Abstract
In northeastern Canada, at the ecotonal limit of the forest tundra and lichen woodland, a rise of the regional water table in the peatland systems was registered since Little Ice Age resulting in increasing pool compartment at the expense of terrestrial surfaces. We hypothesized that, with a mean water table closer to peat surface and higher pool density, these ecosystems would be great CH4 emitters. In summers 2009 and 2010, methane fluxes were measured in a patterned fen located in the northeastern portion of the La Grande river watershed to determine the contribution of the different microforms (lawns, hollows, hummocks, string, pools) to the annual CH4 budget. Mean seasonal CH4 fluxes from terrestrial microforms ranged between 12.9 and 49.4 mg m−2 day−1 in 2009 and 15.4 and 47.3 mg m−2 day−1 in 2010. Pool fluxes (which do not include ebullition fluxes) ranged between 102.6 and 197.6 mg CH4 m−2 day−1 in 2009 and 76.5 and 188.1 mg CH4 m−2 day−1 in 2010. Highest fluxes were measured in microforms with water table closer to peat surface but no significant relationship was observed between water table depth and CH4 fluxes. Spatially weighted CH4 budget demonstrates that, during the growing season, the studied peatland emitted 66 ± 31 in 2009 and 55 ± 26 mg CH4 m−2 day−1 in 2010, 79 % of which is accounted by pool fluxes. In a context where climate projections predict greater precipitations in northeastern Canada, these results indicate that this type of peatlands could contribute to modify the methane balance in the atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Between 1978 and 1999, methane (CH4) atmospheric concentration has risen globally by 13 % (Dlugokencky et al. 2009). CH4 atmospheric burden has then stabilized between 1999 and 2006 but has shown a global increase of 8.3 ± 0.6 ppb in 2007 and of 4.4 ± 0.6 ppb in 2008 (Dlugokencky et al. 2009). This increase has serious implication for climate as CH4 global warming potential is 25 times higher than carbon dioxide (CO2) on a 100-years timescale (Solomon et al. 2007; Wuebbles and Hayhoe 2002). Moreover, methane oxidation to CO2 represent an important addition to the atmospheric carbon concentration (Wuebbles and Tamaresis 1993).
With emissions ranging between 100 and 260 Tg CH4 year−1 (Lelieveld et al. 1998; Mikaloff Fletcher et al. 2004; Wuebbles and Hayhoe 2002), wetlands comprise the largest single source of methane (Solomon et al. 2007). Methane emissions to the atmosphere are the result of the balance between CH4 production, oxidation and transport. Methane is produced by methanogenic bacteria under anoxic conditions while it is oxidized by methanotrophic bacteria under oxic conditions during its diffusive transport towards peat surface. Several studies have reported a link between peat temperature and CH4 production and oxidation. Q10 values associated with these two mechanisms (ranging from 0.6 to 35 for methane production and between 1.5 and 2.9 for methane oxidation) show that methane production is more responsive to temperature than methane oxidation (Segers 1998; Whalen 2005) which leads to higher fluxes under warmer conditions (Bubier et al. 1995; Moore and Dalva 1993; Treat et al. 2007). However, Duc et al. (2010) have shown that methane oxidation can adapt to methane formation with increasing methane oxidation in ecosystems with higher methane formation induced by warmer temperature.
Vegetation also controls CH4 emissions. Vascular plants act as a substrate for methanogenesis by providing fresh and labile organic matter from root exudates and litter deposition (Whiting and Chanton 1992). Some studies have also shown that the aerenchymous tissue of some plant species such as Carex may act as a gas conduit. Hence, CH4 produced in the rhizosphere can bypass the oxidation zone and directly be released to the atmosphere (Christensen et al. 2003; Van den Pol-van Dasselaar et al. 1999; Whiting and Chanton 1992, 1993). In contrast, oxygen can also be transported from the atmosphere to the rhizosphere and decrease methanogenic activity while stimulating methanotrophic bacteria (Joabsson et al. 1999; Waddington et al. 1996). Several studies have reported that water table depth, by controlling the thickness of the acrotelm, may influence CH4 fluxes (Daulat and Clymo 1998; Moore and Knowles 1989; Pelletier et al. 2007; Strack et al. 2006; Turetsky et al. 2008) and that pools are stronger emitters than vegetated surfaces (Bartlett et al. 1992; Hamilton et al. 1994; McEnroe et al. 2009). However, some studies found opposite results or no significant trends between water table depth and CH4 fluxes, mainly in the saturated sites (Bellisario et al. 1999; Kettunen et al. 1996; Moosavi and Crill 1997; Shannon and White 1994; Treat et al. 2007).
Methane emissions from wetlands respond to changes in hydro-climatic conditions and vegetation composition. However, due to diversity in peatland types and microtopography, there is a high uncertainty in predicting methane fluxes in changing climate (Gorham 1991; Moore et al. 1998). In the northeastern portion of the La Grande river watershed, a regional hydrological disequilibrium registered since the Little Ice Age lead to a rise of the water tables in the overall peatlands. This rise has caused tree mortality, string decomposition and influences pool dynamics, development and expansion in these ecosystems. Consequently, the pool surfaces have increased and became more important over the vegetated surface. This disequilibrium of the peatlands’ hydrologic conditions has been characterized with the neologism ‘aqualysis’ (Arlen-Pouliot 2009; Dissanska et al. 2009; Tardif et al. 2009). This phenomenon has been observed in the peatlands of northeastern Canada at the ecotonal boundary of the forest tundra and lichen woodland where abundant annual precipitation, low mean temperatures and short growing season result in low evapotranspiration. Knowing that Canadian Regional Climate Model (CRCM) (Plummer et al. 2006) predicts an increase of winter (20 % ± 5) and summer precipitation (10 % ± 2.5) for northern Quebec in 2041–2070, this phenomenon will likely continue to evolve.
