Abstract
Bacterial isolates from sludge samples collected at a local municipal sewage treatment plant were screened for bacteria producing polyhydroxyalkanoates (PHA). Initially Sudan black B staining was performed to detect lipid cellular inclusions. Lipid-positive isolates were then grown in a nitrogen limitation E2 medium containing 2% (w/v) glucose to promote accumulation of PHA before the subsequent staining with Nile blue A. The positive isolates were quantified initially with a u.v. spectrophotometer, for a very large number of isolates (105) and among them high PHA-producing isolates (15) were selected and were confirmed by gas chromatographic analysis. The GC analysis showed the polymers produced by 13 of the selected isolates to be polyhydroxybutyrate (PHB), and the remaining two isolates produced polyhydroxybutyrate-co-hydroxyvalerate (PHB-co-HV) copolymer. The proportion of the PHA-positive bacterial isolates showed variability in the number of PHA accumulators during various months. The correlation of PHB production with the cell dry weight (CDW) was found to be statistically significant. The metabolism of PHB in these selected 15 isolates was studied using the Nile blue A staining, which showed an initial increase in the fluorescence followed by a decline, on further incubation. All the selected 15 isolates were classified to genus level by studying their morphological and biochemical characteristics. There were seven Bacillus species, three Pseudomonas species, two Alcaligenes species, two Aeromonas species, and one Chromobacterium species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The problem of environmental pollution caused by indiscriminate dumping of plastic waste has assumed global proportions. These conventional plastics that are synthetically derived from petroleum are not readily biodegradable (Huang et al. 1991; Young 1981) and are harmful wastes. In search of environmentally friendly materials to substitute for conventional plastics, different biodegradable plastics have been developed either by incorporating natural polymers into conventional plastics, by chemical synthesis, or by microbial fermentations (Chau et al. 1995; Chang 1994) or else isolated and extracted from natural organisms of different ecosystems.
Polyhydroxyalkanoic acids (PHA) are common intracellular granules found in prokaryotes. PHA are biodegradable polymers with properties that may be of use as bulk commodity plastics. PHA can be produced biologically from renewable resources. The main hindrance in the use of PHA is their cost of production. Bacteria produce PHA as an intracellular carbon and energy storage material by various pathways (Dawes and Senior 1973; Anderson and Dawes 1990; Verlinden et al. 2007). There is great interest in developing optimum PHA-producing organisms for inexpensive production.
Accumulation of these polymers under aerobic conditions often occurs when the carbon source is in excess but one or several other nutrients are limited. Under anaerobic conditions, a few facultative aerobic bacteria or anaerobic bacteria have been reported to accumulate PHA (Lillo and Rodriguez-Valera 1990; Liebergesell et al. 1993; Girbal et al. 1997). The first studied and most common PHA is polyhydroxybutyrate (PHB) (Verlinden et al. 2007), which is commonly synthesized from acetate or butyrate via β-hydroxybutyryl-CoA. The stored PHA provides bacteria with a reserve source of carbon and energy that enhances survival during stress. It has been suggested that PHA acts as an electron sink that helps to regulate the balance of reducing equivalents when cells are grown under O2 limited conditions (Dawes and Senior 1973; Anderson and Dawes 1990; Hustede et al. 1993; Vincenzini et al. 1990).
The bacterial flora in the presence of rich nutrients tends to accumulate certain storage materials like volutin granules, lipids and polyhydroxyalkanoates (Du et al. 2004). Such a rich and diverse ecosystem as sewage sludge, despite its potentials has not been adequately explored for bacteria accumulating polyhydroxyalkanoates (PHA) and hence we considered it to be a potential environment for screening of bacteria accumulating PHA.
Activated sludge or extended aeration treatment involves a continuous system where aerobic biological growths are mixed with wastewater and then separated in a gravity clarifier. Therefore, waste treatment systems such as the sewage sludge systems depend on the activities of communities of living organisms. Hence, sewage sludge becomes a very prominent ecosystem for the isolation of potential PHA-producing organisms.
