Abstract
Wetland ecosystems are a major natural source of the important greenhouse gas methane (CH4). Among these ecosystems, fens have been shown to release high quantities of CH4. Data on CH4 emissions from alpine fens are scarce and mainly limited to the United States and China. Therefore, static chambers were used to quantify CH4 emissions from 14 fens located in the Swiss Alps. The aims of this study were to determine the spatial variability of the emissions and to identify potential key factors which influence CH4 turnover. The fens were located at altitudes between 1,800 and 2,600 m a.s.l., the pore water varied from acidic to slightly acidic (pH 4.5–6.4) and the vegetation was dominated by plants of the genus Carex. In addition, the underlying bedrock was either siliceous or calcareous. Methane emissions ranged from 74 ± 43 to 711 ± 212 mg CH4 m−2 day−1. The type of bedrock, the plant biomass above the water table and the CH4 pore water concentrations at depths from 0 to 20 cm were the main factors influencing CH4 emissions. Detailed measurements in three selected fens suggested that more than 98 % of the total CH4 emissions are due to plant-mediated transport.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands are important ecosystems with regard to the global carbon cycle and consequently exert a major impact on climatic change. Wetlands are characterized by waterlogged, anoxic conditions that result in incomplete decomposition of organic material and accumulation of carbon in the form of peat (Lai 2009). The photosynthetically active plants, mosses and algae at the surface function as a sink for atmospheric carbon dioxide. On the other hand, the deeper layers are a source for carbon dioxide and methane (CH4) because organic matter is decomposed under methanogenic conditions (Conrad 1996). Wetlands are the largest natural sources of atmospheric CH4, with annual emissions of 100–230 Tg CH4, representing 20–40 % of estimated global emissions (Denman et al. 2007). This considerable level of uncertainty arises, in part, from site-specific spatial and seasonal variability (Bubier et al. 1995a; Christensen et al. 1995; Bellisario et al. 1999; Joabsson and Christensen 2001; Whalen 2005; Sachs et al. 2010).
Wetlands are diverse and heterogeneous ecosystems characterized as bogs and fens (Whalen 2005), which are mainly distinguished by their dominant water source (Limpens et al. 2008). Bogs are ombrotrophic ecosystems with a low water input from precipitation, an acidic and nutrient-poor environment and dominated by bryophytes, such as Sphagnum species, which are slowly decomposed (Moore and Basiliko 2006; Limpens et al. 2008). Minerotrophic fens receive water and nutrients from groundwater, resulting in a more nutrient-rich environment, mainly vegetated by vascular plants that promote the gas-exchange between the subsurface and the atmosphere (Rydin and Jeglum 2006). Thus, as a consequence of the fens’ minerotrophic condition, higher average water table, and vegetation type, CH4 emissions from fens are generally higher than those from bogs (Whalen 2005). Methane emissions range from 10 to 180 mg CH4 m−2 day−1 and from 30 to 400 mg CH4 m−2 day−1 for bogs and fens, respectively (Dise et al. 1993; Bubier et al. 1995b; Bellisario et al. 1999; Chasar et al. 2000).
Most of the studies on CH4 emissions in cold environments have focused on wetlands located in the northern hemisphere (Sebacher et al. 1986; Whalen and Reeburgh 1992; Bubier et al. 1995a; Christensen et al. 1995; King et al. 1998; Bellisario et al. 1999; Waddington and Roulet 2000; Whalen and Reeburgh 2000; Wagner et al. 2003; Saarnio et al. 2007; Sachs et al. 2010), as these ecosystems harbor one third of the global soil carbon (Gorham 1991; Turunen et al. 2002). Alpine wetlands have not been extensively studied and available data are mainly limited to the Rocky Mountains (West et al. 1999; Wickland et al. 2001; Chimner and Cooper 2003) and the Tibetan Plateau (Hirota et al. 2004; Cao et al. 2008; Chen et al. 2009, 2011). Very few reports have dealt with alpine wetlands in the European Alps (Koch et al. 2007; Liebner et al. 2012). Hence, detailed investigations are necessary to accurately estimate the contribution of alpine wetlands to the global CH4 budget (Mast et al. 1998; Koch et al. 2007).
Methane production, oxidation, and transport from the subsurface to the atmosphere are closely linked and depend on a number of environmental factors such as temperature (Bubier et al. 1995a; Heyer et al. 2002; Koch et al. 2007), water table elevation (Freeman et al. 1992; Dise et al. 1993; Christensen et al. 1995), pH (Bubier et al. 1995b) and vegetation type (Schimel 1995; Bellisario et al. 1999; Ström et al. 2003). In order to understand and predict the CH4 cycle in wetlands it is necessary to analyze the impact of each environmental factor on CH4 production, oxidation and transport to the atmosphere (Bridgham et al. 2013). Methane is produced by methanogenic archaea under anoxic conditions, while methanotrophic bacteria in oxic sediment zones use the gas as a carbon and energy source (Conrad 1996).
Temperature is an important factor affecting biologically-mediated processes, and it has been reported that CH4 production is more sensitive than CH4 oxidation to temperature changes (Whalen 2005). In several wetland soils a close relationship between temperature and CH4 emissions has been found, with emissions increasing proportionally to temperature (Bubier et al. 1995b; Heyer et al. 2002; Koch et al. 2007). Water table elevation has been identified as a good parameter to estimate CH4 emissions (Bubier et al. 1995a, b; Koch et al. 2007). The water table has been suggested to affect both CH4 production and CH4 oxidation by regulating the position of the oxic-anoxic interface in the peat (Dunfield et al. 1993; Whalen 2005). Drought conditions have been reported to result in a lower CH4 production, limited to deep anoxic soil layers, while CH4 oxidation during drought was not influenced (Freeman et al. 2002). In addition, the oxic layer resulting from a decrease in the water table might support a higher decomposition of the organic material, influencing the carbon cycle in wetlands (Bardgett et al. 2008). However, the data are contradictory and the correlation between the water table elevation and CH4 emissions was reported to be weak (Bellisario et al. 1999; Trudeau et al. 2013).