Under these climatic conditions, we hypothesized that the intrinsic characteristics of these aqualysed peatlands might enhance methane production and transport and hence lead to higher CH4 emissions. The main purpose of this study is to evaluate the influence of a high mean water table and pool density on the CH4 budget of a peatland of the southern limit of subarctic Quebec in order produce annual methane budgets for 2009 and 2010. Methane fluxes were measured during two growing seasons in vegetated and aquatic surfaces with environmental variables monitored. We identified the main controls on the methane fluxes in order to produce a CH4 model at the microform scale that will be extrapolated at the ecosystem level using GeoEye satellite imaging (Dribault et al. 2011).
Methods
Study area and sites
The Laforge region is located in the eastern part of the James Bay territory, in the northeastern section of the La Grande river watershed, at the ecotonal limit of the forest tundra and lichen woodland (Fig. 1). The landscape has been shaped by last glaciation leaving superficial post-glacial deposits as eskers, drumlins and moraines (Dyke and Prest 1987). Biogeographically, the region belongs to the lichen woodland, characterized by acidic soils and dominated by black spruce (Picea mariana (Mill) BSP) and lichens mainly Cladina spp. and Cladonia spp. (Payette et al. 2000). In this region, peatland cover reaches approximatively 15 % of the land surface and is characterised by oligotrophic patterned fens (Tarnocai et al. 2002). The 1971–2003 climate serie show that the region is influenced by humid subarctic conditions. Mean annual precipitation average is 738 mm and mean annual temperature is −4.28 °C, January being the coldest month with a mean temperature of −24.05 °C and July the warmest with an average temperature of 12.76 °C (Hutchinson et al. 2009) (Fig. 2).
Study area and localisation of the peatland following Payette and Rochefort (2001) classification. The studied region (black dot) is located at the ecotonal limit between FO open boreal forest and TF forest toundra
Monthly average temperature (dots, °C) and precipitation (bars, mm) in 2009, 2010 and 1971–2003 (Hutchinson et al. 2009)
The studied peatland, named ‘Abeille’ is an aqualysed (42 %) patterned oligtrophic fen located 15 km from the Hydro-Quebec Laforge hydroelectric infrastructures (LA-1) (54°06.9′N, 72°30.1′W). The peatland is characterised by parallel and elongated pools and strings perpendicular to the general slope. This peatland was chosen after its regional representativeness determined following an aerial and ground regional survey in 2008. The Abeille peatland covers 3.5 ha and has an average peat depth of 108 cm (Proulx-Mc Innis 2010). The fen vegetation is dominated by sedges (Carex exilis, C. oligosperma, C. limosa). Seven sub-environments (microforms) and two pools representative of the microtopography of the peatland were identified and characterized (Tables 1, 2). These microforms present distinct vegetation assemblages and mean water table depth. In the field, evidences of rising water table were observed. Several Sphagnum fuscum hummocks showed eroded margins and collapsing tops on which dead trees such as Picea mariana and Larix laricina were identified. Decomposed strings were also observed allowing pool coalescence and traces of former strings in some pool centers.
CH4 terrestrial fluxes measurements
In June 2009, seven terrestrial microforms and two pools were selected for flux measurements in the Abeille peatland and two collars (25 cm diameter) were installed in each microform. Boardwalks were placed between the sites to minimize the impacts of sampling. In order to integrate spatiotemporal variability, methane fluxes were measured during two growing seasons (2009 and 2010). In 2009, four field campaigns were carried out respectively in June, July, August and October while three field campaigns were held in June, July and August in 2010. Each of these field campaigns included 10 days of daily measurements. Methane fluxes were measured every 2 days during each of the field campaign. For terrestrial microforms, CH4 fluxes were measured using a 18 L plastic static chamber placed on collars (Crill et al. 1988). Chambers were covered with foil to prevent heating the air inside and water was poured between the collar and the chamber in order to seal it. CH4 samples (4) were taken every 6 min for a 24-min period. Air inside the chamber was mixed using a 60 mL syringe and a sample was taken and injected in a 10 mL pre-evacuated glass vial sealed with a rubber septum (butyl 20 mm septa, Supelco cie.) and a metal crimp (Ullah et al. 2009). Samples were kept at 4 °C until analysis with a gas chromatograph (Shimadzu GC-14B) equipped with a flame ionization detector for CH4. Fluxes were determined from a linear regression between the 4 measured CH4 concentrations during the 24-min sampling. Fluxes with coefficients of determination (r 2) lower than 0.8 were rejected.