Materials and methods
Collection of samples
Samples from the local sewage treatment plant (STP) at Amberpet, Hyderabad were collected at monthly intervals during 2005. The samples collected include: inlet, intermediate, outlet and activated sludge. All the other samples except activated sludge were the sewage samples at different stages of treatment, whereas, the activated sludge was the remaining material after aerobic digestion of the solid materials from the sewage. The inlet sample was an untreated sewage sample, whereas, intermediate sample was obtained from aeration tank at the STP. The third sewage sample, outlet was the finally released sewage after primary treatment. All the samples were collected in fresh plastic bottles.
Isolation of bacterial strains
A total of 48 samples (4 samples × 12 months) were collected during the sampling period. Bacterial isolates were obtained by dilution-pour plate technique. Luria Bertani (LB) agar plates were used. Using different dilutions (10−6, 10−7) each sample from the different sites of the Hyderabad municipal sewage treatment plant were pour plated onto LB agar plates. After incubation of 24 h at room temperature (28°C), around 2–9 isolates were randomly picked from each of these 2 plates (two dilutions) with sterile tooth picks and were transferred onto a fresh second plate. The number of colonies formed on the second plate was similar to the number of colonies transferred from the original plates (two plates) of each sample.
Screening for PHA-producing bacteria
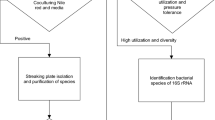
All the colonies formed on the second plate were subjected to an initial Sudan Black B staining (Burdon 1946) to detect the presence of lipid granules. Positively stained isolates were checked for PHA production by specific Nile blue A staining (Kitamura and Doi 1994). However, before this staining, the isolates were first induced to accumulate PHA by culturing in E2 medium, a nitrogen-limiting medium (Lageveen et al. 1988) containing 2% (w/v) glucose. The PHA-accumulating colonies, after Nile blue A staining, showed bright orange fluorescence on irradiation with u.v. light and their fluorescence intensity increased with increase in PHA content of the bacterial cells. The PHA metabolism in the PHA-accumulating isolates was studied by using the microscopic assay method of Ostle and Holt (1982). In this method, heat fixed smears of PHA positive bacterial isolates, grown at different incubation periods, were stained with Nile blue A and observed under an Olympus microscope with fluorescence attachment using a green filter.
Quantitative analysis of PHA
The isolates showing bright orange fluorescence on irradiation with u.v. light after Nile blue A staining were selected as PHA accumulators. These isolates were first grown in E2 broth in 50-ml flasks, and incubated at 28 ± 2°C on orbital shaker at 150 rev min−1. After 48 h of growth, the bacterial cells were harvested and the polymers extracted from the cells according to Rawte and Mavinkurve (2002). In this method, sodium hypochlorite was used for cell hydrolysis, followed by a wash with cold diethyl ether, the PHA from the bacterial cells was precipitated after subsequent centrifugation. The PHA extracted from all the PHA positive isolates by the above method were quantified by u.v. spectrophotometer method (Yilmaz et al. 2005; Aslim et al. 1998; Bonartseva and Myskina 1985). The PHA were acidified in this method with concentrated sulphuric acid to form crotonic acid, the quantity of which was measured by u.v. spectrophotometer at 235 nm.
Confirmation of the amount of PHA
Based on the u.v. spectrophotometer quantifications, 15 maximum PHA-accumulating bacterial isolates were selected from among the Nile blue A-positive isolates (105 isolates). The amount of the polymer in these selected 15 isolates was requantified using the advanced method, gas chromatography. For this assay, methyl esters of the polymers were prepared as described by Lageveen et al. (1988). In this method, small culture samples were centrifuged at 12,000 rev min−1 for 5 min at 4°C, after appropriate incubation period, usually 48 h. The pellets formed were lyophilized or freeze dried at −20°C and suspended in 2 ml of methanol containing 15% (v/v) concentrated H2SO4. After addition of 2 ml of chloroform, the tubes were incubated at 100°C for 2.5 h followed by rapid cooling on ice. On cooling, 1 ml of distilled water was added and the mixture was vortexed. The PHA were separated by centrifugation at 6,000 rev min−1 for 5 min. The bottom organic layer containing PHA was later dried over sodium sulfate before analysis.
