Abstract
Estimation of carbon pools and fluxes were conducted in Bruguiera parviflora dominated naturally growing protected mangrove forest in Kuala Selangor Nature Park of Peninsular Malaysia. Above and below-ground carbon pools in seedlings were estimated from destructive methods. While, carbon pools and fluxes in saplings and trees were estimated from the derived allometric biomass equations. Carbon concentrations in different parts of seedlings, saplings and trees; and litter were measured during the dry, wet and intermediate seasons. Soil cores up to 1 m were analyzed to measure carbon concentrations and bulk densities at different depths. Litter standing crop of the study area was measured at the dry, wet and intermediate seasons and the range of total amount of litter standing crop was from 0.66 to 0.88 Mg/ha. Carbon concentration found to vary with the plant and litter parts; and also with the seasons and the range of mean weighted carbon concentration was 40.19 ± 0.87–56.52 ± 1.01 %. The carbon pools in seedling, sapling, tree and litter were 0.69, 0.51, 82.62 and 0.41 Mg C/ha respectively. However, 13.95 Mg C/ha/year of carbon flux was associated with saplings, trees and litter. The estimated carbon pool in the soil (up to 1 m) of the study area was 488.04 Mg C/ha. The findings of this study are the first estimation of carbon pools and fluxes in B. parviflora dominated sites and suggests the potential of this site as a carbon pool.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At the beginning of industrial revolution, atmospheric CO2 concentration increased from 290 to 370 ppm by the year of 2002 (Keeling et al. 1989; Kimmins 2004). The future prediction of CO2 concentration in the atmosphere is highly variable and depends on the trends of population increase, per capita use of fossil fuels, land use changes and rate of deforestation (Kimmins 2004). Elevated concentration of CO2 in the atmosphere leads to global warming and results in climate change. Climate change and its consequences are the major threats towards sustainable development. Scientific communities are concerning about the climate change problems and looking for different mitigation technologies and measures. The most promising measures are to reduce the emission of CO2 and conservation of forest. Forestry can make significant contributions to mitigate this global risk with adaptation and sustainable development (IPCC 2007). Most of the carbon studies were focused on the terrestrial forests. But, the role of mangroves and wetlands in carbon sink were ignored previously (Rattan 2008; Komiyama et al. 2008; Kridiborworn et al. 2012).
Mangrove forests are serving as carbon sink; and these forests provide feeding, breeding, and nursery ground for a variety of fish and wild life, recreational opportunities, and protection from coastal storms and extreme weather events (Ellison 2008; Alongi 2009). These forests support the dissolved and particulate form of carbon to the aquatic ecosystem which is important for the benthic and pelagic communities in the adjacent waters (Bouillon et al. 2008). The mangrove forests have high root-shoot ratios (Fujimoto et al. 1999; Page et al. 2002; Komiyama et al. 2008), and have the capacity to store great amount of carbon in soil at several meters depth (Bouillon et al. 2003). Mangroves have more carbon in their soil alone than most tropical forests have in all their biomass and soil combined (Kristensen et al. 2008; Donato et al. 2011). In spite of their ecological, economic and social importance, the mangrove forests are threatened by land use changes. Rates of deforestation/conversion are among the highest of all tropical forests, far exceeding rates in upland forests (Valiela et al. 2001; Langner et al. 2007).
