Abstract
Methane emissions and below ground methane pore water concentrations were determined in an alpine fen at 1,915 m a.s.l. in central Switzerland. The fen represented an acidic (pH 4.5–4.9), nutrient-poor to mesotrophic habitat dominated by Carex limosa, Carex rostrata, Trichophorum caespitosum and Sphagnum species. From late fall to late spring the fen was snow-covered. Throughout winter the temperatures never dropped below 0°C at 5 cm below the vegetation surface. Methane emissions in June, July, August and September were in the range of 125 (±26)–313 (±71) mg CH4 m−2 day−1 with a tendency to decrease along the summer season. Mean methane pore water concentrations at a depth of 20–40 cm below the vegetation surface were 526 (±32) μM in June and in the range of 144 (±10)–233 (±7) μM in July, August and September. At a depth of 0–20 cm the mean methane pore water concentrations dropped back to <20 μM with an almost linear decrease between 0 and 15 cm. Oxygen pore water concentrations were close to air saturation in the first few centimeters and dropped back below detection limit at a depth of 20 cm. In July and August the pore water concentrations of dissolved organic carbon (DOC) were in the range of 7.2–10.1 mg C l−1 at all depths. The pore water concentrations of acetate, formate and oxalate were in the range of 2.0–8.2 μM at all depths. Methanotrophic and methanogenic communities were quantified using pmoA and mcrA, respectively, as marker genes. The abundances of both communities showed a distinct peak at a depth of 10–15 cm below the vegetation surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the context of the current debate on global change, methane emissions from peatlands and their impact on the earth’s climate system are of great interest. These emissions result from the typically anaerobic carbon turnover and methane production in natural peatlands. More than 80% of the earth’s peatland area is located in temperate-cold climates in the northern hemisphere, particularly in Russia, Canada, Scandinavia and the United States (Harriss et al. 1985; Limpens et al. 2008). Northern peatlands store between 370 and 455 Gt of carbon (Gorham 1991; Turunen et al. 2002). Methane release from these peatlands is highly variable seasonally and at spatial scales ranging from microtopographic to regional (e.g., Bartlett et al. 1992; Morrissey and Livingston 1992; Shannon and White 1994; Bubier et al. 1995; Christensen et al. 1995; Moore and Roulet 1991; Bellisario et al. 1999; Joabsson and Christensen 2001; Kutzbach et al. 2004; Whalen 2005; Strack and Waddington 2008).
To comprehend variations in methane emissions from peatland ecosystems and to predict their response to climate change, the intimate linkage between carbon cycling and methane turnover needs to be understood. As stated by the recent IPCC report (2007), the major contributors within the atmospheric methane budget and basic processes of methane production, consumption and transport in wetlands have been well described before. The quantification of these contributors, however, is often very difficult because turnover and release rates are highly variable (Saarnio et al. 2009). Data on methane emissions from European main wetland ecosystem types are scarce (Saarnio et al. 2009). To our knowledge only one study dealt with methane emissions from alpine fens in Europe so far (Koch et al. 2007). However, that report did not consider methane concentration profiles in the subsurface. More comprehensive studies were conducted in the Rocky Mountains (Neff et al. 1994; Mast et al. 1998; Wickland et al. 1999; West et al. 1999; Chimner and Cooper 2003) in Alaska (Sebacher et al. 1986) and on the Tibetan Plateau (Hirota et al. 2004; Cao et al. 2008; Chen et al. 2008).
The specific goal of our study was to provide a first dataset of above-ground methane fluxes combined with pore water methane concentration profiles from a fen in the central Alps of Switzerland. Additionally, the study site was characterized with respect to vertical variations of soil temperature, pH, conductivity, DOC, and the abundance of methane-producing (methanogenic) archaea and methane-oxidizing (methanotrophic) bacteria.
Materials and methods
Study site
The study site (46°38′58″N, 8°29′40″E, 1,915 m a.s.l.) is located in the central Alps of Switzerland near Goeschenen in an alpine wetland complex (Fig. 1) with a total surface area of about 170 ha (FOEN: Federal Office for the Environment 2009). The underlying crystalline bedrock is granite and belongs to the autochthonous geological formation of the “Aarmassiv—Zentraler Aaregranit”. The mean annual air temperature in the period November 2008 to November 2009 was 3.3 (±7.2)°C with maximum values in August 2009 (absolute: 21.9°C, mean: 13.1°C) and minimum values in February 2009 (absolute: −16°C, mean: −5.5°C) (meteorological station “Grimsel-Hospiz”, ANETZ-Stations of the Federal Office of Meteorology and Climatology MeteoSwiss 2008, 2009; 46°34′18″N, 8°19′59.5″E, 1,980 m a.s.l.). A mean annual liquid precipitation of approximately 1,800 mm was recorded. Further description of the valley and its habitats can be found on the FOEN webpage and in related studies within the same area (Miniaci et al. 2007; Duc et al. 2009).
According to Rydin and Jeglum (2006), this alpine wetland complex consists of ombrotrophic to minerotrophic open bogs and intermediate open fens interspersed with small ponds. Our study site had a size of approximately 50 m2 within this wetland area (Fig. 1). The study site itself is an acidic, nutrient-poor to mesotrophic fen, comprising an area of 15.9 ha (Rydin and Jeglum 2006; Joosten 2008; FOEN 2009). The mean C/N value of bulk soil of the study site was 39 (±5) within the first 30 cm of peat soil with an average pH of 4.7 (see below). The vascular plant vegetation was dominated by species of the Cyperaceae family: Carex limosa, Carex rostrata and Trichophorum caespitosum. C. limosa and C. rostrata covered between 30 and 50% of the surface area. The mosses Sphagnum sp. and Jungermanniales sp. formed mats on the surface of the peatland.
