Abstract
In this study, the influence of bacteria and activated carbon adjacent was investigated on treatment and decolorization of synthetic wastewater in a Biological Activated Carbon process. Pseudomonas putida was selected as a bacterial strain capable of degrading aromatic compounds and Acid Blue 92 was used as model azo dye. The optimum conditions for environmental factors affecting decolorization of azo dye was achieved from series of batch experiments. At 25 °C and pH7 the highest efficiency was obtained. At concentration of 20 mg L−1 in batch process, more than 90 % of Acid Blue 92 was reduced in color within just 3 h under optimum conditions. Dual-beam UV–Visible spectrometry and plate assay confirmed biodegradation of the dye by P.putida. The optimum values were then used for continues process. It was found that flow rate and thereby retention time has a critical role in biofilter efficiency. A lab-scale submerged downflow GAC column was sustained by bacterial strain to form the biofilter. About 70 % decolorization was obtained in continuous biological activated carbon process within 2 h with 60 mL h−1 flow rate while adsorption of dye reached the equilibrium and no further dye removal observed. Our results suggest the potential use of P. putida in azo dye decolorization. The combination of activated carbon adsorption and biodegradation by bacterial strains capable of degrading xenobiotic proved to be a promising technique for treatment of dye-contaminated wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Azo dyes are regarded as xenobiotic compounds and they are hazardous for the environment (Barragán et al. 2007). Synthetic dyes are widely used in industries like textile, leather, cosmetics, food and paper (Demir et al. 2007; Olya et al. 2012). A large amount of dye is lost in the manufacturing and utilization process and often causes environmental pollution problems (Marandi et al. 2013). Color present in dye-contaminated wastewater of the industries gives an obvious mark of water being polluted, and this effluent discharge can damage the receiving water or soil (Chen et al. 2003). The effluents are variable in chemical composition (Olya et al. 2013). So, evaluation and optimization of main parameters for wastewater treatment is essential. Parameters such as pH and temperature directly influence the bacterial decolorization of the effluent (Saratale et al. 2011). In addition, microbial decolorization effectiveness depends on the activity and adaptability of selected microorganisms (Chen et al. 2003). In fact, development of efficient dye removal process requires a suitable strain and its use under favorable conditions to realize its potential (Biyik et al. 2012). Biological processes have received growing interest due to their cost effectiveness and environmental friendliness (Haq et al. 2016; Jadhav et al. 2010; Saratale et al. 2010). However, developing a practical bioprocess for dye-contaminated effluent treatment is of great importance (Chen et al. 2003). Azo dye biodegradation mechanism involves the cleavage of azo bonds (–N=N–) which occurs with the help of azoreductase enzymes (Du et al. 2015; Ogugbue et al. 2012). It is supposed that under anoxic condition reductase activity is higher and more efficient for azo dye degradation (Saratale et al. 2011). In fact, this process is restrained by the presence of oxygen through its inhibition of the azo bond reduction (Ogugbue et al. 2012). It proposes that agitation and aeration, which intensify oxygen concentration in the medium, would not be utilized for effective color removal by bacteria strains (Chang and Lin 2001; Kumar et al. 2015). However, a latter aerobic treatment is needed for mineralization of degraded azo dyes. So, an anaerobic process with subsequent aerobic treatment could be the most effective approach for treatment of wastewaters containing azo dyes (Khehra et al. 2006; You and Teng 2009). In a Biological Activated Carbon (BAC) process, adsorbable organic compounds are removed by adsorption and biological degradation (Santos and Boaventura 2015). So, BAC process is applicable for compounds that are both biodegradable and readily adsorbable. The biomass shapes as bacterial colonies on the surface of the activated carbon (Walker and Weatherley 1998). Activated carbon provides a great area for biofilm attachment and growth due to its irregular surface with many holes, ridges, and crevices (Herzberg et al. 2004b). The bacterial activity would be through consumption of substrates either in liquid phase or adsorbed phase as two potential sources of substrate. Substrate desorption from activated carbon can increase specific activity caused by an expansion in the active biofilm surface area under condition of substrate partial penetration in the biofilm (Herzberg et al. 2004a). An adsorbing carrier, like granulated activated carbon (GAC), can be the source of an extra flux of pollutant to the biofilm in addition to the bulk liquid. This extra flux can improve the performance of a biological GAC reactor as compared to a non-adsorbing carrier supplemented reactor. This improvement is achieved only under conditions of pollutant partial penetration in the biofilm (Herzberg et al. 2003).