Pool fluxes measurements
Water samples were collected every 2 days during field campaigns following Hamilton et al. (1994) methodology. Samples were taken in glass bottles (Wheaton 125 mL) sealed with a rubber septum. These bottles were prepared in laboratory prior to sample: (1) 8,9 g of KCl was added in order to inhibit biological activity, (2) air contained in the bottle was removed with an electric pump and (3) 10 ml of UHP N2 was added to the bottle to liberate a headspace. In the field, samples were taken by submerging the bottle in water and by piercing the septum with a 18 gauge needle. At sampling time, air and water temperature and wind speed were measured.In laboratory, samples were shaken for 3 min in order to equilibrate water and N2. Air from headspace was then sampled and analysed with a gas chromatograph (Shimadzu GC-14B) to determine CH4 partial pressure (Demarty et al. 2009; Hamilton et al. 1994). As a dissolved gas concentration in a solution is directly proportional to this gas partial pressure in the solution, Henry’s law was used to calculate CH4 concentration in the solution (Hamilton et al. 1994) using two equations:
where;
- CH4wc:
-
CH4 concentration in the water
- CH4wp:
-
CH4 partial pressure
- KH(CH4):
-
CH4 solubility in water in mol L−1 atm−1
- TK :
-
Water temperature in Kelvin
CH4 fluxes were calculated using the 〈〈Thin boundary layer〉〉 as described by Demarty et al. (2009).
where:
- F:
-
The gas flux at water/air interface
- αCH4ac:
-
The gas concentration in water exposed to the atmosphere calculated with Eq. 2 and global mean atmospheric partial pressure of 1,745 for methane (Houghton et al. 2001)
- K:
-
The gas exchange coefficient
To be applied to CH4 and ambient conditions, we adjusted k values with temperature, viscosity and CH4 diffusion coefficients with the Schmidt number (obtained by dividing water kinematic viscosity at temperature ‘a’ by gas diffusion coefficient at temperature ‘a’) following Cole and Caraco (1998):
where
- K600 :
-
The exchange coefficient measured with SF6, normalized to Schmidt number (Sc number) of 600, which is the Sc number for SF6 at 20 °C (Crusius and Wanninkhof, 2003) and U10 represents wind speed at 10 m.
Finally, k was calculated with the following equations (Crusius and Wanninkhof 2003):
If wind speed <3 m/sec:
If wind speed ≥3 m/sec
where
- kCH4 :
-
The gas coefficient exchange for CH4 and ScCH4 is Schmidt number for CH4
Environmental variables
Meteorological data were collected continuously using an automated station located in a nearby peatland (<1 km) for 2009 and in Abeille peatland for 2010. For every terrestrial microform and pool, water table depth and peat temperature were measured continuously between June 13th 2009 and September 1st 2010. Water table depth was measured using level loggers (Odyssey Capacitance Water Level Logger) placed in PVC tubes inserted in peat and pool bottoms. Peat temperature was measured using HOBO probes (TMC6-HD Air/Water/Soil Temp Sensor) inserted at different depths (5–10–20–40 cm). In the pools, temperature was measured at the center and on the border both at the surface and bottom. At the end of 2010, aboveground vegetation inside collars was clipped and kept in plastic bags. Vascular plants stems and leaves were separated and only the capitula were kept for Sphagnum species. Vegetation was then dried in the oven at 80 °C and weighted to calculate biomass (Moore et al. 2002).
Data analysis
To determine the relationship between the environmental variables (water table depth, peat temperature, biomass) and CH4 fluxes, data were analysed along different timescales: mean daily, monthly and seasonal fluxes for 2009 and 2010 respectively and then for the 2 years combined. Statistical software JMP7 (SAS institute inc.) was used for statistical analysis. CH4 fluxes data were log-transformed in order to reduce skewness and approximate normal distribution. The Shapiro–Wilk test was used to determine normality of the distribution and Cook’s distance was used to detect outliers. A total of 12 data were rejected for terrestrial microforms and 6 for pools during the 2 years. For the vegetated surfaces, 291 methane fluxes were kept for analysis (172 in 2009 and 119 in 2010) while 92 fluxes were conserved for pools (47 in 2009 and 45 in 2010). In order to compare means, one-way ANOVA tests were conducted at α = 0.05 (two-tailed). To determine relationships between independent variables and CH4 fluxes, linear regression were realized (α = 0.05). For the final model, stepwise regression was used and the AIC criterion lead to choose the independent variables for the model. For the pools, we analysed relationship between environmental variables and CH4 concentrations in water.
Results
Environmental conditions
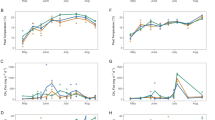
Between June 1st and September 1st 2009, total precipitation was 269 mm and average temperature was 13.21 °C. During the same period in 2010, precipitation was 477 mm and average temperature 12.97 °C (Fig. 2). Mean peatland water table depth varied between −6.9 and −13 cm under the peat surface in 2009 and between −5.4 and −16.3 cm in 2010. In 2009, mean water table decreased slightly in June and July and increased in August whereas in 2010 it fell significantly in June and increased throughout summer (Fig. 3a). Mean peat temperature at 20 cm ranged between 7.5 and 17.35 °C in 2009 and between 9 and 15.5 °C in 2010. Mean peat temperature at 20 cm increased from June to July and lowered in August in 2009 while it increased from June to August in 2010 (Fig. 3b).
CH4 flux measurements and variability
During the growing season, average daily methane fluxes in terrestrial microforms ranged between 1.6 and 106.9 mg CH4 m−2 day−1 in 2009 and between 3.4 and 127.3 mg CH4 m−2 day−1 in 2010. One-way ANOVA test revealed that mean seasonal fluxes of 31 and 28.8 mg CH4 m−2 day−1 for 2009 and 2010 respectively are not significantly different (0.436).