For analysis of the PHA, a capillary gas chromatograph (Shimadzu) equipped with an auto sampler and integrator was used. A 30-m DB5 capillary column (5% phenylmethylsiloxane) was used. Samples were injected in split less mode. The injection split ratio was 120:1 and 1 μl injections were made. The injection port temperature was 180°C and the detector temperature was 200°C. The initial oven temperature was 90°C (maintained for 1 min), with an increase of 8°C min−1 to a final temperature of 150°C (maintained for 5 min). The flow rate of the nitrogen carrier gas was 2 ml min−1. The PHB polymer from Sigma was used as an external standard and benzoic acid was used as an internal standard.
Identification of PHA-producing bacteria
The PHA-positive bacterial isolates were identified according to the routine biochemical tests (Table 1) as described in Bergey’s Manual of Determinative Bacteriology (Holt et al. 2000).
Statistical analysis
The correlation between the production of PHB and dry cell weight of the isolates was determined by Spearman’s correlation coefficient test (Conover 1971).
where d i = x i – y i is the difference between the ranks of corresponding values X i and Y i , and n is the number of values in each data set (same for both sets).
Results and discussion
Biological waste water treatment generates large quantities of biomass as activated sludge. Only a few reports have focused on the potential of utilizing resident bacterial species from activated sludge in PHB production (Law et al. 2001). This paper discusses, a few potential PHB-accumulating bacteria isolated from municipal sewage sludge samples.
A total of 480 bacterial isolates were screened for isolation of PHA-producing bacteria. These (480) were the total number of isolates obtained from the second plates of isolation of all (4) the samples (Table 2). The heterotrophic bacterial counts varied from month to month, with a variation of bacterial count increment in the winter month of January, 2005, in all of the four samples studied. The activated sludge sample yielded the highest number of bacterial isolates, about 29.16% of the total isolates. This was followed by intermediate sewage sample (28.33%), inlet sewage sample (22.5%) and outlet sewage sample (20%). During the month of January, the environmental conditions of the sampling sites like temperature (25–32°C) and pH (6.8–7.2) are favorable for the growth of many microorganisms. Such favorable conditions can show a positive influence on the huge number of microorganisms present in the sewage ecosystem, which can increase the biological activity of these samples. The high counts of bacteria accumulating PHA during January signify the availability of necessary nutrients and small molecular weight components, due to the increased biological activity of the microorganisms. These nutritious substances formed by the microorganisms are most helpful for the growth of bacteria and accumulation of PHA. These nutrients are made available through the sequential action of fungi, yeast and bacteria in the sludge samples.
The study of the cultural characteristics of the isolates revealed the presence of significant number (21.4% of total isolates) of pigment-producing organisms (Table 2). Pigments have a great commercial value and are used immensely as a colorant in numerous industries, such as, plastics, gums, food, dyes and stains etc. (Martin and Williams 2003). The activated sludge sample showed a maximum number of pigment producers (36.8%), followed by intermediate sewage sample (28.1%), inlet sewage sample (19.4%) and outlet sewage sample (15.5%) (Table 2).