Peat acidity can affect CH4 production and oxidation, with lower pH reducing CH4 production. For example, a significant reduction in CH4 production rates were reported for peat slurries incubated at a pH of 5.5 compared to incubations at pH of 7.0 (Valentine et al. 1994). However, partial adaptation of methanogenic archaea and methanotrophic bacteria to sub-optimal pH has been observed (Dunfield et al. 1993).
CH4 emissions from wetlands are strongly dependent on vegetation type, as plants influence CH4 production, oxidation, and transport from the subsurface to the atmosphere (Christensen et al. 2003; Ström et al. 2003). A positive correlation between plant productivity, plant biomass and CH4 emission has been reported in different wetlands (Whiting and Chanton 1993; Bellisario et al. 1999; Joabsson and Christensen 2001; von Fischer et al. 2010; Chen et al. 2011).
Many vascular plants in fens have developed air channels in stems, leaves and roots (i.e. aerenchyma) to transport oxygen to submerged organs in anoxic soil (Armstrong et al. 1991; Joabsson et al. 1999). As a consequence, the aerenchyma may also act as conduits for CH4 transport to the atmosphere, bypassing the oxic layer where biological CH4 oxidation is taking place (Bellisario et al. 1999; Whalen 2005). Several studies addressing atmospheric CH4 emission therefore focused on wetlands dominated by Carex spp. (Whiting and Chanton 1992; Chasar et al. 2000; Ding et al. 2004). It has been reported that more than 90 % of the CH4 emitted in Carex spp. dominated wetlands passes through their aerenchyma system (Whiting and Chanton 1992). In contrast, in a laboratory study using peat cores dominated by Sphagnum spp. and cores dominated by Juncus spp. lower CH4 emissions were observed from the cores vegetated by vascular plants (Roura-Carol and Freeman 1999). Vascular plants also stimulate CH4 production through secretion of root exudates and provision of plant litter to the methanogenic archaea (Whiting and Chanton 1992). In addition, vascular plants transport oxygen to the rhizosphere which stimulates the activity of methanotrophic microorganisms (Bellisario et al. 1999).
A field survey was conducted to quantify CH4 emissions from fens located in the Swiss Alps. The Swiss Alps are characterized by two main bedrock types (siliceous and calcareous) which are the dominant influence on the soil properties (Lazzaro et al. 2009). Calcareous bedrock is composed of large amounts of CaCO3 that result in a more alkaline soil and a lower availability of nutrients compared to siliceous bedrock.
The specific goals of this study are (i) to assess the spatial variability of CH4 emissions from alpine fens located on siliceous or calcareous bedrock in Switzerland, (ii) to quantify CH4 pore water concentrations of these fens, and (iii) to conduct a statistical analysis in order to relate CH4 emissions to selected environmental factors (e.g. bedrock and vegetation type, plant biomass, soil and air temperature, and dissolved organic carbon [DOC] concentrations).
Materials and methods
Sampling sites
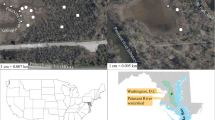
Fourteen different alpine fens in the Swiss Alps were selected (Fig. 1). The main criteria for choosing these fens were (i) a water table above the soil surface and (ii) a vegetation cover mainly composed of plants of the genus Carex. The 14 alpine fens in this study varied in altitude, bedrock type, yearly mean precipitation, as well as type of plant cover (Table 1). The sites are generally characterized as snow-covered in winter and with a 3–4 month long snow-free summer. The average annual precipitation data at each site was obtained from the CLIMAP database of the Swiss Federal Office of Meteorology and Climatology (http://meteoswiss.ch; Table 1). Altitudes ranged from 1,800 to 2,600 m a.s.l.; six fens were located on calcareous bedrock and eight fens on siliceous bedrock. Most fens on siliceous bedrock were dominated by Carex nigra, while C. rostrata was the main vegetation on the calcareous bedrock (Table 1). Fens with an area ranging from 10 to 800 m2 were chosen, with the exception of the Oberaar fen one, which covers an area of 3,000 m2. There was one study site per fen and these study sites were characterized as nutrient-poor to mesotrophic (Rydin and Jeglum 2006). Most of the study sites were sampled once, in either 2010 or 2011, between July and August (Table 2). However, two sites (GA and OA1) were sampled twice, once in 2010 and once in 2011. The microbiological structures of the fens at Oberaar and Göschener Alp have been described by Franchini and Zeyer (2012) and by Liebner et al. (2012), respectively.
Quantification of methane emissions
Methane emissions into the atmosphere were measured between noon and 3.00 p.m. using static transparent chambers (30 × 30 × 30 cm, volume of 0.027 m3), as described in Liebner et al. (2012). At each site, four chambers were gently placed into standing water within a radius of 5 m. Sampling was performed using portable walkways to minimize disturbance. For each chamber, the average height between the water table elevation and the top of the chamber was measured and the volume of the headspace was recorded. After several minutes of equilibration, the openings on the top of the chamber were carefully closed with butyl rubber stoppers. A needle connected to a 50 mL syringe was inserted through a stopper. The chambers were not equipped with fans and therefore the piston of the syringe was pulled and pushed five times to mix the gas in the chamber. Immediately after mixing, a 50 mL gas sample was taken and injected into a 20 mL glass vial, which had been previously flushed with N2 and evacuated. This procedure was repeated every 5 min up to 30 min (seven samples in total).