Fluxes showed a large variability during the growing season (Fig. 4). For both years, the highest mean monthly fluxes were measured in July and August while the lowest mean monthly fluxes were measured at the beginning and the end of the growing season (Fig. 3). Fluxes also varied between microforms. Lowest mean seasonal CH4 emissions were measured in the Cyperaceae string, forest border and hummock while highest fluxes were measured in the Sphagnum lawn, pool border and hollow (Table 3).
During the growing season, CH4 concentrations measured in pools waters varied between 1.77 and 68.66 μmol L−1 with a mean concentration of 16.65 μmol L−1 in 2009. In 2010, CH4 concentrations varied between 1.53 and 43.79 μmol L−1 with a mean of 13.47 μmol L−1. Individual pool fluxes derived from those concentrations (see Method section) ranged between 11.4 and 545.9 mg CH4 m−2 day−1 in 2009 and 4.2 and 578.4 mg CH4 m−2 day−1 in 2010. ANOVA test revealed that seasonal mean of 151.9 and 132.3 mg CH4 m−2 day−1 for 2009 and 2010 respectively are significantly similar (p = 4701). Pool fluxes varied in space and time. Mean monthly fluxes were higher in July and August and lower in June and October (Fig. 5) and mean seasonal fluxes were higher in the shallow pool than in the deep pool (Table 3).
Environmental correlates
Water table depth (WTD)
For the whole peatland and when compared to single microform, relationship between water table depth (WTD) and terrestrial CH4 fluxes was weak or insignificant at all timescales. The only significant relationship that linked individual fluxes and WTD revealed decreasing CH4 fluxes with a lower water table (r 2 = 0.05; p = 0.03). In pools, CH4 concentration decreased with pool depth (r 2 = 0.63; p = 0.02). Significant relationship between mean seasonal fluxes and WTD was obtained when using combined data from aquatic and terrestrial microsites (r 2 = 0.53; p < 0.001).
Peat temperature
Peat temperature at 20 cm showed a positive correlation with CH4 fluxes in terrestrial microforms (daily r 2 = 0.22; p < 0.001, monthly r 2 = 0.32; p < 0.001). The strength of the relationship varied according to microforms with coefficients of correlation ranging between 0.22 for the hummock and 0.94 for the Sphagnum lawn (monthly scale). Microforms with deeper water table such as the hummock and forest border showed better correlation with peat temperature at 40 cm. Temperature was not correlated to pool CH4 concentration, explaining less than 10 % of the variation.
Vegetation
Vegetation composition and biomass differed between microforms. Based on the dominant vegetation, three subgroups were defined: (1) hummocks dominated by shrubs (2) lawns dominated by sedges and (3) hollows dominated by Sphagnum cuspidatum. Fluxes from the three subgroups were significantly different (p < 0,001). Highest mean seasonal fluxes were measured in the hollows while lowest mean seasonal fluxes were measured in the lawns.
No correlation was found between sedges biomass and CH4 fluxes. A negative correlation between end-of-season biomass and mean seasonal fluxes was observed: microforms with high biomass such as hummock and forest border showed lower emission than those with low biomass such as pool border and hollow which were the strongest emitters. In contrast, in aquatic compartments, pool with higher biomass (shallow pool) emitted more methane than deep pool with low biomass.
CH4 budget
The scaling up of the methane budget was carried out based on the spatial mapping of GeoEye satellite image (Dribault et al. 2011). Mean spatially weighted fluxes were extrapolated for the growing season and annually. For each microform, standard error was derived from CH4 model equation (Table 4) and did not take into account the error derived from the spatial classification. Length of the growing season was determined using peat temperature at 5 cm to determine date of spring thaw and autumn freeze. 2009 growing season lasted from May 12th to October 9th (151 days) and 2010 growing season lasted from April 28th to September 30th (155 days).
A stepwise regression was used to identify independent variables for the model equations using AIC criterion to evaluate the strength of each variable. To represent the spatial variability and obtain stronger models, one model per terrestrial microform was used to project monthly fluxes using peat temperature at 20 and 40 cm as independent variables (Table 4). Peat temperature between January 1st 2009 and June 11th 2009 and between September 1st 2010 and December 31st 2010 was modeled using the relationship with air temperature. Winter fluxes in terrestrial microforms were also modelled with peat temperature at 20 and 40 cm but as pool fluxes did not vary significantly with temperature, it was not possible to model their winter emissions. Thus, only growing season budget includes pool emissions for which we used pool seasonal average fluxes (shallow and deep). We assumed that pools did not emit CH4 during winter because of their thick ice cover. However, annual budget missed pool emissions during spring thaw and autumn freeze (Gažovič et al. 2010).
Results showed that Abeille peatland (vegetated and pool surfaces) emitted an average of 7.9 ± 3.8 g CH4 m−2 for the 2009 growing season and 6.6 ± 4.3 g CH4 m−2 for 2010. Annually, the projected emissions are 8.9 ± 2.2 g CH4 m−2 year−1 in 2009 for which 12 % is accounted for the winter fluxes. In 2010, the annual projected flux of methane is 7.8 ± 2.3 g CH4 m−2 year−1 for which 11 % accounted for winter fluxes.