The bacterial flora is divided into two groups, based upon their Gram’s character. Overall, the Gram-positive bacteria tended to dominate the sewage sludge, nearly 58.75% of the total 480 isolates showed Gram-positive character (Table 2). The intracellular lipid-producing organisms were about 45.62% of the total 480 isolates. These isolate percentages were higher in activated sludge samples (33.34%) when compared to other samples, intermediate sample (31.5%), inlet sample (21.0%), and outlet sample (14.15%) (Table 2). Isolates with multiple enzyme activity, though small in number are important in such ecosystems due to their potentiality in industrial applications (Matsusaki et al. 1998). For example, bacteria with intracellular lipids and pigment-producing activity could be of immense use in the detergent industry. Bacteria tend to accumulate intracellular lipids as reserve materials like PHA (polyhydroxyalkanoates), which are generally utilized by the bacterium itself when the growth conditions are adverse. Here, 105 isolates of the sewage sludge bacteria accumulated PHA as well as intracellular lipids. Accumulation of PHA occurs in the presence of excess carbon, which is available for the organisms from the degraded products of diverse nutrients in the sludge.
Selection of the potential PHA-accumulating bacteria
A wide variety of bacteria are known to accumulate PHA. These bacteria have been reported from various environments, but only a few from the sewage ecosystems. In the present study, 105 PHA positive isolates were selected by Nile blue A staining, from a total of 480 bacterial isolates screened from different samples. The PHA-accumulating bacteria were 21.87% of the total 480 bacterial isolates. In the four samples studied, activated sludge sample was dominated by PHA accumulators with 43.8% of total PHA accumulators. The intermediate sewage sample followed with 27.61%, the inlet sewage and outlet sewage samples produced 17.14% and 11.42% PHA accumulators, respectively (Table 2).
PHA accumulation in bacterial cells increases as the incubation period increases reaches maximum at the late exponential stage of the growth curve and declines on further incubation. The pattern of PHB metabolism in the cells can be observed by the change in intensity of fluorescence (of PHA-producing colonies) under u.v. light after staining with Nile blue A. The Kitamura and Doi plate assay method was more suitable for rapid screening of PHA-accumulating bacteria, hence, it was employed for the purpose. The microscopic method of Ostle and Holt (1982) was used to study the change in intensity of fluorescence with increasing incubation period. The intensity of fluorescence was seen to increase with the time, followed by a decline (Table 3).
Quantitative assay of PHA
These PHA-positive isolates selected after Nile blue A staining were first grown in E2 broth in 50-ml flasks, and were employed to extract PHA after 2 days of incubation on orbital shaker as described previously. The PHA from the isolates was extracted by the hypochlorite method, developed by Rawte and Mavinkurve (2002).
After this initial quantitative analysis with u.v. spectrophotometer method (Yilmaz et al. 2005; Aslim et al. 1998; Bonartseva and Myskina 1985), 15 such isolates were obtained which accumulated more than 0.78 g/l of PHA (Table 4). These were selected as the potential PHA accumulators for further study.
The selection of the final 15 isolates was based on the highest amount of PHA produced by these isolates in E2 media with 2% glucose and as measured by u.v. spectrophotometer. Initially these isolates produced PHA from 0.78 to 1.65 g/l, amounting to about 16.89–64.32% PHA of cell dry weight (Table 4). An important finding was that all these 15 isolates were derived from three sample sites, except the outlet sample, which did not show any high count of PHA-accumulating bacteria. These 15 isolates were further studied for their morphological and biochemical characteristics (Table 1).
Since all the cultures were quantitated after a defined period of 48 h, it is possible that the low yield of PHA obtained for certain cultures is due to the time of selection of harvesting the cells, which was either prior to late exponential stage of the growth curve or after onset of PHA hydrolysis.
Among all the bacterial CDW, 64.32% cell dry weight PHB, is the highest report of PHA-accumulating bacteria from sewage sludge, when grown on the media supplemented with 2% glucose, whereas, Rohini et al. (2006) reported 64.10% PHA of CDW from soil bacteria, when grown on the media supplemented with glycerol. It was investigated whether any relation existed between the dry weight and PHA production and the correlation was found P = 0.954. When this value was compared with the table value (critical value at 13 degree of freedom and 0.005 significance level), it was seen that the relationship was significant (P = 0.954 > 0.745).