Malaysia contributes about 4 % of the world mangrove forest and these are under sustainable yield management practices. Peninsular Malaysia contains only 17 % of the country’s total mangrove forests, which mostly concentrates on the sheltered west coast, and almost continues from Kedah to Singapore. Of these forests, only 0.3 % of mangrove areas are legally gazetted as protected areas (Spalding et al. 1997). Total mangroves area in Malaysia was about 650,000 ha during 1982 (Ong 1982) and this area coverage was reduced to about 641,000 ha at the end of 1993 (ISME 1993). During this time, about 9,000 ha of mangrove forest were destroyed, which constituted 1.26 % of yearly destruction and remains higher than the world average destruction rate. The destruction of mangrove areas in Malaysia is mainly due to the construction of sea ports, airports, and industrial estates and agricultural land use. The depletion of mangroves results in serious environmental and economic constraint in the coastal region (Ong 1982). These forests may be of high value in terms of carbon storage, which is particularly relevant to climate change mitigation strategies focusing on reducing carbon emissions through reduction of destruction. Reduced Emissions from Deforestation and Forest Degradation (REDD+) in developing countries has emerged as a likely component of the next international policy effort addressing climate change, to be implemented when the Kyoto Protocol expires in 2012 (Kanninen et al. 2007). This programme would offer economic incentives for conserving forests and associated carbon stocks, intended to offset the short-term economic factors that promote deforestation. Malaysian mangrove forests can be ideal candidates for REDD+ strategies. However, the viability of such a programme depends heavily on having sound information on carbon storage in forests, and how much carbon may be released when these forests are converted. The quantification of carbon emission from deforestation and conversely their values as carbon reservoirs, it is important to quantify the total ecosystem carbon stock. Only one study (e.g. Ong 1993) has quantified the carbon stock (above-ground and below-ground) in Malaysian mangroves. Recent reviews of coastal carbon dynamics and energetics contained little or incomplete data on the total ecosystem carbon storage of mangroves (Bouillon et al. 2008; Alongi 2009; Laffoley and Grimsditch 2009). Therefore, the aims of this study were to estimate the carbon pools and fluxes (above and below ground) in the naturally growing protected mangrove forest in Peninsular Malaysia.
Materials and methods
Description of the study site
The study area is Bruguiera parviflora Wight & Arn. dominated naturally growing mangrove forest in Kuala Selangor Nature Park (Latitudes 3°19′–3°20′N and 101°14′–101°15′E) on the west coast of the Malay Peninsula. It is a fringing mangrove growing at the mouth of the Selangor River, which has an average width of 200 m between sea and land interface. This mangrove forest belongs to Watson’s (1928) tidal inundation class 4 (the maximum tidal height is 4 m above the datum). Bruguiera parviflora, Avicennia alba Blume, Rhizophora apiculata Blume, R. mucronata Lam and Sonneratia caseolaris (L.) Engl. are the common species in this forest. The mean annual rainfall is about 1,790 mm. The wet season (September–December), intermediate season (January–April) and dry season (May–August) contribute about 46, 31 and 23 % of the annual rainfall respectively. The mean minimum and maximum temperatures are 24 and 32 °C respectively. Soil texture is clay and pH varies from slightly acidic to neutral (6.77–7.07). Soil salinity varies from 39.03 to 59.03 m.e./100 g and cation exchange capacity varies from 34.53 to 38.23 m.e./100 g (Mahmood 2004).
Standing biomass and annual biomass increment
Above-and below-ground biomass of B. parviflora seedlings was estimated from destructive sampling method. A total of eighteen plots (1 m × 1 m) were selected randomly and seedlings of B. parviflora inside the plots were collected with their root system. Low-pressure water jet was used to loosen the soil from the collar region of seedlings and the surrounding areas at 50 cm radius. Once the soil was sufficiently loosened, the seedlings were uprooted. The collected seedlings from each plot were then separated into leaves, stems and roots. The fresh mass of each part was measured and recorded in the field at plot basis. Sub-samples from each part (about 0.25 kg) were brought back to the laboratory to obtain fresh to oven dry mass conversion ratios at 80 °C. The oven-dry mass (kg) of seedling parts was calculated from the respective fresh to oven-dry mass (kg) conversion ratios. The above and below-ground biomass and annual biomass increment of B. parviflora saplings and trees were taken from Mahmood et al. (2008).
Litter standing crop and litter production
Natural litter bed (leaves, small branches, bracts and propagules) of the study area was observed from 18 randomly selected plots (1 m × 1 m) during the wet, intermediate and dry seasons. All litter from the individual plot was collected. The collected litter was washed thoroughly to remove all the dirt and sediment particles and sorted into leaves, small branches (diameter <2 cm), bracts and propagules. Leaves were again sorted into intact and broken leaves. The sorted litter was oven dried at 80 °C until constant mass. Total amount of litter standing crop and their parts in different seasons were compared by one way analysis of variance followed by Duncan Multiple Range (DMRT) Test by using SAS (6.12) statistical software. The amount and rate of litter production of the study area was taken from Mahmood et al. (2005).