Field work and sampling
On the study site, four GPS-reference points (46°38′58.34″N/8°29′39.98″E, 46°38′58.18″N/8°29′40.03″E, 46°38′58.28″N/8°29′39.37″E, 46°38′58.73″N/8°29′40.13″E) were marked with poles. This allowed for precise allocation of all the sampling spots. Within this area, one permanent sampling spot was set up to conduct profile and soil temperature measurements. This spot will be denoted as “main profile spot” throughout this paper. A core was sampled three meters away from the main profile spot. Static flux chamber measurements were conducted within a radius of approximately 10 m around the main profile spot.
The field measurements were performed on Oct 20, 2008; Nov 11, 2008; May 9, 2009; Jun 2, 2009; Jul 7, 2009; Aug 24, 2009 and Sep 30, 2009. During the winter season, the region could not be accessed due to the danger of avalanches. According to WSL Institute for Snow and Avalanche Research SLF (http://www.slf.ch/english_EN, MDW database export Bergsee, 2009) the snow cover period ranged from end of October 2008 until end of May 2009, with a mean snow height of 63.5 (±17.6) cm. Snowmelt took place between May 25 and 27, 2009. On Oct 20, 2008; Nov 11, 2008 and May 9, 2009 the vegetation was partially died off, and/or the soil was frozen and snow covered. On these 3 days we were only able to take a few sporadic measurements on the methane fluxes and the pore water chemistry. These data are statistically not relevant and are therefore not shown in detail throughout the paper. If not snow covered, the study site was water saturated, i.e., that water table was 0.75–2 cm above the moss surface.
Gas analysis
Analysis of methane concentrations was performed by gas chromatography (Thermo Electron Corporation, Trace GC Ultra) using a Porapak N column (material: 100/120 mesh, column length 2 m, diameter 4 1/16″, 1 mm) and a flame ionization detector (FID). The column and detector temperatures were set to 30 and 250°C, respectively. Nitrogen served as carrier gas with a flow-velocity of 30 ml min−1. Peak areas were calibrated with Messer CAN-Gas standards (methane in synthetic air 5.0, Messer Schweiz AG) and integrated with the software Chromeleon (Vers. 6.8, Dionex). Calibration was performed over a concentration range from 1 to 10,000 ppm, using ten calibration points at concentrations of 1, 2, 5, 10, 50, 100, 500, 1,000, 4,000 and 10,000 ppm. Gas was sampled directly from the headspace of the pore water sampling vials (serum vials, see below) using an auto sampler with a 1 ml glass syringe (CTC Analytics, HTX PAL, 1 ml MSH 01-00B).
Pore water sampling and methane concentration analysis
Profiles for pore water methane concentrations were obtained at the main profile spot by extracting small samples of pore water with a series of brass tubes (3 mm inner diameter). The tubes were sealed at one end and perforated within the first 10 mm from the base (side ports). The tubes were vertically inserted into the fen at 5–10 cm intervals and connected to three-way valves (Discofix C-3, Braun Melsungen AG, Germany) by Tygon tubing (2.79 mm inner diameter, Ismatec). Pore water was sampled with 10 ml plastic syringes avoiding exposure to oxygen during sampling by sucking the pore water into the tubes, closing the three-way valves, and holding the water within the sampling device. Depending on the dead volume of the tubes, 2.5, 5 or 7 ml of extracted soil water were initially discarded to flush the tubes. Subsequently, 5 ml soil water was sampled and immediately transferred to gas tight 20 ml glass serum vials pre-flushed with nitrogen (N2) (Frenzel and Karofeld 2000; Joabsson and Christensen 2001) and pretreated with 0.1 ml 1 M HCl. In this N2 atmosphere, water samples were stored at 4°C, and headspace methane concentrations were measured by gas chromatography as stated above.
The dissolved methane concentration in the soil pore water was calculated from (i) the measured and pressure corrected methane concentration in the headspace of the sampling vials (serum vials), (ii) the volumes of headspace and water phase in the serum vial and (iii) Henry’s Law, using a headspace method slightly altered from Bossard and Joller (1981). Temperature corrected Henry’s Law constants were taken from Sander (1999) and compared against values from Stumm and Morgan (1981) and values from Perry’s Chemical Engineers’ Handbook 7th Edition (Perry and Green 1997).
In situ oxygen and temperature profiling with optical sensors (Optode)
Oxygen depth-profiles were measured based on the quenching of light in the presence of O2 using a Fibox 3-trace v3 planar trace oxygen minisensor (Fa. Presens, Regensburg, Germany). Oxygen measurements were compensated for temperature. The sensor was inserted into a rod equipped with a hollow tip. The rod was pushed into the soil. When equilibrated to ambient conditions, oxygen concentrations and temperature were logged. Alternatively, oxygen sensitive spots were attached to the end of carbon rods (1,000 mm × 8.0 mmø × 6.0 mmø) that were permanently installed. A SMA-Plug 2 mm glass fiber wire channeling light (excitation wavelength 505 nm) to the spot was attached. Throughout this paper the oxygen concentration is expressed as “% air saturation”. A value of 100% refers to the concentration of oxygen in the water after equilibration with ambient air.
Determination of methane emission using static flux chambers
Methane fluxes across the soil–atmosphere interface were measured with static transparent chambers (30 cm × 30 cm × 30 cm) modified from Moore and Roulet (1991). At the top, the chambers were fitted with butyl rubber stoppers serving to equilibrate pressure and to provide sampling ports. One static flux chamber was always positioned right next to the main profile spot where pore water methane, 11 oxygen and temperature profiles were determined. The other chambers were distributed around the main profile spot within a radius of 10 m. Chambers were gently placed into standing water that made a seal. Effective chamber volume was calculated from the average height of the surface to the top of the chamber. Portable walkways and styrofoam collars on the chambers sidewalls were used to minimize disturbance during the measurements.