Spatially weighted budget of CH4 showed that the patterned fen emitted 66 ± 31 mg CH4 m−2 day−1 during the 2009 growing season for which 79 % can be accounted for pool fluxes. In 2010, mean daily spatially weighted flux was 55 ± 26 mg CH4 m−2 day−1 from which 79 % was emitted from the pools. For the yearly model, 2009 spatially weighted average daily flux reached 29.4 ± 13.3 mg CH4 m−2 day−1 with winter fluxes accounting for 3.02 ± 1.9 mg CH4 m−2 day−1 while in 2010 the projected emission reached 24.8 ± 16.8 mg CH4 m−2 day−1 on average with winter fluxes of 2.4 ± 1.2 mg CH4 m−2 day−1 (Table 5).
Discussion
CH4 fluxes
As hypothesized, microforms with water table closer to surface such as hollows and pool borders and aquatic sites were large CH4 emitters. When combined, their surface represented 55 % of the peatland area but contributed to 85 % of the CH4 fluxes, the other 15 % is accounted by lawns and hummocks. The individual range of terrestrial CH4 fluxes (2.2–127.3 mg CH4 m−2 d−1) and mean seasonal fluxes for vegetated surfaces (28.8–31 mg CH4 m−2 day−1) measured are consistent with other studies. In poor and moderately rich fens of northeastern Ontario, Bubier et al. (1993b) measured mean seasonal fluxes of 32.6 and 44.1 mg CH4 m−2 day−1 while Moore et Knowles (Moore and Knowles 1990) measured daily fluxes ranging between <10–100 mg CH4 m−2 d−1 in a subarctic patterned fen. Mean seasonal pool fluxes ranging between 89.9 (deep pool) and 192.9 mg CH4 m−2 day−1 (shallow pool) are similar of those of 160 and 185 mg CH4 m−2 day−1 measured by Hamilton et al. (1994) in Hudson Bay Lowland fens and to those of 34.6 to 156.2 mg CH4 m−2 day−1 measured by Bubier (1995) in pools from peatlands located in mid-boreal Ontario. Ebullition fluxes were not measured for this study. Considering that studies have reported that this type of fluxes play an important role in peatlands (Strack et al. 2005; Tokida et al. 2007), it is probable that the pools contribution to the CH4 budget would have been higher if taken into account.
CH4 controls
Water table depth
Several studies have observed large spatial variability of CH4 fluxes related to microtopography (Bubier et al. 1993a; Kettunen 2003; Moore et al. 1994; Pelletier et al. 2007). As methane is produced in anaerobic peat and is oxidised in aerobic peat, most studies found that microforms with water table close to surface tend to be stronger emitters because production dominates over oxidation (Daulat and Clymo 1998; Moore and Knowles 1989; Pelletier et al. 2007; Strack et al. 2006; Turetsky et al. 2008). Hence, fluxes generally follow the sequence hummock < lawn < hollow. However, if combined in three subgroups, fluxes measured in the Laforge area followed this sequence: lawn ≤ hummock < hollow which partly explains the non-significance of the relationship between CH4 fluxes and WTD. In this aqualysed peatland, water table encompassed a small range of values. If wetter sites tended to be stronger emitters, variability of the water table did not explain the large variability of the fluxes between and inside the microforms. These results are similar to those of Bellisario et al. (1999), Moosavi and Crill (1997), Shannon and White (1994) and Heikkinen et al. (2002) who found weak or non-significant trends between WTD and CH4 fluxes in peatlands with water table close to the surface. When terrestrial data were combined with those of pools, encompassing a larger range of water table variability, the relationship was significant (p < 0.001) and explained 53 % of the mean seasonal fluxes variation. In pools, mean monthly CH4 concentration was negatively correlated to pool depth, shallow pools being stronger emitters than deep pools. This is consistent with results of Pelletier et al. (2007) and McEnroe et al. (2009) who showed that deeper (and older) pools present lower decomposition rates due to colder water and low availability of labile organic matter.
Peat temperature
A strong correlation was observed between peat temperature at 20 cm and 40 cm and both daily and monthly CH4 fluxes, warmer temperatures leading to higher fluxes and explaining a large part of the seasonal variations. This is in agreement with other northern peatlands studies where higher methane fluxes are associated with warmer peat temperatures (Bubier 1995; Moore et al. 1994; Turetsky et al. 2008). Higher temperature coefficient (Q10) implied that methane production is more sensitive to temperature than methane oxidation (Segers 1998; Whalen 2005). Thus, higher temperatures lead to a greater increase in methane production than methane oxidation, resulting in higher CH4 fluxes under warmer peat temperature. This association varied with microforms: dryer sites such as hummock and forest border showed stronger relationships with peat temperature at 40 cm while wetter sites were more related to peat temperature at 20 cm. Thus, better correlation is obtained when fluxes were related with temperature close to the water table depth. The relationship between peat temperature and seasonal CH4 fluxes was not significant (r 2 = 0) suggesting that peat temperature is a good indicator of CH4 fluxes only on short-term scale. Hence, peat temperature explained temporal variation of the fluxes but did not explain their spatial variation. In pools, air, water and sediment temperature did not explain variation of CH4 concentration (r 2 < 0.1) which corroborates results of McEnroe et al. (2009) in the southwestern part of the La Grande river watershed.