Carbon in plant and litter parts; and soil
Six seedlings were randomly selected, uprooted and separated into parts as described earlier. Six saplings were randomly selected and uprooted by low pressure water jet and portable winch. Collected saplings were then separated into different parts (leaves, buds, branches, bark, stems and roots). Six trees were selected randomly and sub-samples of leaves, buds, and small branches (diameter <2 cm) were collected by using sharp knife attached with long pole. Bark samples were collected by using sharp knife and stem samples were collected by using stem borer. One buttress root was selected randomly from each tree and roots were excavated followed by the buttress root by using water jet. Sub-samples (about 100 g) of different parts of seedlings, saplings and trees were collected randomly during the dry, wet and intermediate seasons. Sub-samples were then oven-dried at 80 °C until constant mass. Eighteen litter traps (1 m × 1 m) were suspended under the tree canopy at a height of 1.5 m above the ground level (beyond the reach of high tides). The trapped litter was collected at monthly to get the litter samples during the dry, wet and intermediate seasons. Season wise litter samples were sorted into leaves, small branches, bracts, flowers and propagules and oven dried at 80 °C until constant mass. Nine samples of soil were collected randomly for the determination of bulk density and carbon concentration at the following depths: 0–15, 15–30, 30–50 and 50–100 cm by using core sampler of 5 cm diameter. The different samples of seedlings, saplings, trees and litter parts; and soil were processed according to Allen (1974). Carbon concentrations in the samples were measured by using Leco CR—12 Carbon determinator, USA. Carbon concentration in different parts of seedlings, saplings and trees and litter at different seasons were compared by one way analysis of variance followed by DMRT (p < 0.05) by using SAS (6.12) statistical software. Carbon pool in plant parts was estimated from their weighted mean carbon concentration and the respective oven-dry biomass. Carbon pool in each soil layer was estimated as the product of the carbon concentration and their corresponding bulk density of each layer.
Results
Biomass and annual biomass increment
The above and below-ground biomass of seedlings, saplings and trees of B. parviflora were 1.12, 0.89 and 142.46 Mg/ha; 0.25, 0.14 and 18.93 Mg/ha respectively (Table 1). Mahmood et al. (2008) reported the yearly average biomass increments for saplings and trees were 0.58 and 16.51 Mg/ha/year respectively (Table 2).
Litter standing crop and litter production
The amount of different parts of litter standing crop showed significant (p < 0.05) variation among the seasons. Comparatively (p < 0.05) higher amount of leaf litter standing crop was observed during the intermediate seasons and lower amount during the dry seasons. The amount of total litter standing crop was ranged from 0.66 to 0.88 Mg/ha (Table 3). Litter production was 10.35 t/ha/year and the rate of leaf litter, small branches, bracts, flowers, propagules and total litter production varied from 1.63 to 3.07, 0.08 to 0.38, 0.13 to 0.39, 0.01 to 0.23, 0.03 to 2.29 and 2.31 to 4.46 g/m2/day respectively (Mahmood et al. 2005).