Using a 50 ml luer-lok syringe (BD Plastipak, Becton-Dickinson S.A., Madrid, Spain), 40 ml of gas was sampled from the chamber headspace after pumping the piston several times (Bubier et al. 1993, 1995). The gas sample was injected into 20 ml glass vials previously flushed with N2 and evacuated. Methane concentrations in the vials were analyzed by gas chromatography as stated above. One flux measurement consisted of 4–6 time points sampled in intervals of 5–20 min. Methane fluxes were calculated from changes of methane concentrations with time in the chamber headspace using linear regression analysis (Van der Nat and Middleburg 1998; Ding et al. 2005). We verified the choice of a linear model by comparing it to an exponential model with respect to the Akaike information criterion following the method by Kutzbach et al. (2007) adapted for methane flux calculation by Forbrich et al. (2010).
High resolution, seasonal temperature measurements
Annual vertical temperature profiles of the fen were obtained down to a depth of 55 cm using iButton® temperature loggers (DS 1922L#F50, 8kB datalog memory, Dallas Semiconductor MAXIM). The iButtons® were coated in waterproof silicone enclosures (SL50-ACC06, Signatrol.com, Data Logging Solutions) and discharged into wells, which were drilled in a wooden pole (1,000 mm, 35 mm ø) every 10 cm. Temperature sampling was conducted continuously in the time period between Oct 20, 2008 and Sep 30, 2009 in intervals of 4 h.
Pore water chemistry
DOC and organic acids
Pore water samples for dissolved organic carbon (DOC) and organic acid analysis were collected every 5–10 cm with the same brass tube probes used for pore water methane sampling. 20 and 10 ml of pore water were withdrawn for DOC and organic acid samples, respectively, using a 50 ml luer-lok syringe (BD Plastipak, Becton-Dickinson S.A., Madrid, Spain). Samples were immediately filtered in the field with 0.45 μm nylon filters (Wicom Perfect Flow, Nyl 0.45 μm) and stored at −20°C in the dark. Prior to sampling, the filters were washed with deionized water to avoid contamination through extractable residues of organic acids and anions. The glass vials used for DOC sampling were acidified with 0.1 ml of a 1 M HCl solution (King et al. 1998). To sterilize the organic acid samples, 0.2 ml of a 1 M NaOH solution was added to the Falcon Tubes which were used as vessels. DOC samples were analyzed by a Shimadzu TOC-5000 analyzer equipped with a Shimadzu auto sampler ASI-5000 A. The measurement of organic acids was performed by a Dionex DX320 ion-chromatograph equipped with an OH− eluent gradient generator EG40 (Dionex, Sunnvalley CA).
Conductivity and pH
Vertical profiles of conductivity and pH were analyzed in situ with the multi parameter probe Multi 350i from WTW (Laboratory and Field Products, Nova Analytics). The conductivity cell LR 325/01 and the pH electrode SenTix 51 were used for measurements. Pore water extraction was carried out as described above using slightly thicker stainless steel tubes (1,000 mm, 5 mm ø) and a flow-through vessel allowing for a constant flow of pore water along the sensors.
Core sampling, treatment and analysis
A core was sampled on Nov 11, 2008 three meters away from the “main profile spot” using a sharp-edged cylinder. The core had a length of ~35 cm (slightly compressed because of sampling) and a diameter of ~12 cm and was stored for 10 weeks at −20°C prior to microbial analysis. The core was thawed at room temperature and the top 5 cm consisting of moss were removed due to disturbance during sampling. The core was sectioned in 2 cm slices with sterile saw and knifes and borders were removed. The remaining part was thoroughly homogenized manually. About 10 g of soil of each section were used for the determination of water content, total carbon and total nitrogen. The remaining soil was aliquoted into 6 × 10 ml Falcon tubes for quantification of total, methanotrophic and methanogenic cell numbers (DAPI and qPCR as stated below). All samples were stored at −20°C.
Content of water, total carbon and nitrogen
For determination of the water content of the core, 10 g of each core section were put in a petri dish and dried at 57°C for 48 h in total. Weight was determined after 24 and 48 h to check for remaining water loss. Dried soil of each depth was ground in an agate rings mill (Retsch RS1, Haan, Germany) for 30 s at 700 U/min. 0.5 g of soil was then analyzed for total carbon and nitrogen with an automated dry combustion instrument LECO CNS 2000 (Mönchengladbach, Germany).
DAPI staining and determination of total microbial cell counts (TCC)
Of each homogenized core section, 0.5 g of wet soil was fixed in a 4% paraformaldehyde (PFA) solution for 3 h at 4°C according to Pernthaler et al. (2001). Samples were diluted (1:1 and 1:5) in 0.1% natriumpyrophosphate (NaPiP) and subsequently sonicated (Ultrasonic Cleaner, VWR) for 20 s at low intensity. 10 μl of each dilution was transferred to diagnostic slides (Thermo Scientific, Braunschweig, Germany) in duplicates and dried at 37°C. After washing in an ethanol series (3 min in 50, 80 and 96%), the slides were dried at room temperature and each well was provided with 10 μl of DAPI (20 μg ml−1) and incubated at 4°C for 10 min. Total cell counts were determined using a UV-microscope (Carl Zeiss) equipped with a color chilled 3CCD camera (Hama-matsu) and using a 100× magnification objective. A minimum of 800 cells per soil depth were counted. The numbers of cells per gram of dry soil were calculated by
where TC is the total cell number counted per g dry soil, CC is the cells counted, M is the microscopy factor (area of applied sample/area of field of sight), D is the dilution factor and W is the mass of wet sample.
DNA extraction and quantification (SYBR-Green assay)
Total genomic DNA was extracted with the PowerSoil™ DNA Isolation Kit (MoBio Laboratories, USA) following the manufacturer’s protocol. The size of genomic DNA was checked by electrophoresis on a 1% agarose gel with SYBR®Safe (Invitrogen) staining. DNA concentrations were determined through a SYBR-Green assay based fluorescence intensity relative to a standard dilution series.