This study aimed to investigate the ability of P.putida for decolorization of azo dye Acid Blue 92 (AB92) in the presence of activated carbon. The effect of various factors on the color removal efficiency was examined. Optimum condition was obtained and utilized for BAC column. BAC process was formed by injection of the bacterial strain into the laboratory scale GAC column. UV–Visible spectrometry and plate assay used for decolorization investigation and confirmed biodegradation of the dye by P.putida. In order to verify bacterial attachment on activated carbon structure, microorganisms were observed using SEM.
Materials and methods
Microorganism and growth medium
The bacterial strain Pseudomonas putida (ATCC 12633) was purchased from the Persian Type Culture Collection (PTCC) (Iran). Pure culture was maintained on BHI agar slants and also grown on BHI agar plate for daily practice Luria–Bertani (LB) media was used in this study for bacteria growth and inoculum preparation (10 g trypton, 10 g NaCl, and 5 g yeast extract) (Han et al. 2012). A loopful of strain seed was taken from a plate culture and transferred into 10 mL LB broth medium. This was then incubated statically at 27 °C for 19 h to achieve inoculum.
Dye stuff and chemicals
Acid Blue 92 (C.I.13390) used in this study was obtained from AlvanSabet Co. (ASC) (Iran) and utilized as a model dye in synthetic wastewater. The chemical structure of the dye is shown in Fig. 1. The composition of mineral salt medium (MSM) was (g L−1): KH2PO4 (0.4); NH4Cl (0.5); MgSO4.7H2O (0.2); FeSO4.2H2O (0.1); NaCl (0.5); Yeast Extract (1). The MSM was prepared by dissolving proper amount of salts to distilled water. Synthetic waste water (SWW) was made by adding azo dye solution to MSM. The pH of the medium was adjusted to desired value using 2 M NaOH.
Adsorbent
A commercial grade crush-type activated carbon with mean particle size of 2 mm was used as adsorbent and support matrix for the strain growth and activity. The activated carbon particles were washed with distilled water to remove the fines; they were dried in oven at 70 °C for 10 h in order to be prepared for the study.
Effect of different environmental conditions
AB92 decolorization was studied at different pH (5–8), temperature (25–35 °C), agitation (0–150 rpm), and initial dye concentration (20–100 mg L−1) in batch process to evaluate the effects of environmental factors on color removal. The experiments were performed in 250-mL Erlenmeyer flasks containing 100 ml of SWW, activated carbon particles, and inoculum. These batch experiments were assumed to be started by inoculating the SWW medium. The medium was incubated at 27 °C for 24 h under anoxic condition. Samples were taken at known time intervals and the absorbance of them was determined at the dye absorbance maxima to obtain decolorization efficiency.
Determination of decolorization
The samples from decolorization cultures test flasks were collected and analyzed using a 6300 Jenway visible scanning spectrophotometer (UK). The dye removal efficiency was stated as the ratio of dye concentration to that of initial one. Decolorization efficiency was determined by the following equation:
where A initial is the absorbance of samples before the process of decolorization and A final is the absorbance at equilibrium.
Analytical methods
Dye removal was studied by analysis of sample absorbance at 0, 1, 2, and 3 h during the decolorization of AB92 under optimum conditions of batch system using a dual-beam UV–Visible spectrophotometer (Perkin-Elmer lambda 25, USA). The absorbance of each sample was measured at maximum absorption wavelength of the dye (λ max = 571 nm) after 10,000 rpm centrifugation for 90 s. The spectra of samples and changes in the absorption spectrum of control and products were recorded.
A plate test was done to assess the decolorizing ability of P. putida. The culture was plated on LB agar containing 100 mg L−1 AB92. The plate was incubated at 27 °C for 24 h and observed then for color removal surrounding the streak.
Scanning electron microscopy (SEM)
In order to verify bacterial attachment on activated carbon structure, micrographs were taken by scanning electron microscopy. The activated carbon sample which used in an enhanced biological decolorization was selected from a batch process. Observations were made with a scanning electron microscope (LEO 1455vp) after sample dehydration.