Concentrations of CH4 were measured by gas chromatography (Trace GC Ultra; Thermo Electron Corporation, Rodano, Italy) using a 2 m Porapak N 100/120 mesh column (1/16″ outer diameter and 1 mm inner diameter) and a flame ionization detector (FID). The column and detector temperatures were set at 30 and 250 °C, respectively. The carrier gas (N2) flow velocity was 30 mL min−1 (30 kPa); the FID hydrogen was set at 35 kPa and the synthetic air flow at 350 kPa. Peak areas calibration was performed with Messer CAN-Gas standards (CH4 in synthetic air 5.0, Messer Schweiz AG, Lenzburg, Switzerland) over a concentration range from 1 to 5,000 ppm and integrated using the software Chromeleon (Vers. 6.8, Dionex AG, Olten, Switzerland).
The emission of CH4 into the atmosphere was calculated from the CH4 accumulated in the chamber over 30 min using linear regression (van der Nat and Middelburg 1998; Ding et al. 2005; Liebner et al. 2012). Linear regression with r2 < 0.95 was omitted from further analyses. The increase in CH4 concentrations was linear and no ebullition was observed in our measurements.
Plant species and biomass
After each emission measurement the plant biomass covered by the chamber was harvested by clipping the plants at the water table with scissors. The plant material was wrapped in aluminum foil and taken to the laboratory. Dead biomass (i.e. brown and black residues of stems and leaves) was discarded and the remaining live biomass (i.e. green stems and leaves) was dried at 70 °C for 48 h and weighed. Throughout this report the term “plant biomass” refers exclusively to the dry weight (units: g m−2) of live plant biomass above the water table. The plant species reported in Table 2 made up more than 95 % of the live biomass for each emission measurement.
Pore water sampling and physico-chemical characteristics
Pore water was sampled to obtain a profile of DOC and CH4 pore water concentrations to a depth of 50 cm. The pore water was extracted along the profile using brass tubes. These tubes (0.3 cm inner diameter) were closed at one end and perforated with 0.1 cm diameter closely spaced holes for the first 1 cm from the closed end. The perforations allow the extraction of pore water and avoid the co-extraction of peat material. Nevertheless, at some sites sampling was not possible below certain depths as the peat material caused severe clogging of the brass tubes.
A series of brass tubes were inserted vertically into the water/soil to allow extraction of the pore water profile at the following depths below the water table: 1.0 (considered to reflect the surface water conditions), 2.5, 5.0, 7.5, 10.0, 15.0, 20.0, 30.0, 40.0 and 50.0 cm. Each tube was connected to a three-way valve (Discofix C-3, Braun Melsungen AG, Melsungen, Germany) by silicon tubing. Seven mL of pore water (equivalent to the dead volume of the tube) was pulled through the tube with a 10 mL syringe. The valve was subsequently closed to hold the pore water inside and to exclude oxygen contaminations from the atmosphere. After 1 h of equilibration, 7 mL of pore water was extracted and discarded.
For DOC measurements, 20 mL of pore water was collected from each depth and immediately filtered on-site through 0.45 μm nylon filters (Wicom Perfect Flow, Meienfeld, Switzerland). Before sampling, the filters were washed with 10 mL of distilled water to remove contaminants. Each filtered sample was acidified with 0.1 mL of a 1 M HCl solution (King et al. 1998) and later stored in a glass vial at −20 °C. DOC analysis was performed with a Shimadzu TOC-5000 analyzer (Shimadzu SSI, Columbia, MD).
To determine the concentration of dissolved CH4 in pore water along the profile, 5 mL of pore water was collected with a 10 mL plastic syringe as described above and immediately injected into to previously N2-flushed vials containing 0.1 mL of a 1 M HCl solution (Frenzel and Karofeld 2000; Joabsson and Christensen 2001). The vials were stored at 4 °C until analysis. Methane concentrations in the vial headspace were measured by gas chromatography, and concentrations of dissolved CH4 were subsequently calculated using the headspace method according to Liebner et al. (2012). At each site in 2010, one or two independent profiles of CH4 pore water concentrations were measured. However, for the sites sampled in 2011, four independent profiles were measured.
Similarly, depth profiles of conductivity and pH were analyzed in situ according to Liebner et al. (2012) using a Multi 350i probe (WTW, Laboratory and Field Products, Nova Analytics, Woburn, MA) connected to a LR 325/01 conductivity cell and a SenTix 51 pH electrode. Prior to using the LR 325/01 conductivity cell, its response was tested using KCl solutions. This test demonstrated that up to 1,200 μS cm−1 the signal is a linear function of the KCl concentration.
Oxygen and temperature measurements along the profile
Oxygen pore water concentrations along the profiles were measured in situ with a Fibox 3-trace v3 planar trace oxygen minisensor (PreSens, Regensburg, Germany), as previously reported (Liebner et al. 2012). Temperature and oxygen pore water concentrations were logged after an equilibration time of about 15 min at the same depths as for DOC and CH4. However, at some sites oxygen pore water concentrations were determined colorimetrically using a DRr890 colorimeter (HACH Lange, Rheineck, Switzerland) with high and low range AccuVac Dissolved Oxygen Reagent Ampules (HACH Lange). 25 mL of pore water was extracted from the brass tubes as previously described. Approximately 5 mL was slowly discarded to avoid exposure of the water samples to air, and 20 mL was transferred immediately to the AccuVac glass ampules and measured using the DRr890 colorimeter (HACH Lange).
The colorimetric method was used at sites BI, MA, VR1, VR2 and VR3, whereas the oxygen minisensor method was used at all other sites. Both methods have been applied in environmental studies (e.g. Kleikemper et al. 2005; Liebner et al. 2012); however, each method also has limitations: The minisensor method is temperature dependent and requires long equilibration times. Moreover, in peat soil the measuring device can clog. On the other hand, the colorimetric method requires large volumes of pore water, and the samples can be exposed to air prior to measurement. Preliminary tests comparing the two methods showed deviations in the range of 2–25 % from each other. This is in agreement with data reported by Bagshaw et al. (2011), who also compared different methods.