Vegetation
Sedge-dominated lawns presented an original fluxes behaviour. Several studies have identified lawns and strings as strong CH4 emitters because of a water table close to the peat surface and of sedges acting as gas conduits (Bubier et al. 1993a, b; Kettunen 2003). In the Abeille peatland, lawns were the lowest emitters. When excluded from the linear regression between WTD and mean seasonal fluxes, the relationship becomes significant (p = 0.04) and explains 40 % of the variability of the fluxes. These results suggest that controls other than water table depth and Carex biomass can explain the CH4 fluxes in strings and lawns. In specific case, peat substrate was covered with a dense carpet of Gymnocolea inflata that may have inhibited CH4 diffusion. It is also possible that oxygen produced by the liverworts increased CH4 oxidation in this substrate but we have no data to confirm this assumption. In aquatic compartments, shallow pools with higher biomass were stronger CH4 emitters which is consistent with Bartlett et al. (1992) who suggest that, as in vegetated surfaces, vascular plants act as a conduit between the methane production zone and the atmosphere and hence increase CH4 emissions.
CH4 budget
Estimated growing season CH4 budgets for 2009 and 2010 are in agreement with fluxes measured in similar environments in Europe, as no budget was published for such region in Canada. Nilsson et al. (2001) measured an annual average of 8.2 ± 0.8 g m−2 year−1 in a low sedge fen of northern Sweden which is similar to our annual average of 9.1 ± 2.2 and 7.8 ± 2.3 g m−2 year−1 measured in 2009 and 2010 respectively. In a subarctic aapa mire in Finland, Heikkinen et al. (2002) measured mean spatially-weighted flux of 55.3 mg CH4 m−2 d−1 for the growing season which concords with our mean spatially-weighted growing season fluxes of 66.1 CH4 m−2 day−1 for 2009 and 55.3 mg CH4 m−2 day−1 in 2010.
The average spatially weighted modelled winter emission of 3.02 ± 1.9 mg m−2 day−1 in 2009 and of 2.4 ± 1.2 mg m−2 day−1 in 2010 are similar to those of 2.3 ± 1.1 mg m−2 day−1 measured by Pelletier et al. (2007) in Radisson, at the southwestern limit of the watershed. The winter fluxes represented 11–12 % of the annual fluxes which agrees with the 8–17 % obtained by Alm et al. (1999) and 13 % by Pelletier et al. (2007).
Conclusions
The objective of this study was to assess seasonal and annual methane budgets for 2 years in an oligotrophic fen at the ecotonal limit of the forest tundra and lichen woodland in northeastern Canada. The aqualysis process represents a long term rise of the regional water tables, which leads to an increase of pool density at the expense of the terrestrial surface. By influencing water table depth, this phenomenon modifies peatland surface dynamics and related ratio of aquatic/terrestrial microforms. A water table close to peat surface favours pool, hollow, lawn and string development at the expense of hummocks. In the studied peatland, eroded hummock margins, dying mature trees and drowned strings confirm the strength of the phenomenon. It would be important to define if hydroclimatic changes that influence CH4 emissions to the atmosphere are also reported in other regions of the northern hemisphere such as in aapa mire.
The aqualysis phenomenon is increasing the proportion of pools and high water table microforms and has an indirect effect on the methane fluxes: higher fluxes were measured in high water table microforms and pools fluxes were five times higher than those of the vegetated surface. Contribution of pools to the methane budget is non-negligible in the studied peatland. Pools covered 42 % of the surface and represented 79 % of the growing season area-extrapolated methane budget. By its influence in microforms dynamics, the aqualysis process will play a role in long term methane balance of the boreal patterned fens, while peat temperature will control variation of the fluxes at the growing season scale. The climate scenarios provided from the CRCM for the 2041–2070 (Plummer et al. 2006) predict an increase of temperature and precipitation in the boreal and subarctic region of northeastern Canada, which suggests a potential rise in methane emissions from these systems under predicted wetter and warmer conditions.