Biofilter set-up and operation conditions
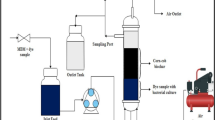
A lab-scale submerged downflow GAC column was used as biofilter, consisting of a tubular glass column (40 cm in height and 1.5 cm inner diameter) which was filled with crushed activated carbon. The height of packing was 5 cm which provides adsorption media and also a favorable place for bacterial growth and activity. It was supported by a polymeric mesh in the bottom of the column to avoid carbon particles gush. A U-shaped pipe was jointed to the bottom of the glass column and was extended up parallel to the column as shown in Fig. 2, in order to increase the residence time. Table 1 provides the details of the set-up and different operating conditions. The process was made up of three operational phase which are identified as A, B, and C. Phase A is the startup of the process in which SWW flow to the column containing fresh activated carbon with flow rate of 300 mL h−1 for 14 h. In phase A, decolorization of AB92 was expected to be exclusively attributed to the adsorption on activated carbon, as no inoculum added to the filter yet. The endpoint of phase A assumed as the breakpoint of the adsorption process. Phase B started by injection of 10 mL inoculum from top of the biofilter which lasts for 4 h and phase C was afterward started by flow rate reduction to 60 mL h−1. Phase C continued for additional 21 h. The inlet concentration of the dye in the SWW was fixed at 50 mg L−1 and flow rate was adjusted using a polymeric mini valve. The SWW was directed into the column and no aeration was used in the process to make microaerophilic condition. The absorbance of the dye was measured at the outlet of the filter using a 6300 Jenway visible scanning spectrophotometer (UK). Optimum environmental factors (pH and temperature) of decolorization obtained from batch process were employed for continues operation of biofilter. The schematic diagram of the biofilter system that was constructed for this study is shown in Fig. 2.
Results
Decolorization properties of the strain were investigated in batch system as a function of pH, temperature, agitation, and initial dye concentration. The results are given as decolorization yield.
Effect of initial pH on decolorization
The results indicate pH of the medium has a very significant effect on the decolorization activity of the cells as the decolorization efficiency increased with pH, being 3-fold when the pH was raised from 5 to 7, and reduced thereafter. It exhibited maximum decolorization at neutral pH. Decolorization of azo dye at various pH values are shown in Fig. 3.
Effect of initial temperature on decolorization
The effect of 25, 30, and 35 °C incubation temperatures on decolorization of AB92 was investigated at optimum pH obtained previously. The maximum decolorization efficiency was achieved at 25 °C. The effect of various temperatures on bacterial activity and decolorization is shown in Fig. 4.
Effect of agitation on decolorization
85 % decolorization of AB92 was achieved within just 4 h of static incubation (Fig. 5) and further incubation did not enhance decolorization. In the case of agitated incubation with 150 rpm, only 28 % decolorization obtained within 4 h and it could not rise above 45 % within 24 h of incubation.
Effect of initial dye concentration on decolorization
Decolorization activity of the strain was studied at different initial concentration of AB92 (Fig. 6). Decolorization efficiency decreased with increase in dye concentration and the highest decolorization was obtained in lowest initial concentration of AB92. Lower efficiency is because of higher inhibition at high dyestuff concentration (Gurulakshmi et al. 2008).
Biodecolorization and biodegradation analysis
Figure 7 shows the UV–Visible spectral scans which obtained at 0, 1, 2, and 3 h during the decolorization of AB92 under optimum conditions of batch system. The characteristic peak of absorbance spectrum within visible range (at 571 nm) reduced gradually with time. The spectrum showed no departure toward wavelength axis.
Colorless area surrounding the streak of the strain in the plate test demonstrated the decolorizing ability of P. putida (Fig. 8).
SEM observation
The SEM micrograph of bacterial strain attached on the surface of activated carbon pellet is presented in Fig. 9. The erratic structure of the surface provided a suitable condition for bacterial attachment and enhanced their stability in the medium.
Biofilter process consequences
The concentration of the dye solution was measured at the outlet of the filter for determination of decolorization percentage and the ratio of effluent concentration to influent concentration (S/S 0). According to the batch experiments outcome, Optimum values of environmental factors were pH7 and 25 °C which were employed for continues operation of biofilter. Figure 10 shows the results in the biofilter consisting of three operational phases. The first phase (A) was caused by conventional adsorption of dye by activated carbon. The second phase (B) was started by inoculation, when adsorption of dye reached the equilibrium and no further dye removal observed. At this point of operation the S/S 0 ratio was about 0.84. This means that the effluent reached to 84 % of influent SWW in dye concentration. Inoculation was performed without any alteration in the mass transfer zone. As no tangible change was observed within elapsed time of 4 h, flow rate was diminished to 60 mL h−1 (start of phase C). A significant decolorization was observed within 2 h after flow rate reduction and the S/S 0 ratio reached to 0.29 swiftly. This ratio is equal to about 70 % decolorization of the solution. This phase (C) continued for 21 h till the S/S 0 ratio reached to 0.74. No aeration was used in the column to provide microaerophilic condition, as efficient decolorization would be obtained in such condition (Chen et al. 2003).