Statistical analysis
The statistical analysis was performed with the software R (version 2.15.2) (R Development Core Team 2012). Methane emissions, plant biomass, CH4, oxygen and DOC pore water concentrations, as well as data for conductivity were logarithmically transformed to approximate normal distributions. Normality of the data was tested using the Shapiro–Wilk test. Differences between bedrock or vegetation type, and CH4 emissions, plant biomass and physico-chemical parameters (e.g. soil and air temperature, DOC pore water concentrations) were identified with two-sample t-tests.
Linear mixed models were used to perform stepwise multiple linear regression analyses. First, the relationship between CH4 emissions (dependent variable) and the following fixed parameters were evaluated: altitude, bedrock and vegetation type, plant biomass, air temperature and pressure, precipitation, and the mean values (depth 0–20 cm) of conductivity, pH, soil temperature, and CH4, oxygen and DOC pore water concentration. Second, the relationships between CH4 pore water concentration (0–20 cm, dependent variable) and the following fixed parameters were evaluated: altitude, bedrock and vegetation type, plant biomass, air temperature and pressure, precipitation, conductivity, pH, soil temperature, and oxygen and DOC pore water concentration. These linear mixed models were implemented using R package “lme4” (function “lmer”) (Bates et al. 2011). The sites were included as a random effect to account for the variability observed between sites. Normality and homogeneity were examined by visually inspecting plots of the residuals against fitted values. Selection of the best-fitting models was performed based upon Akaike Information Criterion (AIC) values, and calculated with the Restricted Maximum Likelihood (REML) (Johnson and Omland 2004). Thus, the simplest method that performed the best was applied. In addition, to assess the validity of the mixed parameter analyses, ANOVA analyses were performed comparing the models with fixed parameters to the null models with only the random effect. Results in which the model including fixed effects did not differ significantly from the null models were rejected. The significance level for all tests was P = 0.05. Pore water below 20 cm was not included in this analysis because of difficulty sampling below this depth.
Results
Physico-chemical characteristics of the fen pore water
Physico-chemical characteristics are summarized in Table 2. At all sites the water table elevation varied from 1 to 6 cm above the soil surface. From 0 to 20 cm, soil temperatures differed substantially between the study sites (9.2 to 20.7 ± 4.8 °C, Table 2). These temperatures partially reflected the air temperatures at the time of sampling. In deeper soil layers the temperatures of the different sites were more equal (9.1 to 15.2 ± 1.9 °C).
The pH values of pore water ranged from slightly acidic (max. pH 6.4 ± 0.3) to acidic (min. pH 4.5 ± 0.2), and conductivities ranged broadly from 14.5 ± 1.7 to 826.3 ± 123.1 μS cm−1. DOC pore water concentrations varied from 2.0 ± 1.4 to 15.4 ± 15.9 mg C L−1 for the first 20 cm and from 2.9 ± 1.6 to 41.1 ± 25.3 mg C L-1 in deeper soil layers. No significant difference in water table elevation between sites located on siliceous and those on calcareous bedrocks was detected (P = 0.314). Parameters, such as pH (P = 0.982), conductivity (P = 0.872), DOC pore water concentrations from 0 to 20 cm (P = 0.477) and 20 to 50 cm (P = 0.763), and soil temperatures from 0 to 20 cm (P = 0.148) and 20 to 50 cm (P = 0.853) did not vary significantly. Mean altitudes were 2,257 ± 243 m a.s.l. and 1,948 ± 77 m a.s.l. for fen sites located on siliceous and calcareous bedrock, respectively.
Plant species and plant biomass
C. nigra and C. rostrata dominated the vegetation at the study sites. Sites on siliceous bedrock were dominated by C. nigra, while calcareous sites were dominated by C. rostrata (Table 1). This difference was not significant (P = 0.094). The average plant biomass for C. nigra ranged from 44.4 ± 33.5 to 101.7 ± 15.8 g m−2 and for C. rostrata from 67.0 ± 17.8 to 430.1 ± 361.0 g m−2 (Table 3). C. rostrata biomass was significantly higher than C. nigra biomass (P = 0.001). Moreover, plant biomass on siliceous bedrock was lower than on calcareous bedrock (P = 0.002).
Methane emissions
Methane emissions ranged from 74 ± 43 mg CH4 m–2 day−1 at VR3 to 711 ± 212 mg CH4 m−2 day−1 at BI during the 2010 campaign, and from 153 ± 31 mg CH4 m−2 day−1 at GA to 539 ± 58 mg CH4 m−2 day−1 at TA during 2011 (Table 2). At the sites that were sampled twice, GA and OA1, CH4 emissions in 2011 were roughly 25 % lower than emissions in 2010 (Table 2), however, the emissions were not significantly different (P = 0.122; P = 0.217, respectively). An average CH4 emission of 162 ± 76 (74 ± 43 to 246 ± 45) mg CH4 m−2 day−1 was observed at siliceous sites, while the average emission at calcareous sites was 503 ± 176 (328 ± 120 to 711 ± 212) mg CH4 m−2 day−1. In addition, an average CH4 emission of 232 ± 190 (74 ± 43 to 544 ± 147) mg CH4 m−2 day−1 was measured at sites covered by C. nigra, whereas the average emission at C. rostrata sites was 398 ± 218 (153 ± 31 to 711 ± 212) mg CH4 m−2 day−1. Significant differences were found between bedrock types and CH4 emissions (P < 0.001) and between vegetation types and CH4 emissions (P = 0.005).
Methane and oxygen pore water concentrations
Methane pore water concentrations were determined along a profile from the water table down to 50 cm, where extraction to this depth was possible. At all sites (except CA and OA2), in the first few cm below the water table, CH4 pore water concentrations were low but they steadily increased down to 20 cm (27 ± 34–273 ± 236 μM). From 20 to 50 cm, CH4 pore water concentrations remained high (64 ± 71–627 ± 555 μM) (Table 4). High CH4 pore water concentrations (>250 μM) were detected near the surface of the water table at CA and OA2, but at these sites pore water could not be extracted below 25 cm due to clogging. Furthermore, at CA, gas bubbles (50 % of the total volume extracted) were present in pore water samples extracted from 15 to 20 cm, resulting in high CH4 pore water concentrations (Table 4). At several sites, pore water could not be extracted below 15–25 cm (12 profiles) or 30–40 cm (five profiles) (Table 4, footnotes). No significant differences were found between CH4 pore water concentrations from 0 to 20 cm and bedrock types (P = 0.294) or vegetation types (P = 0.143). Similarly, CH4 pore water concentrations at a depths 20–50 cm were not significantly different between bedrock types (P = 0.993) or vegetation types (P = 0.347).
