, pp 265–296
Enkhtuya B, Rydlová J, Vosátka M (2000) Effectiveness of indigenous and non-indigenous isolates of arbuscular mycorrhizal fungi in soils from degraded ecosystems and man-made habitats. Appl Soil Ecol 14:201–211 doi:10.1016/S0929-1393(00)00057-3
Eriksen M, Bjureke KE, Dhillion SS (2002) Mycorrhizal plants of traditionally managed boreal grasslands in Norway. Mycorrhiza 12:117–123
Fisher JB, Jayachandran K (2002) Arbuscular mycorrhizal fungi enhance seedling growth in two endangered plant species from south Florida. Int J Plant Sci 163(4):559–566 doi:10.1086/340428
Fisher JB, Jayachandran K (2005) Presence of arbuscular mycorrhizal fungi in South Florida native plants. Mycorrhiza 15:580–588 doi:10.1007/s00572-005-0367-0
Fuchs B, Haselwandter K (2004) Red list plants: colonization by arbuscular mycorrhizal fungi and dark septate endophytes. Mycorrhiza 14:277–281 doi:10.1007/s00572-004-0314-5
Gemma JN, Koske RE, Habte M (2002) Mycorrhizal dependency of some endemic and endangered Hawaiian plant species. Am J Bot 89(2):337–345 doi:10.3732/ajb.89.2.337
Helgason T, Merryweather JW, Denison J, Wilson P, Young JPW, Fitter AH (2002) Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J Ecol 90:371–384 doi:10.1046/j.1365-2745.2001.00674.x
Hildebrandt U, Janetta K, Bothe H (2002) Towards growth of arbuscular mycorrhizal fungi independent of a host plant. Appl Environ Mirob 68(4):1919–1924 doi:10.1128/AEM.68.4.1919-1924.2002
Hildebrandt U, Ouziad F, Marner FJ, Bothe H (2006) The bacterium Paenibacillus validus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiol Lett 254:258–267
Johnson TR, Case CL (2004) Laboratory experiments in microbiology, 7th edn. Pearson, Benjamin Cummings, San Francisco, Boston, New York, pp 407–410
Kaźmierczakowa R, Zarzycki K (eds) (2001) Polish red data book of plants (in Polish with English summaries). W. Szafer Institute of Botany, Institute of Nature Conservation, Polish Academy of Sciences, Kraków
McGonile TP, Fitter AH (1990) Ecological specificity of vesicular-arbuscular mycorrhizal associations. Mycol Res 94:120–122
Mirek Z (1991) Senecio umbrosus (Compositae)-a new species in the flora of the Tatra Mountains. Pol Bot Stud 2:213–216
Orłowska E, Ryszka P, Jurkiewicz A, Turnau K (2005) Effectiveness of arbuscular mycorrhizal fungal (AMF) strains in colonization of plants involved in phytostabilisation of zinc wastes. Geoderma 129:92–98 doi:10.1016/j.geoderma.2004.12.036
Panwar J, Vyas A (2002) AM fungi: A biological approach towards conservation of endangered plants in Thar desert, India. Curr Sci India 82(5):576–578
Phillips J, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Piękoś-Mirkowa H, Kaczmarczyk D (1990) Pulsatilla slavica Reuss-ecology, threat and conservation (in Polish with English summary). Stud Naturae ser A 33:133–166
Piękoś-Mirkowa H, Mirek Z, Miechówka A (1996) Endemic vascular plants in the Polish Tatra Mountains – distribution an ecology. Pol Bot Stud 12:1–107
Pinior A, Grunewaldt-Stöcker G, von Alten H, Strasser RJ (2005) Mycorrhizal impact on drought stress tolerance of rose plants probed by chlorophyll a fluorescence, proline content and visual scoring. Mycorrhiza 15:596–605 doi:10.1007/s00572-005-0001-1
Sastry MSR, Sharma AK, Johri BN (2000) Effect of an AM fungal consortium and Pseudomonas on the growth and nutrient uptake of Eucalyptus hybrid. Mycorrhiza 10:55–61 doi:10.1007/s005720000057
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic, London
Strasser RJ (1978) The grouping model of plant photosynthesis. In: Akoyunoglou G, Argyroudi-Akoyunoglou JH (eds) Chloroplast development. Elsevier/North-Holland Biomedical, Amsterdam, Netherlands, pp 513–524
Strasser RJ (1981) The grouping model of plant photosynthesis: heterogeneity of photosynthetic units in thylakoids. In: Akoyunoglou G (ed) Photosynthesis III. Structure and molecular organization of the photosynthetic apparatus. Balaban. International Science Services, Philadelphia, pp 727–737
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M (ed) Probing photosynthesis: mechanisms, regulation and adaptation. Taylor and Francis, London, pp 445–483
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis, advances in photosynthesis and respiration series (Govindjee-Series Editor) vol 19. Kluwer Academic, Rotterdam, pp 321–362
Strasser RJ, Tsimilli-Michael M, Dangre D, Rai M (2007) Biophysical phenomics reveals functional building blocks of plants systems biology: a case study for the evaluation of the impact of mycorrhization with Piriformospora indica. In: Varma A, Oelmuller R (eds) Advanced techniques in soil biology. Soil Biology Series, Springer, Germany, pp 220–221