Quantitative (real-time) PCR
Quantitative PCR (qPCR) was applied to determine the vertical abundance of methanotrophic bacteria and of methanogenic archaea within the core. In particular, copy numbers of the pmoA and mcrA genes were determined. These genes are functional marker genes for methanotrophs and methanogens, respectively (refer to review by McDonald et al. (2008) for pmoA and to review by Juottonen et al. (2006) for mcrA gene).
SybrGreen based qPCR assays were run on a real-time polymerase chain reaction system (Typ 7300, Applied Biosystems, Foster City, CA). Briefly, real-time PCR was performed in 20 μl reactions in PCR microplates (PCR-96-FLT-C; Axygen, California, USA) sealed with ABsolute qPCR Seal (Thermo scientific; Surrey, UK). Template dilutions (1 μl, 0.5–50 ng) were added to 19 μl of master mix containing 8.52 μl DEPC treated water (Invitrogen), 10 μl KAPA SYBR® FAST qPCR Master Mix (2×) ABI Prism™ (KAPA Biosystems), 0.04 μl of each primer (final concentration 200 nM) and 0.4 μl BSA (final concentration 1 mg ml−1).
PCR conditions, primer details, reference strains and qPCR efficiencies are summarized in Table 1. Each qPCR run included calibration standards and blanks and was performed in triplicates.
Results
Profiles of temperature and pore water concentrations of methane and oxygen
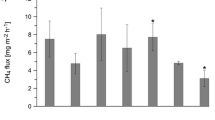
In June, July, August and September 2009 the methane pore water concentrations displayed a steep gradient between 0 and 20 cm on every sampling date with decreasing concentrations towards the vegetation surface (Fig. 2, left panel). In the uppermost 15 cm, pore water methane concentrations decreased almost linear (r 2 = 0.95 ± 0.05) with a mean slope of 22 (±20) μM cm−1. Below 20 cm soil depth, methane pore water concentrations at a given date varied little, covering value ranges of approximately 144 (±10) − 233 (±7) μM in July, August and September and of 526 (±32) μM in June after snowmelt. An integration over depth revealed that the total pore water methane contents were by far higher in June than in July, August and September (Fig. 3, upper panel).
Oxygen concentrations (% air saturation) in the water saturated profile typically decreased to zero within the first 10–20 cm below the vegetation surface (Fig. 2, right panel). Oxygen profiles varied little except in August 2009 when oxygen already got depleted within the uppermost 5 cm. In June, August and September surface temperatures were between 15 and 25°C. They decreased with depth and reached values in the range of 10–16 at −40 cm.
Methane emissions into the atmosphere
Net average daily fluxes of methane across the vegetation–atmosphere boundary decreased from 313 (±71) mg CH4 m−2 day−1 measured in June to 125 (±26) mg CH4 m−2 day−1 in September (Fig. 3). Methane fluxes in July and August were 258 (±127) and 220 (±71) mg CH4 m−2 day−1, respectively. The high methane fluxes measured in June coincided with high subsurface concentrations of pore water methane (Fig. 2).
Seasonal soil temperature dynamics
The vertical annual temperature series indicates that monthly mean temperatures of the soil below 5 cm were always positive. Only the upper few centimeters were temporarily frozen during the period with ice and snow cover (end of October 2008 until end of May 2009). Temperature variations among seasons and monthly extremes increased markedly towards the vegetation surface (Fig. 4). Pronounced fluctuations in soil temperature were not observed during winter, most likely because of the insulating properties of snow and the great heat capacity of the water-saturated peat.
During summer (June–August 2009), the uppermost 5 cm of the fen experienced temperature maxima of approximately 30°C on single days. Mean temperature values for this period at 5 cm soil depth varied between 13.8 (±6.4) in June, 15.5 (±4.4) in July and 16.0 (±3.8)°C in August. At a soil depth of 15 cm the corresponding values varied between 12.4 (±2.2) in June, 14.4 (±1.8) in July and 15.5 (±1.5)°C in August (Fig. 4).
Pore water chemistry
Chemical pore water analysis was performed on samples from June, July, August, and September, 2009. A summary of the measured DOC, pH, conductivity, and concentrations of organic acids is given in Tables 2 and 3.
The concentrations of pore water DOC ranged from 6.1–10.6 mg C l−1. Pore water pH and conductivity values varied little throughout the sampling period. Lowest pH values of 4.5 were measured in September 2009, and highest values of 4.9 were detected in July 2009. Conductivity was lowest in June 2009 with a value of 11.2 μS cm−1 and highest in September 2009 with 17.3 μS cm−1. A vertical gradient of pore water DOC, pH, and conductivity values could not be observed.
Concentrations of the organic acids acetate, formate, and oxalate were determined on two sampling days (Jul 7, 2009 and Aug 24, 2009). Their concentrations were close to the detection limit, ranging from 2.0 to 8.2 μM and varied little vertically and between the sampling dates.
Core characteristics
Water content, total carbon and total nitrogen
Consistent to the conditions in the field, the water content of the sampled soil core was very high increasing slightly from 88% between 0 and 15 cm to 91% between 15 and 30 cm. Complete water saturation as occurring in the field was not observed in the core likely due to a loss of water during sampling.Total carbon (expressed as % of dry weight) ranged from 42.4 (±5.5)% (0–15 cm soil depth) to 46 (±0.5)% (15–30 cm soil depth). Total nitrogen in the same depths ranged from 1.4 (±0.3)% to 1.1 (±0.2)%, respectively.