Discussion
This study investigated the Acid Blue 92 decolorization activity of P. putida in cooperation with activated carbon. Azo dye decolorization by bacteria is often initiated by enzymatic reduction of azo bonds (Zimmermann et al. 1982). The presence of oxygen usually restrains the activity of azoreductase since it may dominate the utilization of NADH thus impede the electron transfer from NADH to azo bonds (Chen and Lin 2007). P. putida exhibited more decolorizing activity under static incubation and its activity reduced with increase of agitation as shown in Fig. 5. According to UV–Visible spectral scans, for treated dye solution, after 3 h, the absorbance peak in the visible region disappeared which indicating complete decolorization. The peaks in the UV spectra changed extremely and the peaks at 227 and 275 nm were replaced by new ones. A reduction in absorbance within the visible range indicates dye decolorization through adsorption or biosorption. It is also stated that this reduction is due to cleavage of chromophore group (–N=N–) (Ogugbue et al. 2012). In addition, the result of plate assay proved the capability of P. putida on decolorization of AB92 via biodegradation by making colorless area surrounding the streak of the strain. Figure 8 shows that colorless area went beyond the border of the streak. It could be due to transpire of azoreductase in the agar.
In biofilter process, no tangible change was observed within elapsed time of 4 h after inoculation at the higher flow rate. A significant decolorization was observed within 2 h after flow rate reduction. It could be due to lack of enough time for dye and bacteria adjacent. In this case, every unit of solution drained away and bacteria lost the chance of activity on substrate. It could be also because of azoreductase gush out. No aeration was used in the column to provide microaerophilic condition. It is supposed that under anoxic condition reductase activity is higher and more efficient for azo dye degradation (Saratale et al. 2011). The profile vibration observed in phase C may due to dye desorption and activity of free cells above the packing bed which unsettled the profile uniformity. This phase showed the importance of flow rate for the performance of bioreactor. In a BAC biofilter, adsorbed dye supplies a higher substrate concentration which is also stagnant. This stagnancy of substrate helps the enzyme and bacterial activity on a specific unit of contaminant. In fact, the mass transfer rate of dye could be enhanced with activated carbon incorporation, as more dye could be transported to the cells and less resistance existed in the biofilter. This would increase the substrate removal rate (Wang and Li 2007).
Conclusion
In this study, the synergic performance of P. putida adjacent to activated carbon was investigated and a novel method was tried to parade in biofiltration. It was demonstrated that the role of the bacterial strain in dye removal by inoculation to a GAC column reached the equilibrium and no further dye removal observed. A significant decolorization was observed again at preferable flow rate. Environmental parameters (pH, temperature, aeration, and initial concentration) had significant effect on biodecolorization. The result of plate assay proved the capability of P. putida on decolorization of AB92 via biodegradation by making colorless area surrounding the streak of the strain.
References
Barragán BE, Costa C, Carmen Marquez M (2007) Biodegradation of azo dyes by bacteria inoculated on solid media. Dyes Pigments 75:73–81
Biyik H, Basbulbul G, Kalyoncu F, Kalmis E, Oryasin E (2012) Biological decolorization of textile dyes from isolated microfungi. J Environ Biol 33:667–671
Chang J-S, Lin C-Y (2001) Decolorization kinetics of a recombinant Escherichia coli strain harboring azo-dye-decolorizing determinants from Rhodococcus sp. Biotechnol Lett 23:631–636
Chen J-P, Lin Y-S (2007) Decolorization of azo dye by immobilized Pseudomonas luteola entrapped in alginate–silicate sol–gel beads. Process Biochem 42:934–942
Chen K-C, Wu J-Y, Liou D-J, Hwang S-CJ (2003) Decolorization of the textile dyes by newly isolated bacterial strains. J Biotechnol 101:57–68
Demir G, Ozcan HK, Tufekci N, Borat M (2007) Decolorization of Remazol Yellow RR Gran by white rot fungus Phanerochaete chrysosporium. J Environ Biol 28:813–817