Sylvia DM, Williams SE (1992) Vesicular-arbuscular mycorrhiza and environmental stress. In: Bethlenfalvay GJ, Linderman RG (eds) Mycorrhiza in sustainable agriculture. ASA No 54, Madison, WI, USA, pp 101–124
Sylvia DM, Jarstfer AG, Vosátka M (1993) Comparison of vesicular-arbuscular mycorrhizal species and inocula formulations in a commercial nursery and on diverse Florida beaches. Biol Fertil Soils 16:139–144 doi:10.1007/BF00369416
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA, Paris, pp 217–221 http://www.dijon.inra.fr/bbceipm/Mychintec/Mycocalc-prg/download.html
Tsimilli-Michael M, Eggenberg P, Biró B, Köves-Pechy K, Vörös I, Strasser RJ (2000) Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. Appl Soil Ecol 15:169–182 doi:10.1016/S0929-1393(00)00093-7
Tsimilli-Michael M, Strasser RJ (2008) In vivo assessment of plants’ vitality: applications in detecting and evaluating the impact of mycorrhization on host plants. In: Varma A (ed) Mycorrhiza: state of the art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics (3rd edn). Springer, pp 679–703
Turnau K, Haselwandter K (2002) Arbuscular mycorrhizal fungi, an essential component of soil microflora in ecosystem restoration. In: Gianinazzi S, Schűepp H, Barea JM, Haselwandter K (eds) Mycorrhizal technology in agriculture. From genes to mycorrhiza application. Birkhauser Verlag, Switzerland, pp 137–149
Van Aarle JM, Olsson PA, Soderstrom B (2001) Microscopic detection of phosphatase activity of saprophytic and arbuscular mycorrhizal fungi using a fluorogenic substrate. Mycologia 93:17–24 doi:10.2307/3761601
Van der Heijden MGA (2002) Arbuscular mycorrhizal fungi as determinant of plant diversity: in search for underlying mechanisms and general principles. In: Van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 243–266
Vivas A, Marulanda A, Ruiz-Lozano JM, Barea JM, Azcón R (2003) Influence of Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant response to PEG-induces drought stress. Mycorrhiza 13:249–256 doi:10.1007/s00572-003-0223-z
Vosátka M (1995) Influence of inoculation with arbuscular mycorrhizal fungi on the growth and mycorrhizal infection of transplanted onion. Agric Ecosyst Environ 53:151–159 doi:10.1016/0167-8809(94)00563-T
Vosátka M, Gryndler M (2000) Response of micropropagated potatoes transplanted to peat media to post-vitro inoculation with arbuscular-mycorrhizal fungi and soil bacteria. Appl Soil Ecol 15:145–152 doi:10.1016/S0929-1393(00)00090-1
Vosátka M, Dodd JC (2002) Ecological considerations for successful application of arbuscular mycorrhizal fungi inoculum. In: Gianinazzi S, Schűepp H, Barea JM, Haselwandter K (eds) Mycorrhizal technology in agriculture. From genes to mycorrhiza application. Birkhauser Verlag, Switzerland, pp 235–247
Zaady E, Okon Y, Perevolotsky A (1994) Growth response of Mediterranean herbaceous swards to inoculation with Azospirillum brasilense. J Range Manage 47(1):12–15 doi:10.2307/4002833
Zubek S, Turnau K, Błaszkowski J (2005) Arbuscular mycorrhiza of plants from the Mountain Botanical Garden in Zakopane. Acta Mycol 40(1):25–41
Zubek S, Turnau K, Błaszkowski J (2008) Arbuscular mycorrhiza of endemic and endangered plants from the Tatra Mts. Acta Soc Bot Pol 77(2):149–156
Acknowledgements
The present research was financially supported by the Polish Ministry of Science and Higher Education, Project No. 2 P04G 00628 (2005–2006), and the Jagiellonian University funds DS/758/UJ. Support by the Swiss National Science Foundation, Project Nr: 200021-116765, to M.T.-M. and R.J.S. is acknowledged. The authors thank Prof. H. Bothe and Dr. U. Hildebrandt (Institute of Botany, University of Cologne) for providing us with the bacterial strains. Prof. H. Piękoś-Mirkowa, M. Pacyna, M.Sc. Eng. A. Delimat, and M.Sc. Eng. E. Walusiak (Institute of Nature Conservation of the Polish Academy of Sciences, Kraków) are acknowledged for providing us with the seeds of threatened taxa and for their useful remarks concerning plant cultivation. We are also grateful to the authorities of the Tatra National Park (TPN) for the permission for soil samples collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zubek, S., Turnau, K., Tsimilli-Michael, M. et al. Response of endangered plant species to inoculation with arbuscular mycorrhizal fungi and soil bacteria. Mycorrhiza 19, 113–123 (2009). https://doi.org/10.1007/s00572-008-0209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-008-0209-y