Gas chromatography is generally used to rapidly determine the amount of PHA in the cells and identify the constituents of the different hydroxyalkanoic acids. All these final 15 isolates were confirmed to accumulate polyhydroxybutyrate (PHB) by GC analysis, since the reagent using methods can also act with other lipid inclusions, and thus are not specific (Ciesielski et al. 2006). For this purpose a PHB standard from Sigma was used, and the methyl esters of the 3-hydroxybutyrate were prepared as described by Lageveen et al. (1988).
All these 15 bacterial samples tested by GC were first grown in E2 medium with 2% glucose as the carbon substrate. Almost all of them produced only PHB, except the isolates 88/D and 303/A which yielded an additional peak in GC spectra besides that of 3-hydroxybutyrate methyl ester (Fig. 1). Repeated analysis of polymers produced by these two isolates (88/D & 303/A) with GC/MS confirmed the second peak detected in GC analysis to be of 3-hydroxyvalerate methyl ester, a copolymer of PHB (results not shown). The GC analysis was also used to estimate the amount of PHB accumulated by these final 15 isolates. The results of this quantitative estimation of PHB accumulated by these 15 isolates were presented in Table 4.
By using Bergey’s Manual of Determinative Bacteriology (Holt et al. 2000), all these 15 isolates were classified up to genus level using the morphological and biochemical characteristics (Table 1). Seven of the fifteen isolates have been identified as Bacillus species, three as Pseudomonas species, four isolates each two of Alcaligenes and Aeromonas genus, along with one isolate from genus Chromobacterium.
Conclusion
In the present study, nitrogen limitation was shown to enhance the PHA accumulation rate, which can supply additional energy for the biosynthesis of cell constituents. PHA accumulation followed a growth-associated pattern, cell growth and PHA accumulation occurred simultaneously till some extent, later the PHA production was enhanced by nutrient limitation. The sewage sludge ecosystem yielded 15 optimum PHB-producing bacteria, which produced PHB in amounts greater than 0.78 g/l. The highest amount of PHB produced by these isolates was 1.65 g/l, corresponding to 64.32% of cell dry weight. Two isolates from this study produced hydroxyvalerate copolymer, in a medium with glucose as a sole carbon source, a rare finding in wild strains of any bacteria. In view of all the above findings, these isolates can be utilized to achieve cost-effective production of biodegradable polymers, which is a great hindrance in the commercial use of PHA.
The further study involves both the characterization of the selected isolates through molecular methods as well as the characterization of the polymers produced by these selected isolates.
References
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Aslim B, Caliskan F, Beyatli Y, Gunduz U (1998) Poly-β-hydroxybutyrate production by lactic acid bacteria. FEMS Microbiol Lett 159:293–297
Bonartseva GA, Myskina VL (1985) Fluorescence intensity of strains of nodule bacteria (Rhizobium melliloti, R. phaseoli) differing in activity, grown in the presence of the lipophilic vital stain phosphine 3R. Microbiology (English Translation of Mikrobiologiya) 54(4):535–541
Burdon KL (1946) Fatty acid material in bacteria and fungi revealed by staining dried, fixed, slide preparation. J Bacteriol 52:665–678
Chang HN (1994) Biodegradable plastics and biotechnology. In: Teo WK (ed) Better living through innovative biochemical engineering. Singapore University press, Singapore, pp 24–30
Chau H, Yu PHF, Xing S, Ho LY (1995) Potential of biodegradable plastics as environmentally friendly substitute for conventional plastics in Hong Kong. Presented in the 17th Symposium on Biotechnology for Fuels and Chemicals, May 1995, Colorado, USA