Total microbial cell counts (TCC), methanotrophic, and methanogenic abundance
TCC numbers per gram of dry soil obtained through DAPI staining and fluorescence microscopy comprised 1.6 (±1.0) × 108 between 0 and 15 cm and 2.1 (±0.8) × 108 between 15 and 30 cm soil depth. Maximum abundance of both methanotrophic bacteria and methanogenic archea were found in depths between 10 and 15 cm. Abundance of both microbial communities decreased towards the vegetation surface and towards deeper soil layers. Copy numbers of the pmoA gene, representing the abundance of methanotrophic bacteria, were generally more than an order of magnitude higher (up to 5 × 105 copies per nanogram of DNA) than those of the mcrA gene, representing methanogenic archaea (Fig. 5).
Discussion
Subsurface temperatures
The alpine fen peat studied here was at an altitude of 1,915 m a.s.l., and the measurements reported were done between October 2008 and September 2009. The coldest month in the study period was February 2009 (mean air temperature around −5.5°C) and even then the monthly mean soil temperatures of the fen were slightly above the freezing point (0.1°C at −5 cm and 1.6°C at −35 cm). The site was covered with snow from end of October 2008 to end of May 2009 and the mean snow height was around 63 cm. Surface air and soil temperatures fell below 0°C at Oct 28, 2008, when the snow-cover was only a few centimeters thick. The onset of freezing, however, overlapped with heavy snowfall. Taras et al. (2002) estimated that a decoupling of soil temperature from air temperature in Arctic Alaska requires snow depths of >80 cm while soil and air temperatures are strongly coupled at snow depth of <25 cm. These authors calculated an increase of soil temperature at the snow–soil interface by 0.5–3°C coinciding with 15 cm increase in snow depth. Considering that the subsurface temperatures at our study site were above the freezing point even in winter, the fen most likely remained microbiologically active all year round. Year-round soil temperatures above the freezing point as a result of the insulation through snow were also reported by Mast et al. (1998) for a subalpine wetland in the Rocky Mountains at 3,200 m a.s.l. and by Koch et al. (2007) for an alpine site at 2,250 m a.s.l. in Austria.
Methane fluxes and profiles
The alpine fen is representative for an acidic, nutrient-poor to mesotrophic alpine wetland dominated by Carex species. The methane emissions obtained through static chambers during the snow-free period (~125–313 mg CH4 m−2 day−1) are in the range of what was observed for fens of the Tibetan Plateau (Hirota et al. 2004; Chen et al. 2008) and of the Rocky Mountains (Sebacher et al. 1986; Wickland et al. 1999; Chimner and Cooper 2003) and were similar to methane emissions from wet Arctic tundra during summer (Sebacher et al. 1986; Whalen and Reeburgh 1988, 1990; Morrissey and Livingston 1992; Bartlett et al. 1992; Whalen and Reeburgh 1992; Christensen 1993; Shannon and White 1994; Christensen et al. 1995, Shannon et al. 1996, Sachs et al. 2010). The emissions were slightly higher than those observed by Koch et al. (2007) for alpine fens from Europe. In their study, maximum methane emissions were below 150 mg CH4 m−2 day−1 and average methane emissions in the snow-free period were in the range of 19–116 mg CH4 m−2 day−1.
In our study, the mean pore water concentrations of methane at depths below 20 cm were above 500 μM in June 2009, around 250 μM in July and around 150 μM in August and September 2009. Bartlett et al. (1992) and Knoblauch et al. (2008) reported pore water concentrations of methane in the range of 250–500 μM at permafrost-affected sites in Alaska (Yukon Delta and Toolik Lake) and Siberia (Lena Delta). Pore water methane concentrations reported by Koch et al. (2007) for fens in the Eastern Alps peaked around 90 μM (at −20 cm). The soil temperatures were comparable. In the snow free season at a depth of 35 cm Koch et al. (2007) measured temperatures in the range of 8–10°C whereas we recorded values in the range of 9°C (June)–13°C (August). At our site an integration of the methane pore water concentration over depths shows that the total amount of pore water methane in the subsurface is by far higher in early June (note that snowmelt was end of May) than in July, August and September. In early June, also methane emissions were highest. This trend is consistent with studies from subarctic and alpine lakes and mires (Hargreaves et al. 2001; Huttunen et al. 2003; Koch et al. 2007) where sediments and soil, respectively, remained unfrozen also during winter. However, this trend is different from what is observed in permafrost-affected tundra where highest methane emissions are mostly observed in summer (e.g., Whalen and Reeburgh 1992; Sachs et al. 2010) or in autumn during the onset of freezing (Mastepanov et al. 2008). Our calculations on the soil methane concentrations, however, have to be interpreted with great care. First, we do not have enough replicas to allow for statistically significant conclusions on the methane emissions and pore water profiles. Second, the profiles only consider the methane pore water concentrations. Although these concentrations are far below the solubility limit of methane (i.e., about 2,000 μM at 10°C), we cannot exclude that some methane might be entrapped as bubbles in the rhizosphere. Strack and Waddington (2008) estimated that more than 50% of the subsurface methane stock can be entrapped in bubbles. Third, we have no detailed data on the development of the vegetation from June to September and therefore we cannot assess of whether the plant mediated transport of methane has an impact on the shape of the profiles and the amount of methane in the subsurface. However, since we observed a near linear decrease of pore water methane concentrations between 0 and 15 cm, we determined the diffusive flux of methane towards the vegetation surface applying Fick’s first law of diffusion neglecting porosity and tortuosity. Based on this, the diffusive methane flux towards the vegetation surface only accounts for about 2% of the methane flux into the atmosphere (4.56 vs. 230 mg CH4 m−2 day−1).
As mentioned above, the study site was snow-covered from late October 2008 to late May 2009, and accessibility was very limited due to avalanches. Methane emissions and the methane pore water concentrations during the snow-covered period were only measured on Oct 20, 2008; Nov 11, 2008 and May 9, 2009 (data not shown). However, there were no replicas and the data have to be interpreted with great care. The methane emissions were below 70 mg CH4 m−2 day−1 on all three sampling dates also at sites where the snow had been removed. The winter fluxes were thus low compared to the snow-free period which was probably due to the die-off and dormancy of the vascular plants. The profiles of the methane pore water concentrations on Oct 20, 2008 and Nov 11, 2008 were similar to the ones on Aug 24, 2009 and Sep 30, 2009 presented in Fig. 2. However, the profile on May 9 revealed methane pore water concentrations far beyond 750 μM. This may indicate an accumulation of methane below the frozen surface which may still be evident on Jun 2, 2009 (Fig. 2) a few days after snowmelt.