Carbon concentration in plants and litter parts; carbon pool and fluxes
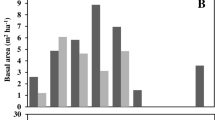
Seasonal fluctuation in carbon concentration in different parts of seedlings was observed and stems (48.64 ± 0.08–56.24 ± 0.03) contained relatively (p < 0.05) higher carbon followed by leaves and roots (Fig. 1). In saplings parts, stems (46.53 ± 0.06–52.40 ± 0.08 %) and branches (48.05 ± 0.03–50.28 ± 0.06 %) showed comparatively (p < 0.05) higher carbon concentrations at different seasons and leaves contained lower carbon (Fig. 2). In trees, stems (45.26 ± 0.01–57.52 ± 0.02 %) and roots (46.06 ± 0.01–53.68 ± 0.03 %) contained comparatively (p < 0.05) higher carbon concentration during different seasons (Fig. 3). However, comparatively (p < 0.05) higher carbon concentration (48.77 ± 0.07–54.92 ± 0.02 %) was found in leaves of litter production in different seasons (Fig. 4) and all the parts of litter standing crops showed wide range in carbon concentration among the seasons (Fig. 5). Most of the parts of seedlings, saplings, trees and litter were found to contain higher carbon concentration during the dry seasons (Figs. 1, 2, 3, 4, 5). Considerable variations in weighted mean carbon concentrations (40.19 ± 0.87–56.52 ± 1.01) were observed for different parts of seedlings, saplings, trees and litter (Table 4). The carbon pools in above and below-ground plant material were 74.45 and 9.78 Mg C/ha respectively. The estimated carbon pool in the soil (up to 1 m) of the study area was 488.04 Mg C/ha. However, 13.95 Mg C/ha/year of carbon flux was associated with different parts of saplings, trees and litter production (Table 5).
Discussion
Carbon concentrations in different parts of plant are strongly related with the biological activities of the plant parts (Santa Regina 2000) and structural components of plant cell (Kaakinen et al. 2004). This could be the reason for observing variation in carbon concentration in different parts of plants and litter components. Moreover, comparatively higher carbon concentrations were usually observed in woody parts of plants (Figs. 1, 2, 3, 4, 5) (Hart et al. 2003) because they contain higher concentrations of cellulose, hemicellulose and lignin (Schädel et al. 2009; Körner 2003). Plant species, types of plant parts and physiological age of the tissue and seasons (dry and wet) may influence the carbon concentration in plant parts (Salazar et al. 2010). Seasonal variation in carbon concentrations in plant parts was also reported and higher concentrations were observed during dry seasons (Figs. 1, 2, 3, 4, 5). Similar observation of carbon concentrations in different parts of Sonneratia apetala, Excoecaria agallocha and Avicennia alba were also described by Mitra et al. (2011) for the Sundarbans mangrove forest. The dry seasons of the study area were characterized by high salinity (30 ppt) of water and lower numbers of tidal inundation (25 tides, May–August) compared to wet seasons (25 ppt water salinity; 45 tides, September–December) (Mahmood 2004). Salinity may have positive relationship with carbon assimilation in salt tolerant species (Asch et al. 2000) and tidal inundation showed negative relationship with net ecosystem exchange of CO2 and carbon assimilation for the salt marshes (Kathilankal et al. 2008). Irrespectively, seasonal variation in carbon concentrations in different parts of plants and litter may be due to the variability in assimilation of CO2 by plants in relation to solar irradiation, moisture, temperature (Kato and Tang 2008), and different stress factors like high salinity, arid environment, and low availability of nutrients (Bouillon et al. 2008; Barr et al. 2010). Higher amounts of net ecosystem exchange of CO2 and net primary productivity are observed during the growing season and expecting higher increment of biomass and carbon concentration in plant parts (Zha et al. 2007; Ardö et al. 2008). Moreover, these variations of carbon concentration in plant parts may affect the carbon pools and fluxes at season as well as annual scales in mangroves as reported by Mitra et al. (2011).