Ciesielski S, Kwiatkowska AC, Pokoj T, Klimiuk E (2006) Molecular detection and diversity of medium-chain-length polyhydroxyalkanoates-producing bacteria enriched from activated sludge. J Appl Microbiol 101:190–199
Conover WJ (1971) Practical non-parametric statistics. Wiley, New York. ISBN 0471168521
Dawes EA, Senior PJ (1973) The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol 10:135–266
Du G, Chen LXL, Yu J (2004) High-efficiency production of bioplastics from biodegradable organic solids. J Polym Environ 12(2):89–94
Girbal L, Orlygsson J, Reinders BJ, Gottschal JC (1997) Why does Clostridium acetireducens not use interspecies hydrogen transfer for growth on leucine? Curr Microbiol 35:155–160
Holt JG, Krieg NR, Sneath PHA, Stanley JT, Williams ST (2000) Bergey’s manual of determinative bacteriology, 9th edn. Lippincot, Williams and Wilkins, Baltimore
Huang T, Zhao JQ, Shen JR (1991) The progress in microbiodegradable plastics. Plast Ind 4:23–27
Hustede E, Steinbuchel A, Schlegel HG (1993) Relationship between the photoproduction of hydrogen and accumulation of PHB in non-sulphur purple bacteria. Appl Microbiol Biotechnol 39:87–93
Kitamura S, Doi Y (1994) Staining method of poly (3-hydroxyalkanoic acid) producing bacteria by Nile Blue. Biotechnol Tech 8:345–350
Lageveen RG, Huisman GW, Preustig H, Ketelaar P, Eggink G, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans; effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54:2924–2932
Law KH, Leung YC, Lawford H, Chau H, Lo WH, Yu PH (2001) Production of polyhydroxybutyrate by Bacillus species isolated from municipal activated sludge. Appl Biochem Biotechnol 91–93:515–524
Liebergesell M, Mayer F, Steinbuchel A (1993) Analysis of polyhydroxyalkanoic acid-biosynthesis genes of anoxygenic phototrophic bacteria reveals synthesis of polyester exhibiting an unusual composition. Appl Microbiol Biotechnol 40:292–300
Lillo JG, Rodriguez-Valera F (1990) Effects of culture conditions on poly (β-hydroxybutyric acid) production by Haloferax mediterranei. Appl Environ Microbiol 56:2517–2521
Martin DP, Williams SF (2003) Medical applications of poly-4-hydroxybutyrate: a strong flexible absorbable biomaterial. Biochem Eng J 16:97–105
Matsusaki H, Manji S, Taguchi K, Kato M, Fukui T, Doi Y (1998) Cloning and molecular analysis of the poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyalkanoate) biosynthesis genes in Pseudomonas sp. strain 61-3. J Bacteriol 180:6459–6467
Ostle AG, Holt JG (1982) Nile blue as a fluorescent stain for PHB. Appl Environ Microbiol 44:238–241
Rawte T, Mavinkurve S (2002) A rapid hypochlorite method for the extraction of polyhydroxyalkanoates from bacterial cells. Indian J Exp Biol 40:924–929
Rohini D, Phadnis S, Rawal SK (2006) Synthesis and characterization of poly-β-hydroxybutyrate from Bacillus thuringiensis R1. Indian J Biotechnol 5:276–283
Verlinden RAJ, Hill DJ, Kenward MA, Williams CD, Redecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102:1437–1449
Vincenzini M, Silli C, de Philippis R, Ena A, Materassi R (1990) Occurrence of poly-β-hydroxybutyrate in Spirulina species. J Bacteriol 172:2791–2792
Yilmaz M, Soran H, Beyatli Y (2005) Determination of poly-β-hydroxybutyrate (PHB) production by some Bacillus spp. World J Microbiol Biotechnol 21:565–566
Young RJ (1981) Synthesis of polymers. In: Introduction to polymers. Chapman and Hall, New York, pp 9–85
Acknowledgments
The authors thank the University Grants Commission and Department of Science and Technology, India for the facilities provided for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vishnuvardhan Reddy, S., Thirumala, M., Kishore Reddy, T.V. et al. Isolation of bacteria producing polyhydroxyalkanoates (PHA) from municipal sewage sludge. World J Microbiol Biotechnol 24, 2949–2955 (2008). https://doi.org/10.1007/s11274-008-9839-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9839-7