Oxygen pore water concentrations generally decreased to zero within the first 20 cm below the water table (Table 4). At most sites, average oxygen pore water concentrations from 0 to 20 cm were above 5.0 ± 4.8 μM. Oxygen pore water concentrations were significantly different between vegetation types (P = 0.026). Higher oxygen pore water concentrations were observed with C. rostrata (75.9 ± 51.4 μM) than C. nigra (24.3 ± 19.0 μM). From 20 to 50 cm average oxygen pore water concentrations were lower than 6.6 ± 9.6 μM (Table 4) at most sites.
Methane and oxygen pore water concentration profiles
Some of the concentrations presented in Table 4 are based on one or two profiles or pore water samples could not be extracted below 15 or 30 cm (Table 4, footnotes) and thus this data is not presented in Fig. 2. However, at four sites sampled in 2011 (GA, GS, OA1 and TA), four independent profiles to a depth of 50 cm were measured. For these four profiles the mean values of CH4 and oxygen pore water concentrations at each depth are shown in Fig. 2. At GA in 2011, CH4 pore water concentrations were generally low (<100 μM) and CH4 pore water concentrations increased with depth (Fig. 2a). The profiles obtained at GS and TA in 2011 were quite similar to each other. The profiles were characterized by an exponential increase of CH4 pore water concentrations from 0 to 7.5 cm, followed by an almost linear increase from 7.5 to 15 or 20 cm, where the highest CH4 pore water concentrations were observed (Fig. 2a). Below 15–20 cm CH4 pore water concentrations decreased again (Fig. 2a).
At GA in 2011, high oxygen pore water concentrations could be detected down to 30 cm (Fig. 2b). At GS, OA1 and TA oxygen pore water concentration profiles displayed a similar trend with concentrations close to zero from 7.5 to 15 cm (Fig. 2b).
Factors related to methane dynamics
Statistical analyses revealed that the CH4 emissions were positively related to plant biomass and average CH4 pore water concentrations from 0 to 20 cm (Table 5; Equation 1). Moreover, CH4 emissions were lower on siliceous bedrock than on calcareous bedrock. Other environmental factors, such as altitude, vegetation type, pH and oxygen pore water concentration had no direct influence on CH4 emissions at the studied sites.
In addition, CH4 pore water concentrations from 0 to 20 cm were negatively related to oxygen pore water concentrations (Table 5; Equation 2), while other environmental factors had no direct influence on CH4 pore water concentrations at shallow depths.
Discussion
Plant-mediated transport of methane from alpine fens
An almost linear increase of CH4 pore water concentrations from 7.5 to 15 cm at GS and TA, and from 5 to 10 cm at OA1 was observed in 2011 (Fig. 2); therefore, the upward CH4 flux in pore water using Fick’s first law of diffusion was calculated similarly to Beer and Blodau (2007) and Hornibrook et al. (2009). The diffusion coefficient of CH4 in water (Dw, methane) was determined from the mean temperature measured in situ across the depth interval of the linear regression using the polynomial regression for Dw, methane in water (83rd Edition of the Handbook of Physics and Chemistry). The diffusion coefficient of CH4 in soil (Ds, methane) was calculated using the equation (Lerman 1979):
where φ stands for porosity which was assumed to be 0.9 at shallow soil depths according to Letts et al. (2000). The calculated diffusive CH4 fluxes were 4 (OA1), 7 (GS) and 6 mg CH4 m−2 day−1 (TA), which accounts only for 2.3, 1.7 and 1.1 %, respectively, of the total CH4 emissions into the atmosphere. These emissions are in the same range of previously reported values from a Swiss alpine fen (Liebner et al. 2012). The results suggest that the CH4 transport through the aerenchyma of C. rostrata is the major emission pathway in the alpine fens analyzed. Similar results have been reported from other wetlands by measuring CH4 emissions in the presence and absence of plants (Whiting and Chanton 1992; Waddington et al. 1996; King et al. 1998).
Within the first 5–7.5 cm below the water table at GS and TA and 2.5–5 cm at OA1, the CH4 pore water concentration curves have a concave shape (Fig. 2a). However, at GS and OA1 for this depth interval there was free water above the soil surface, where the governing transport mechanisms for CH4 remain unknown. Therefore, these sections of the CH4 pore water concentration profiles were not considered for further analyses. The concavity of the profiles at TA from 5 to 7.5 cm, suggested that the shallow layers are a sink for CH4. The CH4 sink or consumption was determined by calculating the difference between upward fluxes at depths from 5 to 7.5 cm and from 7.5 to 15 cm (Fechner and Hemond 1992). A sink of 4 mg CH4 m−2 day−1 accounts for about 67 % of the diffusive flux calculated from 7.5 to 15 cm depth, and might be the result of CH4 consumption by methanotrophic bacteria. However, we are aware that CH4 pore water concentration profiles may be subjected to spatial and temporal variability. While several studies have determined emissions to the atmosphere, these calculations aim to quantify the subsurface processes. The obtained results complement previous findings by Fechner and Hemond (1992) and Hornibrook et al. (2009).
In addition to diffusion and plant mediated transport, ebullition, has been reported to be an important process in fens (Whalen 2005). However, ebullition was not detected during CH4 flux measurements at our field sites, which is in agreement with a recent study quantifying the episodic ebullition to approximately 3 % of the total CH4 emission (Green and Baird 2013).