Quantification of mangrove carbon pools and its monitoring over time can be important information for formulating climate change mitigation strategies. The total carbon pool (572.27 Mg C/ha) (above and below-ground plant material and soil carbon to 1 m) in the study area was comparatively higher than the carbon pool (411–414 Mg C/ha) in Palau and Yap site of Micronesian mangrove forest dominated by S. alba, R. apiculata and B. gymnorrhiza (Kauffman et al. 2011). Moreover, the much lower carbon pools (119.3 Mg C/ha) in Kandelia obovata dominated mangrove stand at Okinawa Island of Japan was reported by Khan et al. (2007). While comparatively higher carbon pool (993.3 Mg C/ha) and higher carbon flux (20 Mg C/ha/year) were recorded from the Indonesian mangrove forests (Murdiyarso et al. 2009) and Matang mangrove forest of Malaysia (Ong 1993) respectively. The variation in carbon pool in different mangrove forest arises from carbon storage in above-ground biomass, forest structure (Bradford et al. 2010), forest age (Kridiborworn et al. 2012; Cerón-Bretón et al. 2011), disturbance history (Goodale et al. 2002), dominant species (Ksawani et al. 2007) and below-ground carbon pool. Below-ground pools contribute to a significant proportion (88–98 %) to the total pool of carbon of mangroves (Murdiyarso et al. 2009). This below ground carbon pool largely depends on the depth of the organic soil, carbon concentration in soil, vegetation cover (Conant et al. 1998). Significant differences in carbon flux in mangrove forests depend on soil conditions (Oren et al. 2001), climatic conditions (Barford et al. 2001), variations in rate of biomass increment among the species and litter production (Bradford et al. 2010). These factors and relationships are essential for understanding the control over forest carbon pools and fluxes over large areas (Waring and Running 1998; Kennedy et al. 2006).
Estimation of carbon pools as well as carbon flux in naturally growing mangrove forests give more insight into the ecological services and role of mangroves. Moreover, this study helps to develop the rapid assessment and monitoring of the carbon pool in Malaysian mangroves. Mangroves appear to be one of the most carbon dense types of tropical forest. The information generated from this study will help in evaluating their values and benefits from conservation and restoration of mangroves. Moreover, mangroves could benefit from REDD+ and should be considered among strategies for climate change mitigation.
References
Allen SE (1974) Chemical analysis of ecological materials. Blackwell Scientific publication, Oxford
Alongi DM (2009) The energetics of mangrove forests. Springer Science and Business Media BV, New York
Ardö J, Mölder M, El-Tahir BA, Elkhidir HAM (2008) Seasonal variation of carbon fluxes in a sparse savanna in semi arid Sudan. Carbon Bal Manag 3:7. doi:10.1186/1750-0680-3-7
Asch F, Dingkuha M, Dorffling K (2000) Salinity increases CO2 assimilation but reduce growth in field-grown, irrigated rice. Plant Soil 218:1–10
Barford CC, Wofsy SC, Goulden ML, Munger JW, Pyle EH, Urbanski SP, Hutyra L, Saleska SR, Fitzjarrald D, Moore K (2001) Factors controlling longand short-term sequestration of atmospheric CO2 in a mid-latitude forest. Science 294:1688–1691
Barr JG, Engel VC, Fuentes JD, Zieman JC, O’Halloran TL, Smith TJ III, Anderson GH (2010) Controls on mangrove forest-atmosphere carbon dioxide exchanges in western Everglades National Park. J Geophys Res Biogeo. doi:10.1029/2009JG001186
Bouillon SM, Frankignoulle F, Dehairs B, Velimirov A, Eiler H, Etcheber G, Borges AV (2003) Inorganic and organic carbon biogeochemistry in the Gautami Godavari estuary (Andhra Pradesh, India) during pre-monsoon: the local impact of extensive mangrove forests. Global Biogeochem CY 17(4):1114. doi:10.1029/2002GB002026