References
Alm J, Saarnio S, Nykänen H, Silvola J, Martikainen P (1999) Winter CO2, CH4 and N2O fluxes on some natural and drained boreal peatlands. Biogeochemistry 44:163–186
Arlen-Pouliot Y (2009) Développement holocène et dynamique récente des tourbières minérotrophes structurées du Haut-Boréal québécois, PhD thesis, Biology department, Université Laval, Québec. http://www.theses.ulaval.ca/
Bartlett KB, Crill PM, Sass RL, Harriss RC, Dise NB (1992) Methane emissions from tundra environments in the Yukon-Kuskokwim Delta, Alaska. J Geophys Res 97:16645–16660
Bellisario LM, Bubier JL, Moore TR (1999) Controls on CH4 emissions from a northern peatland. Glob Biogeochem Cycles 13(1):81–91
Bubier JL (1995) The relationship of vegetation to methane emission and hydrochemical gradients in northern Peatlands. J Ecol 83:403–420
Bubier JL, Costello A, Moore TR, Roulet NT, Savage K (1993a) Microtopography and methane flux in boreal peatlands, northern Ontario, Canada. Can J Bot 71:1056–1063
Bubier JL, Moore TR, Roulet NT (1993b) Methane emissions from wetlands in the midboreal region of northern Ontario, Canada. Ecology 74:2240–2254
Bubier JL, Moore TR, Bellisario L, Comer NT, Crill PM (1995) Ecological controls on methane emissions from a Northern Peatland Complex in the zone of discontinuous permafrost, Manitoba, Canada. Glob Biogeochem Cycles 9:455–470
Christensen TR, Ekberg A, Ström L, Mastepanov M, Panikov N, Öquist M, Svensson BH, Nykänen H, Martikainen PJ, Oskarsson H (2003) Factors controlling large scale variations in methane emissions from wetlands. Geophys Res Lett 30:1414–1418
Cole JJ, Caraco NF (1998) Atmospheric exchange of carbon dioxyde in a low wind oligotrophic lake measured by the additio of SF6. Limnol Oceanogr 43:647–656
Crill PM, Bartlett KB, Harriss RC, Gorham E, Verry ES, Sebacher DI, Madzar L, Sanner W (1988) Methane flux from Minnesota Peatlands. Glob Biogeochem Cycles 2:371–384
Crusius J, Wanninkhof R (2003) Gas transfer velocities measured at low wind speed over a lake. Limnol Oceanogr 48:1010–1017
Daulat WE, Clymo RS (1998) Effects of temperature and watertable on the efflux of methane from peatland surface cores. Atmos Environ 32:3207–3218
Demarty M, Bastien J, Tremblay A (2009) Carbon dioxide and methane annual emissions from two boreal reservoirs and nearby lakes in Quebec, Canada. Biogeosci Discuss 6:2939–2963
Dissanska M, Bernier M, Payette S (2009) Object-based classification of very high resolution panchromatic images for evaluating recent change in the structure of patterned peatland. Can J Remote Sens 25:189–215
Dlugokencky EJ, Bruhwiler L, White JWC, Emmons LK, Novelli PC, Montzka SA, Masarie KA, Lang PM, Crotwell AM, Miller JB, Gatti LV (2009) Observational constraints on recent increases in the atmospheric CH4 burden. Geophys Res Lett 36:L18803
Dribault Y, Chokmani K, Bernier M (2011) Monitoring seasonal dynamic of surface hydrology in minerotrophic peatlands using high resolution satellite imagery. EGU, Vienne
Duc N, Crill P, Bastviken D (2010) Implications of temperature and sediment characteristics on methane formation and oxidation in lake sediments. Biogeochemistry 100:185–196
Dyke AS, Prest VK (1987) Late Wisconsinan and Holocene History of the Laurentide Ice Sheet. Géog Phys Quatern 41:237–263
Gažovič M, Kutzbach L, Schreiber P, Wille C, Wilmking M (2010) Diurnal dynamics of CH4 from a boreal peatland during snowmelt. Tellus B 62:133–139
Gorham E (1991) Northern Peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–195
Hamilton JD, Kelly CA, Rudd JWM, Hesslein RH, Roulet NT (1994) Flux to the atmosphere of CH4 and CO2 from wetland ponds on the Hudson Bay lowlands (HBLs). J Geophys Res 99:1495–1510
Heikkinen JEP, Maljanen M, Aurela M, Hargreaves KJ, Martikainen PJ (2002) Carbon dioxide and methane dynamics in a sub-Arctic peatland in northern Finland. Polar Res 21:49–62
Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA (2001) Climate change 2001: the scientific basis. Contribution of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Hutchinson MF, McKenney DW, Lawrence K, Pedlar JH, Hopkinson RF, Milewska E, Papadopol P (2009) Development and testing of Canada-wide interpolated spatial models of daily minimum–maximum temperature and precipitation for 1961–2003. J Appl Meteorol Climatol 48:725–741
Joabsson A, Christensen TR, Wallén B (1999) Vascular plant controls on methane emissions from northern peatforming wetlands. Trends Ecol Evol 14:385–388
Kettunen A (2003) Connecting methane fluxes to vegetation cover and water table fluctuations at microsite level: a modeling study. Glob Biogeochem Cycles 17:1051
Kettunen A, Kaitala V, Alm J, Silvola J, Nykänen H, Martikainen PJ (1996) Cross-correlation analysis of the dynamics of methane emissions from a boreal peatland. Glob Biogeochem Cycles 10:457–471
Lelieveld JOS, Crutzen PJ, Dentener FJ (1998) Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B 50:128–150
McEnroe NA, Roulet NT, Moore TR, Garneau M (2009) Do pool surface area and depth control CO2 and CH4 fluxes from an ombrotrophic raised bog, James Bay, Canada? J Geophys Res 114:G01001
Mikaloff Fletcher SE, Tans PP, Bruhwiler LM, Miller JB, Heimann M (2004) CH4 sources estimated from atmospheric observations of CH4 and its 13C/12C isotopic ratios: 1. Inverse modeling of source processes. Glob Biogeochem Cycles 18:GB4004
Moore TR, Dalva M (1993) The influence of temperature and water table position on carbon dioxide and methane emissions from laboratory columns of peatland soils. Eur J Soil Sci 44:651–664