Environmental factors related to methane dynamics
Methane emissions (74 ± 43 to 711 ± 212 mg CH4 m−2 day−1) reported in this study showed high variability within and between the sites. High variability in CH4 emissions has been reported in most studies where static chamber techniques have been used to quantify gas-exchanges from the subsurface to the atmosphere. Bubier et al. (1993) reported a within-site variability of CH4 emissions ranging from 86 to 200 % that could be explained mainly by differences in the site microtopography. Methane emissions in our study are in the same range as those in the arctic tundra (Bartlett et al. 1992; Whalen and Reeburgh 1992).
A relationship between CH4 emissions and bedrock type was found in the present study (Table 5, Equation 1). On siliceous bedrock, CH4 emissions ranged from 74 ± 43 to 246 ± 45 mg CH4 m−2 day−1. These emissions are in agreement with data reported by Koch et al. (2007) and Liebner et al. (2012), who also investigated fens on siliceous bedrock. On calcareous bedrock, CH4 emissions ranged from 328 ± 120 to 711 ± 212 mg CH4 m−2 day−1; however, these sites were also located at lower altitudes and plant biomass was higher. Moreover, no significant differences in physico-chemical parameters were found between sites located on siliceous and calcareous bedrock. This supports Chapman et al. (2003) who suggested that the water chemistry of fens is controlled by biologically-mediated processes rather than by water sources.
A positive correlation was found between CH4 emissions and vascular plant biomass (Table 5, Equation 1), which is consistent with results reported from fens located in the northern hemisphere (Bellisario et al. 1999; Joabsson and Christensen 2001; von Fischer et al. 2010) and in alpine regions (Koch et al. 2007; Chen et al. 2011). Moreover, in most cases C. rostrata had higher plant biomass than C. nigra, which is in full agreement with data published by Visser et al. (2000). This may have an indirect influence on the CH4 emissions.
A positive correlation was found between CH4 emissions and CH4 pore water concentrations at shallow depths (Table 5, Equation 1). In addition, CH4 pore water concentrations were negatively related to oxygen pore water concentrations at the same depth (Table 5, Equation 2). Further, the aerenchyma of vascular plants allows for the diffusion of oxygen from the atmosphere down to the rhizosphere, where it stimulates the activity of methanotrophic bacteria and at the same time hampers the activity of methanogenic archaea (Whalen 2005). Furthermore, Epp and Chanton (1993) suggested that 10–90 % of CH4 produced is oxidized by methanotrophic bacteria in the rhizosphere of vascular plants. Thus, the negative relationship found between CH4 and oxygen pore water concentrations might result from both CH4 oxidation and plant-mediated transport. Similarly, King et al. (1998) reported an inverse correlation between CH4 pore water concentration and root density, where low CH4 pore water concentrations were measured at depths of 15–20 cm in zones of high root densities.
Conclusion
The objective of this study was to quantify the CH4 emissions from fens located in the Swiss Alps at 1,800–2,600 m a.s.l. The emissions ranged from 74 to 711 mg CH4 m−2 day−1 and thus were in the same range as emissions from wetlands in northern latitudes (Alaska, Siberia, and northern Canada). Despite the fact that CH4 emissions showed high spatial variability within and between the different sites the emissions could be related to environmental factors, such as bedrock type, plant biomass and CH4 pore water concentration at shallow depths. However, since these environmental factors may be interdependent further study would be required to determine if they have a direct or an indirect effect. This study also demonstrates the importance of aerenchymous plants like Carex spp. These plants not only provide carbon substrates for the methanogenic archaea and oxygen for the methanotrophic bacteria in the subsurface but also act as major conduits for the transport of CH4 from the subsurface into the atmosphere.
References
Armstrong W, Justin SHFW, Beckett PM, Lythe S (1991) Root adaptation to soil waterlogging. Aquat Bot 39:57–73
Bagshaw EA, Wadham JL, Mowlem M, Tranter M, Eveness J, Fountain AG, Telling J (2011) Determination of dissolved oxygen in the cryosphere: a comprehensive laboratory and field evaluation of fiber optic sensors. Environ Sci Technol 45:700–705
Bardgett RD, Freeman C, Ostle NJ (2008) Microbial contribution to climate change through carbon cycle feedbacks. ISME J 2:805–814
Bartlett KB, Crill PM, Sass RL, Harriss RC, Dise NB (1992) Methane emissions from tundra environments in the Yukon-Kuskokwin Delta, Alaska. J Geophys Res Atmos 97:16645–16660
Bates D, Maechler M, Bolker B (2011) lme4: Linear mixed-effect model using S4 classes. R package version 0.999375-39. http://CRAN.R-project.org/package0lme4. Accessed Dec 2012
Beer J, Blodau C (2007) Transport and thermodynamics constrain belowground carbon turnover in a northern peatland. Geochim. Cosmochim. Acta 71:2989–3002
Bellisario LM, Bubier JL, Moore TR, Chanton JP (1999) Controls on CH4 emissions from a northern peatland. Glob Biogeochem Cycles 13:81–91
Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang QL (2013) Methane emissions from wetlands: biogeochemical, microbial, and modeling perspective from local to global scales. Glob Chang Biol 19:1325–1346
Bubier JL, Moore TR, Roulet NT (1993) Methane emissions from wetlands in the mid-boreal region of northern Ontario, Canada. Ecology 74:2240–2254