Bouillon S, Borges AV, eda-Moya EC, Diele K, Dittmar T, Duke NC, Kristensen E, Lee SY, Marchand C, Middelburg JJ, Rivera-Monroy VH, Smith III TJ, Twilley RR (2008) Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochem CY 22:1–12 (GB2013). doi: 10.1029/2007GB003052
Bradford JB, Weishampel P, Smith ML, Kolka R, Birdsey RA, Ollinger SV, Ryan MG (2010) Carbon pools and fluxes in small temperate forest landscapes: variability and implications for sampling design. Forest Ecol Manag 259:1245–1254
Cerón-Bretón RM, Cerón-Bretón JG, Sánchez-Junco RC, Damián-Hernández DL, Guerra-Santos JJ, Muriel-Garcia M, Cordova-Quiroz AV (2011) Evaluation of carbon sequestration potential in mangrove forest at three estuarine sites in Campeche Mexico. Int J Energy Environ 5(4):487–494
Conant RT, Klopatek JM, Malin RC, Klopatek CC (1998) Carbon pool and fluxes along an environmental gradient in northern Arizona. Biogeochemistry 43:43–61
Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Affiliations MK (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geos 4:293–297
Ellison AM (2008) Mangrove ecology–applications in forestry and coastal zone management. Aquat Bot 89:77
Fujimoto K, Imaya A, Tabuchi R, Kuramoto S, Utsugi H, Murosushi T (1999) Belowground carbon storage of Micronesian mangrove forests. Ecol Res 14:409–413
Goodale CL, Apps MJ, Birdsey RA, Field CB, Heath LS, Houghton RA, Jenkins JC, Kohlmaier GH, Kurz W, Liu SR, Nabuurs GJ, Nilsson S, Shvidenko AZ (2002) Forest carbon sinks in the Northern Hemisphere. Ecol Appl 12:891–899
Hart PBS, Clinton PW, Allen RB, Nordmeyer AH, Evans G (2003) Biomass and macro- nutrients (above- and below-ground) in a New Zealand beech (Nothofagus) forest ecosystem: implications for storage and sustainable forest management. Forest Ecol Manag 174(1–3):281–294
IPCC (2007) Summary for policymakers. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
ISME (1993) The economic and environmental values of mangrove forest and their present state of conservation in the South-East Asia and Pacific Region. Mangrove Ecosystems Technical Reports Vol 1. Okinawa: ISME
Kaakinen S, Jolkkonen A, Iivonen S, Vapaavuori E (2004) Growth, allocation and tissue chemistry of Picea abies seedlings affected by nutrient supply during the second growing season. Tree Physiol 24:707–719
Kanninen M, Murdiyarso D, Seymour F, Angelsen A, Wunder S, German L (2007) Do trees grow on money? The implications of deforestation research for policies to promote REDD. CIFOR, Bogor
Kathilankal JC, Mozdzer TJ, Fuentes JD, D’Odorico P, McGlathery KJ, Zieman JC (2008) Tidal influences on carbon assimilation by salt marsh. Environ Res Lett. doi:10.1088/1748-9326/3/4/044010
Kato T, Tang Y (2008) Spatial variability, and major controlling factors of CO2 sink strength in Asian terrestrial ecosystems: evidence from eddy covariance data. Glob Change Biol 14:2333–2348
Kauffman JB, Heider C, Cole TG, Dwire KA, Donato DC (2011) Ecosystem carbon stocks of micronesian mangrove forests. Wetlands 31:343–352
Keeling CD, Bacastow RB, Carter AF, Piper SC, Whorf TP, Heimann M, Mook WG, Roeloffzen H (1989) Aspect of climate variability in the pacific and Western Americans. In: Pererson DH (ed) Geophysical monograph 55. American Geographical Union, Washington DC, pp 165–236
Kennedy RE, Turner DP, Guzy M, Cohen WB (2006) A method to efficiently apply a biogeochemical model to a landscape. Landsc Ecol 21:213–224
Khan MNI, Suwa R, Hagihara A (2007) Carbon and nitrogen pools in a mangrove stand of Kandelia obovata (S., L.) Yong: vertical distribution in the soil–vegetation system. Wetl Ecol Manag 15(2):141–153. doi:10.1007/s11273-006-9020-8
Kimmins JP (2004) Forest ecology: a foundation for sustainable forest management and environmental ethics in forestry. Prentice Hall, NewYork
Komiyama A, Ong JE, Poungparn S (2008) Allometry, biomass and productivity of mangrove forests: a review. Aquat Bot 89:128–137
Körner C (2003) Carbon limitation in trees. J Ecol 91:4–17