Methanogenic and methanotrophic abundance
By enumerating the pmoA and mcrA copy numbers in a core from the fen, we observed that the methanotrophic and methanogenic communities spatially overlap along the entire depth of the core (0–30 cm). This supports related studies reporting potential methane oxidation and production along the rhizosphere of aerenchymatous plants (Frenzel 2000; Popp et al. 2000; Conrad 2004; Wagner et al. 2005). In sediments and in wetlands absent of aerenchymatous plants, methane and oxygen diffuse conversely, and consequently methanotrophic and methanogenic activity is observed at the aerobic/anaerobic sediment or soil interface (Frenzel et al. 1992; Rahalkar et al. 2009). Aerenchymatous vascular plants, however, transport oxygen vital for methanotrophic activity from the atmosphere to the rhizosphere. The surface of plant roots can thus be inhabited by methanotrophic bacteria (King 1994, Gilbert et al. 1998). In turn, methanogens feed on root exudates such as organic acids and are assumed to colonize anaerobic niches along the rhizosphere (Conrad 2004 and papers within). Although we found an overlap of methanotrophic and methanogenic abundance, we also observed a distinct peak of the pmoA and mcrA copy numbers at a depth of 10–15 cm. Popp et al. (2000) could show that (living) root mass as well as methanotrophic and methanogenic activity usually peak within the uppermost 10–15 cm in sites vegetated with Carex species.
Pore water concentrations of organic acids and DOC
In the present study, the organic acids acetate, formate, and oxalate were identified in pore water samples taken in July and August 2009. These acids are direct and indirect precursors for methanogenesis. Very few studies reported pore water values for acetate in wetlands. Those values were in the range of 6–73 μM (Stroem et al. 2005; Whitmire and Hamilton 2008) thus higher than the values reported here for July and August 2009 (below 5 μM). Formate concentrations (<10 μM) were low as well compared to a temperate acidic peatland with values partly exceeding 480 μM below 20 cm soil depth (Kusel et al. 2008). This might indicate high turnover rates of acetate and formate in summer. Pore water concentrations of DOC were in the range of 6.1–10.6 mg C l−1. Values reported for arctic permafrost-affected tundra were below 20 mg C l−1 (King et al. 1998; Wagner et al. 2003). It has to be noted, however, that the values for organic acids and DOC given in Table 2 represent steady state concentrations. The turnover may be very fast and steady state concentrations do not allow assessing the rate and the amount of methane production in the subsurface. Isotopic analyses showed that the methane produced in Carex spp. dominated areas was derived from recent plant compounds rather than the breakdown of older soil organic matter (Chanton et al. 1992, 1995; Bellisario et al. 1999).
References
Babic KH, Schauss K, Hai B, Sikora S, Redžepovic S, Radl V, Schloter M (2008) Influence of different Sinorhizobium meliloti inpcula on abundance of genes involved in nitrogen transformations in the rhizosphere of alfalfa (Medicago sativa L.). Environ Microbiol 10:2922–2930
Bartlett KB, Crill PM, Sass RL, Harriss RC, Dise NB (1992) Methane emissions from tundra environments in the Yukon-Kuskokwim delta, Alaska. J Geophys Res 97:16,645–16,660
Bellisario LM, Bubier JL, Moore TR, Chanton JP (1999) Controls on CH4 emissions from a northern peatland. Glob Biogeochem Cycles 13:81–91
Bossard P, Joller T (1981) Die quantitative Erfassung von Methan im Seewasser. Schweizerische Zeitung Hydrologie 43:200–211
Bubier JL, Moore TR, Roulet NT (1993) Methane emissions from wetlands in the mid-boreal region of northern Ontario, Canada. Ecology 74:2240–2254
Bubier JL, Moore TR, Juggins S (1995) Predicting methane emission from bryophyte distribution in northern Canadian peatlands. Ecology 76:677–693
Cao G, Xu X, Long R, Wang Q, Wang C, Du Y, Zhao X (2008) Methane emissions by alpine plant communities in the Qinghai-Tibet Plateau. Biol Lett 4:681–684
Chanton JP, Whiting GJ, Showers WJ, Crill PM (1992) Methane flux from Peltandra virginica: stable isotope tracing and chamber effects. Glob Biogeochem Cycles 6:15–31
Chanton JP, Bauer JE, Glaser PA, Siegel DI, Kelley CA, Tyler SC, Romanowicz EH, Lazrus A (1995) Radiocarbon evidence for the substrates supporting methane formation within northern Minnesota peatlands. Geochim Cosmochim Acta 59:3663–3668
Chen H, Yao S, Wu N, Wang Y, Luo P, Tian J, Gao Y, Sun G (2008) Determinants influencing variations of methane emissions from alpine wetlands in Zoige Plateau and their implications. J Geophys Res 113:D12303
Chimner RA, Cooper DJ (2003) Carbon dynamics of pristine and hydrologically modified fens in the southern Rocky Mountains. Can J Bot 81:477–491
Christensen TR (1993) Methane emission from Arctic tundra. Biogeochemistry 21:117–139
Christensen TR, Jonasson S, Callaghan TV, Havström M (1995) Spatial variation in high-latitude methane flux along a transect across Siberian and European tundra environments. J Geophys Res 100: 21,035–21,045
Conrad R (2004) Methanogenic microbial communities associated with aquatic plants. In: Varma A, Abbott L, Werner D, Hampp R (eds) Plant surface microbiology. Springer-Verlag, Berlin, pp 35–50