Bubier JL, Moore TR, Juggins S (1995a) Predicting methane emission from bryophyte distribution in Northern Canadian Peatlands. Ecology 76:677–693
Bubier JL, Moore TR, Bellisario L, Comer NT (1995b) Ecological controls on methane emissions from a northern peatland complex in the zone of discontinuous permafrost, Mannitoba, Canada. Glob Biogeochem Cycles 9:455–470
Cao GM, Xu XL, Long RJ, Wang QL, Wang CT, Du YG, Zhao XQ (2008) Methane emissions by alpine plant communities in the Qinghai–Tibet plateau. Biol Lett 4:681–684
Chapman JB, Lewis B, Litus G (2003) Chemical and isotopic evaluation of water sources to the fens of the south park, Colorado. Environ Geol 43:533–545
Chasar LS, Chanton JP, Glaser PH, Siegel DI (2000) Methane concentration and stable isotope distribution as evidence of rhizospheric processes: comparison of a fen and bog in the glacial lake agassiz peatland complex. Ann Bot 86:655–663
Chen H, Wu N, Gao YH, Wang YF, Luo P, Tian JQ (2009) Spatial variations on methane emissions from zoige alpine wetlands of southwest China. Sci Total Environ 407:1097–1104
Chen HA, Wu N, Wang YF, Gao YH, Peng CH (2011) Methane fluxes from alpine wetlands of Zoige Plateau in relation to water regime and vegetation under two scales. Water Air Soil Pollut 217:173–183
Chimner RA, Cooper DJ (2003) Carbon dynamics of pristine and hydrologically modified fens in the southern Rocky Mountains. Can J Bot 81:477–491
Christensen TR, Jonasson S, Callaghan TV, Havstrom M (1995) Spatial variation in high-latitude methane flux along a transect across Siberian and European tundra environments. J Geophys Res Atmos 100:21035–21045
Christensen TR, Ekberg A, Ström L, Mastepanov M, Panikov N, Öquist M, Svensson BH, Nykänen H, Martikainen PJ, Oskarsson H (2003) Factors controlling large scale variations in methane emissions from wetlands. Geophys Res Lett 30:1414–1418
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60:609–640
Denman KL, Brasseur A, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine DA, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, da Silva Dias PL, Wofsy SC, Zhang X (2007) Couplings 1 between changes in the climate system and biogeochemistry. climate change 2007: the physical science basis. Contribution of Working Group I to the fourth assessment report of the intergovernmental panel on climate change
Ding WX, Cai ZC, Wang DX (2004) Preliminary budget of methane emissions from natural wetlands in China. Atmos Environ 38:751–759
Ding WX, Cai ZC, Tsuruta H (2005) Plant species effects on methane emissions from freshwater marshes. Atmos Environ 39:3199–3207
Dise NB, Gorham E, Verry ES (1993) Environmental factors controlling methane emissions from peatlands in northern Minnesota. J Geophys Res Atmos 98:10583–10594
Dunfield P, Knowles R, Dumont R, Moore TR (1993) Methane production and consumption in temperate and subarctic peat soils: response to temperature and pH. Soil Biol Biochem 25:321–326
Epp MA, Chanton JP (1993) Rhizospheric methane oxidation determined via the methyl fluoride inhibition technique. J Geophys Res 98:18422–18423
Fechner EJ, Hemond HF (1992) Methane transport and oxidation in the unsaturated zone of a Sphagnum peatland. Glob Biogeochem Cycles 6:33–44
Franchini AG, Zeyer J (2012) Freeze-coring method for characterization of microbial community structure and function in wetland soils at high spatial resolution. Appl Environ Microbiol 78:4501–4504
Freeman C, Lock MA, Reynolds B (1992) Fluxes of CO2, CH4 and N2O from a Welsh peatland following simulation of water table draw-down: potential feedback to climate change. Biogeochemistry 19:51–60
Freeman C, Nevison GB, Kang H, Hughes S, Reynolds B, Hudson JA (2002) Contrasted effects of simulated drought on the production and oxidation of methane in a mid-Wales Wetland. Soil Biol Biochem 34:61–67
Frenzel P, Karofeld E (2000) CH4 emission from a hollow-ridge complex in a raised bog: the role of CH4 production and oxidation. Biogeochemistry 51:91–112
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–195
Green SM, Baird AJ (2013) The importance of episodic ebullition methane losses from three peatland microhabitats: a controlled-environment study. Eur J Soil Sci 64:27–36
Heyer J, Berger U, Kuzin IL, Yakovlev ON (2002) Methane emissions from different ecosystem structures of the subarctic tundra in Western Siberia during midsummer and during the thawing period. Tellus B 54:231–249
Hirota M, Tang YH, Hu QW, Hirata S, Kato T, Mo WH, Cao GM, Mariko S (2004) Methane emissions from different vegetation zones in a Qinghai-Tibetan plateau wetland. Soil Biol Biochem 36:737–748
Hornibrook ERC, Bowes HL, Culbert A, Gallego-Sala AV (2009) Methanotrophy potential versus methane supply by pore water diffusion in peatlands. Biogeosciences 6:1490–1504
Joabsson A, Christensen TR (2001) Methane emissions from wetlands and their relationship with vascular plants: an Arctic example. Glob Chang Biol 7:919–932
Joabsson A, Christensen TR, Wallen B (1999) Vascular plant controls on methane emissions from northern peatforming wetlands. Trends Ecol Evol 14:385–388
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
King JY, Reeburgh WS, Regli SK (1998) Methane emission and transport by arctic sedges in Alaska: results of a vegetation removal experiment. J Geophys Res Atmos 103:29083–29092
Kleikemper J, Pombo SA, Schroth MH, Sigler WV, Pesaro M, Zeyer J (2005) Activity and diversity of methanogens in a petroleum hydrocarbon-contaminated aquifer. Appl Environ Microbiol 71:149–158
Koch O, Tscherko D, Kandeler E (2007) Seasonal and diurnal net methane emissions from organic soils of the eastern alps, Austria: effects of soil temperature, water balance, and plant biomass. Arct Antarct Alp Res 39:438–448