Kridiborworn P, Chidthaisong A, Yuttitham M, Tripetchkul S (2012) Carbon sequestration by mangrove forest planted specifically for charcoal production in Yeesarn, Samut songkram. J Sustain Energy Environ 3:87–92
Kristensen E, Bouillon S, Dittmar T, Cyril Marchand (2008) Organic carbon dynamics in mangrove ecosystems: a review. Aquat Bot 89:201–219
Ksawani I, Kmarusaman J, Nurum-Nadhirah MI (2007) Biological diversity assessment of Tok Bali mangrove forest, klantan, Malaysia. WSEAS Transac on Environ Develop 3:7–44
Laffoley D, Grimsditch G (2009) The management of natural coastal carbon sinks. IUCN, Gland
Langner A, Miettinen J, Siegert F (2007) Land cover change 2002–2005 in Borneo and the role of fire derived from MODIS imagery. Glob Change Biol 13:2329–2340
Mahmood H (2004) Biomass, litter production and nutrients in Bruguiera parviflora dominated mangrove forest ecosystem at Kuala Selangor, Malaysia. PhD thesis. Universiti Putra Malaysia
Mahmood H, Saberi O, Japar Sidik B, Misri K (2005) Litter flux in Kuala Selangor Nature Park mangrove forest Malaysia. Indian J For 28(3):233–238
Mahmood H, Saberi O, Japar Sidik B, Kusnan M (2008) Net primary productivity of Bruguiera parviflora (Wight & Arn.) dominated mangrove forest at Kuala Selangor, Malaysia. For Ecol Manag 255:179–182
Mitra A, Sengupta K, Banerjee K (2011) Standing biomass and carbon storage of above-ground structures in dominant mangrove trees in the Sundarbans. For Ecol Manag 261:1325–1335
Murdiyarso D, Donato D, Kauffman JB, Kurnianto S, Stidham M, Kanninem M (2009) Carbon storage in mangrove and peat land ecosystems- a preliminary account from plots in Indonesia. Working paper 48, Center for International Forestry Research
Ong JE (1982) Mangroves and aquaculture in Malaysia. Ambio 11(5):252–257
Ong JE (1993) Mangroves -a carbon source and sink. Chemosphere 27:1097–1107
Oren R, Ellsworth DS, Johnsen KH, Phillips N, Ewers BE, Maier C, Schafer KVR, McCarthy H, Hendrey G, McNulty SG, Katul GG (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 411:469–472
Page SE, Siegert F, Rieley JO, Boehm H-DV, Jaya A, Limin S (2002) The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420:61–65
Rattan L (2008) Sequestration of atmospheric CO2 in global carbon pools. Energy Environ Sci 1:86–100
Salazar S, Sanchez LE, Galindo P, Santa-Regina I (2010) Above-ground tree biomass equations and nutrient pools for paraclimex chestnut stand and for a climax oak stand in the Sierra de Francia Mountains, Salamanca, Spain. Sci Res Essays 5(11):129–1301
Santa Regina I (2000) Biomass estimation and nutrient pools in tour Quercus pyrenaica in Sierra de Gata Mountains, Salamanca, Spain. For Ecol Manag 132:127–141
Schädel C, Blöchl A, Richter A, Hoch G (2009) Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiol Bioch 47(1):1–9
Spalding MD, Blasco F, Field CD (1997) World mangrove atlas. The International Society for Mangrove Ecosystems, Okinawa
Valiela I, Bowen JL, York JK (2001) Mangrove forests: one of the world’s threatened major tropical environments. Bioscience 51:807–815
Waring RH, Running SW (1998) Forest ecosystems-analysis at multiple scales. Academic Press, San Diego
Watson GJ (1928) Malayan forest record 6: mangrove forests of the Malay Peninsula. Fraser and Neave Ltd., Singapore
Zha T, Xing Z, Wang KY, Kellomäki S, Barr AG (2007) Total and component carbon fluxes of a scots pine ecosystem from chamber measurement and eddy covariance. Ann Bot 99:345–353
Acknowledgments
The authors wish to thank the Department of Biology, University Putra Malaysia and the Malaysian Natural Society for their financial and logistic support throughout the study period.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hossain, M. Carbon pools and fluxes in Bruguiera parviflora dominated naturally growing mangrove forest of Peninsular Malaysia. Wetlands Ecol Manage 22, 15–23 (2014). https://doi.org/10.1007/s11273-013-9318-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-013-9318-2