Moore TR, Knowles R (1989) The influence of water table levels on methane and carbon dioxide emissions from peatland soils. Can J Soil Sci 69:33–38
Moore TR, Knowles R (1990) Methane emissions from fen, bog and swamp peatlands in Quebec. Biogeochemistry 11:45–61
Moore TR, Heyes A, Roulet NT (1994) Methane emissions from wetlands, southern Hudson Bay lowland. J Geophys Res 99:1455–1467
Moore TR, Roulet NT, Waddington JM (1998) Uncertainty in predicting the effect of climatic change on the carbon cycling of Canadian peatlands. Clim Chang 40:229–245
Moore TR, Bubier JL, Frolking SE, Lafleur PM, Roulet NT (2002) Plant biomass and production and CO2 exchange in an ombrotrophic bog. J Ecol 90:25–36
Moosavi SC, Crill PM (1997) Controls on CH4 and CO2 emissions along two moisture gradients in the Canadian boreal zone. J Geophys Res 102:29261–29277
Nilsson M, Mikkelä C, Sundh I, Granberg G, Svensson BH, Ranneby B (2001) Methane emission from Swedish mires: national and regional budgets and dependence on mire vegetation. J Geophys Res 106:20847–20860
Payette S, Rochefort L (2001) Écologie des tourbières du Québec et du Labrador. Les Presses de l’Université Laval, Ste-Foy
Payette S, Bhiry N, Delwaide A, Simard M (2000) Origin of the lichen woodland at its southern range limit in eastern Canada: the catastrophic impact of insect defoliators and fire on the spruce-moss forest. Can J For Res 30:288–305
Pelletier L, Moore TR, Roulet NT, Garneau M, Beaulieu-Audy V (2007) Methane fluxes from three peatlands in the La Grande Rivière watershed, James Bay lowland, Canada. J Geophys Res 112:G01018
Plummer DA, Caya D, Frigon A, Côté H, Giguère M, Paquin D, Biner S, Harvey R, de Elia R (2006) Climate and climate change over North America as simulated by the Canadian RCM. J Clim 19:3112–3132
Proulx-Mc Innis S (2010) Caractérisation hydrologique, topographique et géomorphologique d’un bassin versant incluant une tourbière minérotrophe fortement aqualysée, Baie-de-James, Québec, MSc thesis, Science de l’eau. INRS, Québec, p 147
Segers R (1998) Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry 41:23–51
Shannon RD, White JR (1994) A three-year study of controls on methane emissions from two Michigan peatlands. Biogeochemistry 27:35–60
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (2007) Climate change 2007: the physical basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Strack M, Kellner E, Waddington JM (2005) Dynamic of biogenic gas bubbles in peat and their effects on peatland biogeohemistry. Glob Biogeochem Cycles 19:1003–1029
Strack M, Waller M, Waddington J (2006) Sedge succession and peatland methane dynamics: a potential feedback to climate change. Ecosystems 9:278–287
Tardif S, St-Hilaire A, Roy R, Bernier M, Payette S (2009) Statistical properties of hydrographs in minerotrophic fens and small lakes in mid-latitude Québec, Canada. Can Water Resour J 34(4):365–380
Tarnocai C, Kettles IM, Lacelle B (2002) Peatlands of Canada database. In: Geological Survey of Canada (ed). Natural Resources Canada, Ottawa
Tokida T, Miyasaki T, Mizoguchi M, Nagata O, Takakai F, Kagemoto A, Hatano R (2007) Falling atmospheric pressure as a trigger for methane ebullition from peatland. Glob Biogeochem Cycle 21:1–8
Treat CC, Bubier JL, Varner RK, Crill PM (2007) Timescale dependence of environmental and plant-mediated controls on CH4 flux in a temperate fen. J Geophys Res 112:G01014
Turetsky MR, Treat CC, Waldrop MP, Waddington JM, Harden JW, McGuire AD (2008) Short-term response of methane fluxes and methanogen activity to water table and soil warming manipulations in an Alaskan peatland. J Geophys Res 113, G00A10
Ullah S, Frasier R, Pelletier L, Moore TR (2009) Greenhouse gas fluxes from boreal forest soils during the snow-free period in Quebec, Canada. Can J For Res 3:666–680
Van den Pol-van Dasselaar A, van Beusichem ML, Oenema O (1999) Determinants of spatial variability of methane emissions from wet grasslands on peat soil. Biogeochemistry 44:221–237
Waddington JM, Roulet NT, Swanson RV (1996) Water table control of CH4 emission enhancement by vascular plants in boreal peatlands. J Geophys Res 101:22775–22785
Whalen SC (2005) Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci 22:73–94
Whiting GJ, Chanton JP (1992) Plant-dependent CH4 emission in a subarctic Canadian fen. Glob Biogeochem Cycles 6:225–231
Whiting GJ, Chanton JP (1993) Primary production control of methane emission from wetlands. Nature 364:794–795
Wuebbles DJ, Hayhoe K (2002) Atmospheric methane and global change. Earth Sci Rev 57:177–210
Wuebbles DJ, Tamaresis JS (1993) The role of methane in the global environment. In: Khalil MAK (ed) Atmospheric methane: sources, sinks, and role in global change. Springer-Verlag, Berlin
Acknowledgments
The authors would like to thank NSERC, FQRNT, Hydro-Québec and Consortium Ouranos for financial support. We gratefully thank Hans Asnong, Éric Rosa and Jean-François Hélie for judicious advices and laboratory assistance. We also thank Yan Bilodeau, Antoine Thibault, Robin Beauséjour, Sébastien Lacoste and Sylvain Jutras for field and laboratory assistance. Special thanks to Gwenael Carrer, Yann Dribault and Sandra Proulx McInnis for sharing data and results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trudeau, N.C., Garneau, M. & Pelletier, L. Methane fluxes from a patterned fen of the northeastern part of the La Grande river watershed, James Bay, Canada. Biogeochemistry 113, 409–422 (2013). https://doi.org/10.1007/s10533-012-9767-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-012-9767-3