Lai DYF (2009) Methane dynamics in northern peatlands: a review. Pedosphere 19:409–421
Lazzaro A, Abegg C, Zeyer J (2009) Bacterial community structure of glacier forefields on siliceous and calcareous bedrock. Eur J Soil Sci 60:860–870
Lerman A (1979) Geochemical processes: water and sediment environments. Wiley, New York
Letts MG, Roulet NT, Comer NT, Skarupa MR, Verseghy DL (2000) Parametrization of peatland hydraulic properties for the Canadian land surface scheme. Atmos Ocean 38:141–160
Liebner S, Schwarzenbach SP, Zeyer J (2012) Methane emissions from an alpine fen in central Switzerland. Biogeochemistry 109:287–299
Limpens J, Berendse F, Blodau C, Canadell JG, Freeman C, Holden J, Roulet N, Rydin H, Schaepan-Strub G (2008) Peatlands and the carbon cycle: from local processes to global implications—a synthesis. Biogeosciences 5:1475–1491
Mast MA, Wickland KP, Striegl RG, Clow DW (1998) Winter fluxes of CO2 and CH4 from subalpine soils in rocky mountain national park, Colorado. Glob Biogeochem Cycles 12:607–620
Moore TR, Basiliko N (2006) Decomposition in boreal peatlands. In: Wieder RK, Vitt DH (eds) Boreal peatland ecosystems. Ecological studies 188. Springer, Berlin, pp 125–144
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/. Accessed Dec 2012
Roura-Carol M, Freeman C (1999) Methane release from peat soils: effect of Sphagnum and Juncus. Soil Biol Biochem 31:323–325
Rydin H, Jeglum JK (2006) The biology of peatlands. Oxford University Press, Oxford
Saarnio S, Morero M, Shurpali NJ, Tuittila ES, Makila M, Alm J (2007) Annual CO2 and CH4 fluxes of pristine boreal mires as a background for the lifecycle analyses of peat energy. Boreal Environ Res 12:101–113
Sachs T, Giebels M, Boike J, Kutzbach L (2010) Environmental controls on CH4 emission from polygonal tundra on the microsite scale in the lena river delta, Siberia. Glob Chang Biol 16:3096–3110
Schimel JP (1995) Plant transport and methane production as controls on methane flux from arctic wet meadow tundra. Biogeochemistry 28:183–200
Sebacher DI, Harriss RC, Bartlett KB, Sebacher SM, Griche SS (1986) Atmospheric methane sources: Alaskan tundra bogs, an alpine fen, and subarctic boreal marsh. Tellus B 38:1–10
Ström L, Ekberg A, Mastepanov M, Christensen TR (2003) The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Glob Chang Biol 9:1185–1192
Trudeau NC, Garneau M, Pelletier L (2013) Methane fluxes from a patterned fen of the northeastern part of the La grande river watershed, james bay, Canada. Biogeochemistry 113:409–422
Turunen J, Tomppo E, Tolonen K, Reinikainen A (2002) Estimating carbon accumulation rates of undrained mires in Finland—application to boreal and subarctic regions. Holocene 12:69–80
Valentine DW, Holland EA, Schimel DS (1994) Ecosystem and physiological controls over methane production in northern wetlands. J Geophys Res 99:1563–1571
van der Nat FJWA, Middelburg JJ (1998) Seasonal variation in methane oxidation by the rhizosphere of Phragmites australis and Scirpus lacustris. Aquat Bot 61:95–110
Visser EJW, Bögemann GM, van de Steeg HM, Pierik R, Blom CWPM (2000) Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation. New Phytol 148:93–103
von Fischer JC, Rhew RC, Ames GM, Fosdick BK, von Fischer PE (2010) Vegetation height and other controls of spatial variability in methane emissions from the arctic coastal tundra at barrow, Alaska. J Geophys Res Biogeosci 115:G00I03
Waddington JM, Roulet NT (2000) Carbon balance of a boreal patterned peatland. Glob Change Biol 6:87–97
Waddington JM, Roulet NT, Swanson RV (1996) Water table control of CH4 emission enhancement by vascular plants in boreal peatlands. J Geophys Res 101:22775–22785
Wagner D, Kobabe S, Pfeiffer EM, Hubberten HW (2003) Microbial controls on methane fluxes from a polygonal tundra of the lena delta, Siberia. Permafr Periglac 14:173–185
West AE, Brooks PD, Fisk MC, Smith LK, Holland EA, Jaeger CH, Babcock S, Lai RS, Schmidt SK (1999) Landscape patterns of CH4 fluxes in an alpine tundra ecosystem. Biogeochemistry 45:243–264
Whalen SC (2005) Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci 22:73–94
Whalen SC, Reeburgh WS (1992) Interannual variation in tundra methane emission: a 4 years time series at fixed sites. Glob Biogeochem Cycles 6:139–159
Whalen SC, Reeburgh WS (2000) Methane oxidation, production, and emission at contrasting sites in a boreal bog. Geomicrobiol J 17:237–251
Whiting GJ, Chanton JP (1992) Plant-dependent CH4 emission in a subartic Canadian fen. Glob Biogeochem Cycles 6:225–231
Whiting GJ, Chanton JP (1993) Primary production control of methane emission from wetlands. Nature 364:794–795
Wickland KP, Striegl RG, Mast MA, Clow DW (2001) Carbon gas exchange at a southern Rocky Mountain wetland, 1996–1998. Glob Biogeochem Cycles 15:321–335
Acknowledgments
We thank A. Gauer, M. Vogt and É. Mészáros for their assistance in the laboratory and the field, as well as J. Schneller for botanical determinations. We are grateful to Kraftwerke Oberhasli AG (KWO) and Centralschweizerische Kraftwerke (CKW) for facilitating access to the field sites at Oberaar and Göschener Alp, respectively. Additionally, we acknowledge A. Lazzaro, R. Henneberger, B. Morris, and C. Hoffman for helpful comments on the manuscript. ETH Zurich supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franchini, A.G., Erny, I. & Zeyer, J. Spatial variability of methane emissions from Swiss alpine fens. Wetlands Ecol Manage 22, 383–397 (2014). https://doi.org/10.1007/s11273-014-9338-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-014-9338-6