Ding W, Cai Z, Tsuruta H (2005) Plant species effects on methane emissions from freshwater marshes. Atmos Environ 39:3199–3207
Duc L, Noll M, Meier BE (2009) High diversity of diazotrophs in the forefield of a receding alpine glacier. Microb Ecol 57:179–190
FOEN (2009) Federal Office for the Environment. http://www.bafu.admin.ch/index.html?lang=en. Accessed Nov 2009
Forbrich I, Kutzbach L, Hormann A, Wilmking M (2010) A comparison of linear and exponential regression for estimating diffusive CH4 fluxes by closed-chambers in peatlands. Soil Biol Biochem 42:507–515
Frenzel P (2000) Plant-associated methane oxidation in rice fields and wetlands [review]. Adv Microb Ecol 16:85–114
Frenzel P, Karofeld E (2000) CH4 emission from a hollow-ridge complex in a raised bog: the role of CH4 production and oxidation. Biogeochemistry 51:91–112
Frenzel P, Rothfuss F, Conrad R (1992) Oxygen profiles and methane turnover in a flooded rice microcosm. Biol Fertil Soils 14:84–89
Gilbert B, Assmus B, Hartmann A, Frenzel P (1998) In situ localization of two methanotrophic strains in the rhizosphere of rice plants. FEMS Microbiol Ecol 25:117–128
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–195
Hales BA, Edwards C, Ritchie DA, Hall G, Pickup RW, Saunders JR (1996) Isolation and identification of methanogen-specific DNA from blanket bog peat by PCR amplification and sequence analysis. Appl Environ Microbiol 62:668–675
Hargreaves KJ, Fowler D, Pitcairn CER, Aurela M (2001) Annual methane emission from Finnish mires estimated from eddy covariance campaign measurements. Theor Appl Climatol 70:201–213
Harriss RC, Gorham E, Sebacher DI, Bartlett KB, Flebbe PA (1985) Methane flux from northern peatlands. Nature 315:652–654
Hirota M, Tang Y, Hu Q, Hirata S, Kato T, Mo W, Cao G, Mariko S (2004) Methane emissions from different vegetation zones in a Qinghai-Tibetan Plateau wetland. Soil Biol Biochem 36:737–748
Holmes AJ, Costello AM, Lidstrøm ME, Murrell JC (1995) Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett 132:203–208
Huttunen JT, Alm J, Saarijaervi E, Lappalainen JS, Martikainen PJ (2003) Contribution of winter to the annual CH4 emission from a eutrophied boreal lake. Chemosphere 50:247–250
IPCC (2007) Climate change 2007: contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, the physical basis of climate change. http://www.ipcc-wg2.org/index.html
Joabsson A, Christensen TR (2001) Methane emissions from wetlands and their relationship with vascular plants: an Arctic example. Glob Change Biol 7:919–932
Joosten H (2008) What are peatlands? In: Parish F, Sirin A, Charman D, Joosten H, Minayeva T, Silvius M, Stringer L (eds) Assessment on peatlands, biodiversity and climate change: main report. Global Environmental Center, Kuala Lumpur and Wetlands International, Wageningen
Juottonen H, Galand PE, Yrjala K (2006) Detection of methanogenic Archaea in peat: comparison of PCR primers targeting the mcrA gene. Res Microbiol 157:914–921
King GM (1994) Associations of methanotrophs with the roots and rhizomes of aquatic vegetation. Appl Environ Microbiol 60:3220–3227
King JY, Reeburgh WS, Regli SK (1998) Methane emission and transport by arctic sedges in Alaska: results of a vegetation removal experiment. J Geophys Res 103:29,083–29,092
Knoblauch C, Zimmermann U, Blumenberg M, Michaelis W, Pfeiffer EM (2008) Methane turnover and temperature response of methane-oxidising bacteria in permafrost-affected soils of northeast Siberia. Soil Biol Biochem 40:3004–3013
Koch O, Tscherko D, Kandeler E (2007) Seasonal and diurnal net methane emissions from organic soils of the Eastern Alps: effects of soil temperature, water balance, and plant biomass. Arct Antarct Alp Res 39:438–448
Kusel K, Blothe M, Schulz D, Reiche M, Drake HK (2008) Microbial reduction of iron and porewater biogeochemistry in acidic peatlands. Biogeosciences 5:1537–1549
Kutzbach L, Wagner D, Pfeiffer EM (2004) Effect of microrelief and vegetation on methane emission from wet polygonal tundra, Lena Delta, Northern Siberia. Biogeochemistry 69:341–362
Kutzbach L, Schneider J, Sachs T, Giebels M, Nykänen H, Shurpali NJ, Martikainen PJ, Alm J, Wilmking M (2007) CO2 flux determination by closed-chamber methods can be seriously biased by inappropriate application of linear regression. Biogeosciences 4:1005–1025
Limpens J, Berendse F, Blodau C, Canadell JG, Freeman C, Holden J, Roulet N, Rydin H, Schaepman-Strub G (2008) Peatlands and the carbon cycle: from local processes to global implications—a synthesis. Biogeosciences 5:1475–1491
Mast MA, Wickland KP, Striegl RT, Clow DW (1998) Winter fluxes of CO2 and CH4 from subalpine soils in Rocky Mountain National Park, Colorado. Glob Biogeochem Cycles 12:607–620
Mastepanov M, Sigsgaard C, Dlugokencky EJ, Houweling S, Stroem L, Tamstorf MP, Christensen TR (2008) Large tundra methane burst during onset of freezing. Nature 456:628–630
McDonald IR, Bodrossy L, Chen Y, Murrell JC (2008) Molecular ecology techniques for the study of aerobic methanotrophs. Appl Environ Microbiol 74:1305–1315
Miniaci C, Bunge M, Duc L (2007) Effects of pioneering plants on microbial structures and functions in a glacier forefield. Biol Fertil Soils 44:289–297