Du L-N, Li G, Zhao Y-H, Xu H-K, Wang Y, Zhou Y, Wang L (2015) Efficient metabolism of the azo dye methyl orange by Aeromonas sp. strain DH-6: characteristics and partial mechanism. Int Biodeterior Biodegrad 105:66–72
Gurulakshmi M, Mani DS, Venba R (2008) Biodegradation of leather acid dye by Bacillus subtilis. Biodegrad Leather ACID Dye Bacillus subtilis 7:12–18
Han J-L, Ng I-S, Wang Y, Zheng X, Chen W-M, Hsueh C-C, Liu S-Q, Chen B-Y (2012) Exploring new strains of dye-decolorizing bacteria. J Biosci Bioeng 113:508–514
Haq I, Kumar S, Kumari V, Singh SK, Raj A (2016) Evaluation of bioremediation potentiality of ligninolytic Serratia liquefaciens for detoxification of pulp and paper mill effluent. J Hazard Mater 305:190–199
Herzberg M, Dosoretz CG, Tarre S, Green M (2003) Patchy biofilm coverage can explain the potential advantage of BGAC reactors. Environ Sci Technol 37:4274–4280. doi:10.1021/es0210852
Herzberg M, Dosoretz C, Tarre S, Beliavski M, Green M (2004a) Biological granulated activated carbon fluidized bed reactor for atrazine remediation. Water Sci Technol 49:215–222
Herzberg M, Dosoretz CG, Tarre S, Michael B, Dror M, Green M (2004b) Simultaneous removal of atrazine and nitrate using a biological granulated activated carbon (BGAC) reactor. J Chem Technol Biotechnol 79:626–631
Jadhav JP, Phugare SS, Dhanve RS, Jadhav SB (2010) Rapid biodegradation and decolorization of Direct Orange 39 (Orange TGLL) by an isolated bacterium Pseudomonas aeruginosa strain BCH. Biodegradation 21:453–463
Khehra MS, Saini HS, Sharma DK, Chadha BS, Chimni SS (2006) Biodegradation of azo dye CI Acid Red 88 by an anoxic–aerobic sequential bioreactor. Dyes Pigments 70:1–7
Kumar AN, Reddy CN, Mohan SV (2015) Biomineralization of azo dye bearing wastewater in periodic discontinuous batch reactor: effect of microaerophilic conditions on treatment efficiency. Bioresour Technol 188:56–64
Marandi R, Mahanpoor K, Sharif AAM, Olya ME, Moradi R (2013) Photocatalytic degradation of azo dye acid yellow 23 in water using nife2o4 nanoparticles supported on clinoptilolite as a catalyst in a circulating fludized bed reactor. J Basic Appl Sci Res 3:347–357
Ogugbue CJ, Morad N, Sawidis T, Oranusi NA (2012) Decolorization and partial mineralization of a polyazo dye by Bacillus firmus immobilized within tubular polymeric gel. 3. Biotech 2:67–78
Olya M, Aleboyeh H, Aleboyeh A (2012) Decomposition of a diazo dye in aqueous solutions by KMnO4/UV/H2O2 process. Progr Color Colorants Coat 5:41–46
Olya ME, Pirkarami A, Mirzaie M (2013) Adsorption of an azo dye in an aqueous solution using hydroxyl-terminated polybutadiene (HTPB). Chemosphere 91:935–940
Santos SC, Boaventura RA (2015) Treatment of a simulated textile wastewater in a sequencing batch reactor (SBR) with addition of a low-cost adsorbent. J Hazard Mater 291:74–82
Saratale R, Saratale G, Chang J, Govindwar S (2010) Decolorization and biodegradation of reactive dyes and dye wastewater by a developed bacterial consortium. Biodegradation 21:999–1015
Saratale R, Saratale G, Chang J, Govindwar S (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157
Walker G, Weatherley L (1998) Bacterial regeneration in biological activated carbon systems. Process Saf Environ Prot 76:177–182
Wang C, Li Y (2007) Incorporation of granular activated carbon in an immobilized membrane bioreactor for the biodegradation of phenol by Pseudomonas putida. Biotechnol Lett 29:1353–1356
You S-J, Teng J-Y (2009) Anaerobic decolorization bacteria for the treatment of azo dye in a sequential anaerobic and aerobic membrane bioreactor. J Taiwan Inst Chem Eng 40:500–504
Zimmermann T, Kulla HG, Leisinger T (1982) Properties of purified Orange II azoreductase, the enzyme initiating azo dye degradation by Pseudomonas KF46. Eur J Biochem 129:197–203
Acknowledgments
The authors would like to thank Mrs. Felor Mazhar for her kind help and guide in the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Araghi, M.S., Olya, M.E., Marandi, R. et al. Investigation of enhanced biological dye removal of colored wastewater in a lab-scale biological activated carbon process. Appl Biol Chem 59, 463–470 (2016). https://doi.org/10.1007/s13765-016-0177-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-016-0177-4