Moore TR, Roulet NT (1991) A comparison of dynamic and static chambers for methane emission measurements from subarctic fens. Atmos Ocean 29(1):102–109
Morrissey LA, Livingston GP (1992) Methane emissions from Alaska Arctic tundra: an assessment of local spatial variability. J Geophys Res 97:16,661–16,670
Neff JC, Bowman WD, Holland EA, Fisk MC, Schmidt SK (1994) Fluxes of nitrous oxide and methane from nitrogen-amended soils in a Colorado alpine ecosystem. Bioegochemistry 27:23–33
Pernthaler J, Gloeckner FO, Schoenhuber W, Amann R (2001) Fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. Meth Microbiol Mar Microbiol 30:207–226
Perry RH, Green DW (1997) Perry’s chemical engineers’ handbook, 7th edn. McGraw-Hill, New york
Popp TJ, Chanton JP, Whiting GJ, Grant N (2000) Evaluation of methane oxidation in the rhizosphere of a Carex dominated fen in north central Alberta, Canada. Biogeochemistry 51:259–281
Rahalkar M, Deutzmann J, Schink B, Bussmann I (2009) Abundance and activity of methanotrophic bacteria in littoral and profundal sediments of Lake Constance (Germany). Appl Environ Microbiol 75:119–126
Rydin H, Jeglum JK (2006) The biology of peatlands. Oxford University Press, Oxford
Saarnio S, Winiwarter W, Leitão J (2009) Methane release from wetlands and watercourses in Europe. Atmos Environ 43:1421–1429
Sachs T, Giebels M, Boike J, Kutzbach L (2010) Environmental controls on CH4 emissions from polygonal tundra on the microsite scale in the Lena river delta, Siberia. Glob Change Biol 16:3096–3110
Sander R (1999). Compilation of Henry’s law constants for inorganic and organic species of potential importance in environmental chemistry (vers. 3). Air Chemistry Department, Max-Planck Institute of Chemistry Mainz. http://www.mpch-mainz.mpg.de/~sander/res/henry.html. Accessed Oct 2008
Sebacher D, Harriss RC, Bartlett KB, Sebacher SM, Grice SS (1986) Atmospheric methane sources: Alaskan tundra bogs, an alpine fen, and a subarctic boreal marsh. Tellus 38B:1–10
Shannon RD, White JR (1994) A three-year study of controls on methane emissions from two Michigan peatlands. Biogeochemistry 27:35–60
Shannon RD, White JR, Lawson JE, Gilmour BS (1996) Methane efflux from emergent vegetation in peatlands. J Ecol 84:239–246
Strack M, Waddington JM (2008) Spatiotemporal variability in peatland subsurface methane dynamics. J Geophys Res 113:G02010
Stroem L, Mastepanov M, Christensen TR (2005) Species-specific effects of vascular plants on carbon turnover and methane emissions from wetlands. Biogeochemistry 75:65–82
Stumm W, Morgan JJ (1981) Aquatic chemistry: chemical equilibria and rates in natural waters (3rd edn). Environmental Science and Technology. Wiley-Interscience Series of Texts and Monographs. Wiley, New York
Taras B, Sturm M, Liston GE (2002) Snow-ground interface temperatures in the Kuparuk River Basin, Arctic Alaska: measurements and model. J Hydrometeorol 3:377–394
Turunen J, Tomppo E, Tolonen K, Reinikainen A (2002) Estimating carbon accumulation rates of undrained mires in Finland—application to boreal and subarctic regions. Holocene 12:69–80
Van der Nat FJWA, Middleburg JJ (1998) Seasonal variation in methane oxidation by the rhizosphere of Phragmites australis and Scirpus lacustris. Aquat Bot 61:95–110
Wagner D, Kobabe S, Pfeiffer EM, Hubberten HW (2003) Microbial controls on methane fluxes from a polygonal tundra of the Lena Delta, Siberia. Permafrost Perigl Proc 14:173–185
Wagner D, Lipski A, Embacher A, Gattinger A (2005) Methane fluxes in permafrost habitats of the Lena Delta: effects of microbial community structure and organic matter quality. Environ Microbiol 7:1582–1592
West AE, Brooks PD, Fisk MC, Smith LK, Holland EA, Jaeger CH III, Babcock S, Lai RS, Schmidt SK (1999) Landscape patterns of CH4 fluxes in an alpine tundra ecosystem. Biogeochemistry 45:243–264
Whalen SC (2005) Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci 22:73–94
Whalen SC, Reeburgh WS (1988) A methane flux time series for tundra environments. Glob Biogeochem Cycles 2:399–409
Whalen SC, Reeburgh WS (1990) A methane flux transect along the trans-Alaska pipeline haul road. Tellus 42B:237–249
Whalen SC, Reeburgh WS (1992) Interannual variations in tundra methane emission: a 4-year time series at fixed sites. Glob Biogeochem Cycles 6:139–159
Whitmire SL, Hamilton SK (2008) Rates of anaerobic microbial metabolism in wetlands if divergent hydrology on a glacial landscape. Wetlands 28:703–714
Wickland KP, Striegl RG, Schmidt SK, Mast MA (1999) Methane flux in subalpine wetland and unsaturated soils in the southern Rocky Mountains. Glob Biogeochem Cycles 13:101–113
Acknowledgments
We sincerely thank Jakob Schneller for botanical determinations, Livio Baselgia and Ladina Muggler for lab work, and Antonin Vydrzel and Hansruedi Scherrer for technical support of the oxygen measurements. This research was supported by ETH Zurich and a DAAD grant (German Academic Exchange Service) to SL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liebner, S., Schwarzenbach, S.P. & Zeyer, J. Methane emissions from an alpine fen in central Switzerland. Biogeochemistry 109, 287–299 (2012). https://doi.org/10.1007/s10533-011-9629-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-011-9629-4